Cataract surgery is the most common ocular surgery in the United Kingdom with approximately 330,000 operations performed annually.1 Notably, 92% of patients without ocular comorbidity and 77% of patients with ocular comorbidity achieved a visual acuity (VA) of 6/12 or better.2 However, one in every 50 cases experience severe complications.3 In this article, we will explore the aetiology, risk factors, differential diagnosis and treatment of the following complications that may occur following cataract surgery:
- Posterior capsular opacification (PCO)
- Cystoid macular oedema (CMO)
- Endophthalmitis
Posterior Capsular Opacification (PCO)
Pathophysiology
Extracapsular cataract surgery (ECCS) with phacoemulsification is the most common method of lens extraction in developing countries.4 The post-operative capsular bag contains residual lens epithelial cells (LECs) that line its interior surface.
Posterior capsular opacification (PCO) is a complication of ECCS in which the anterior or pre-equatorial LECs proliferate, abnormally differentiate, and migrate along the posterior capsule, eventually covering the visual axis and thus impairing vision.5 The incidence of PCO ranges from 5% to 50%.6
Morphologically, PCO can be distinguished by its pearl or fibrous formation. The pearl form is thought to be caused by proliferation of pre-equatorial LECs,5 whilst the fibrous form is the result of LECs lining the anterior capsule undergoing epithelial mesenchymal transformation (EMT).7,8 PCO may be graded by several scales,9 most commonly using retro-illumination, and broadly classified from grade 1 (minimal PCO) to 4 (severe fibrosis or Elschnig pearls covering the visual axis and severely reducing the red reflex).10,11
Risk Factors
Surgical Technique
Any residual LECs must be removed to reduce the likelihood of PCO. Hydrodissection is used to separate the lens cortex from the capsular bag and minimise zonal-capsular rupture;12,13 this reduces the area of central PCO.14
A circular continuous curvilinear capsulorrhexis (CCC), which has a diameter of 5.25 mm, optimises the prevention of PCO.15 Furthermore, designing the CCC during ECCS so that its diameter is slightly smaller than diameter of the intraocular lens (IOL) optic size limits or prevents the migration of LECs to the posterior capsule.12,16
Femtosecond laser-assisted cataract surgery (FLACS) was first performed in 200917 and has been shown to create a more precise corneal incision and capsulorrhexis.18-20 Studies on the influence of FLACS on PCO formation are inconclusive.19, 21-24
Post-operative Inflammation
Any surgical insult to the anterior capsule, such as ECCS, triggers an inflammatory response in the eye due to a breakdown of the blood-aqueous barrier.25 This inflammatory response consists of the release of cytokines, growth factors and hepatocytes, including transformation growth factor β (TGFβ) and basic fibroblast growth factors (bFGFs).26 These inflammatory mediators likely interact with the LECs via complex pathways, resulting in the development of PCO.26
IOL Properties
Hydrophobic IOL implants are commonly used in ECCS as they demonstrate lower rates of PCO than hydrophilic IOLs, mainly because of their sharp edge design and bioadhesive properties.27 The sharp edge at the back of the IOL compresses the posterior capsule and prevents the LECs from migrating further towards the central posterior capsule.12, 28 Additionally, the bioadhesive properties of the IOL encourages adhesion between the LECs and the IOL, thereby limiting LEC migration and resulting in lower rates of visually significant PCO.12, 29
Differential Diagnosis
Anterior capsular phimosis (ACP) is a fibrotic contraction and opacification of the peripheral anterior capsule resulting from the proliferation and differentiation of the anterior LECs when they touch the IOL.30 ACP is mainly managed with neodymium-doped yttrium aluminium garnet (Nd:YAG) laser treatment to break the anterior capsule and release capsular tension (figure 1).30
Figure 1: Anterior capsular phimosis. White arrow indicates region of fibrous contraction and opacification
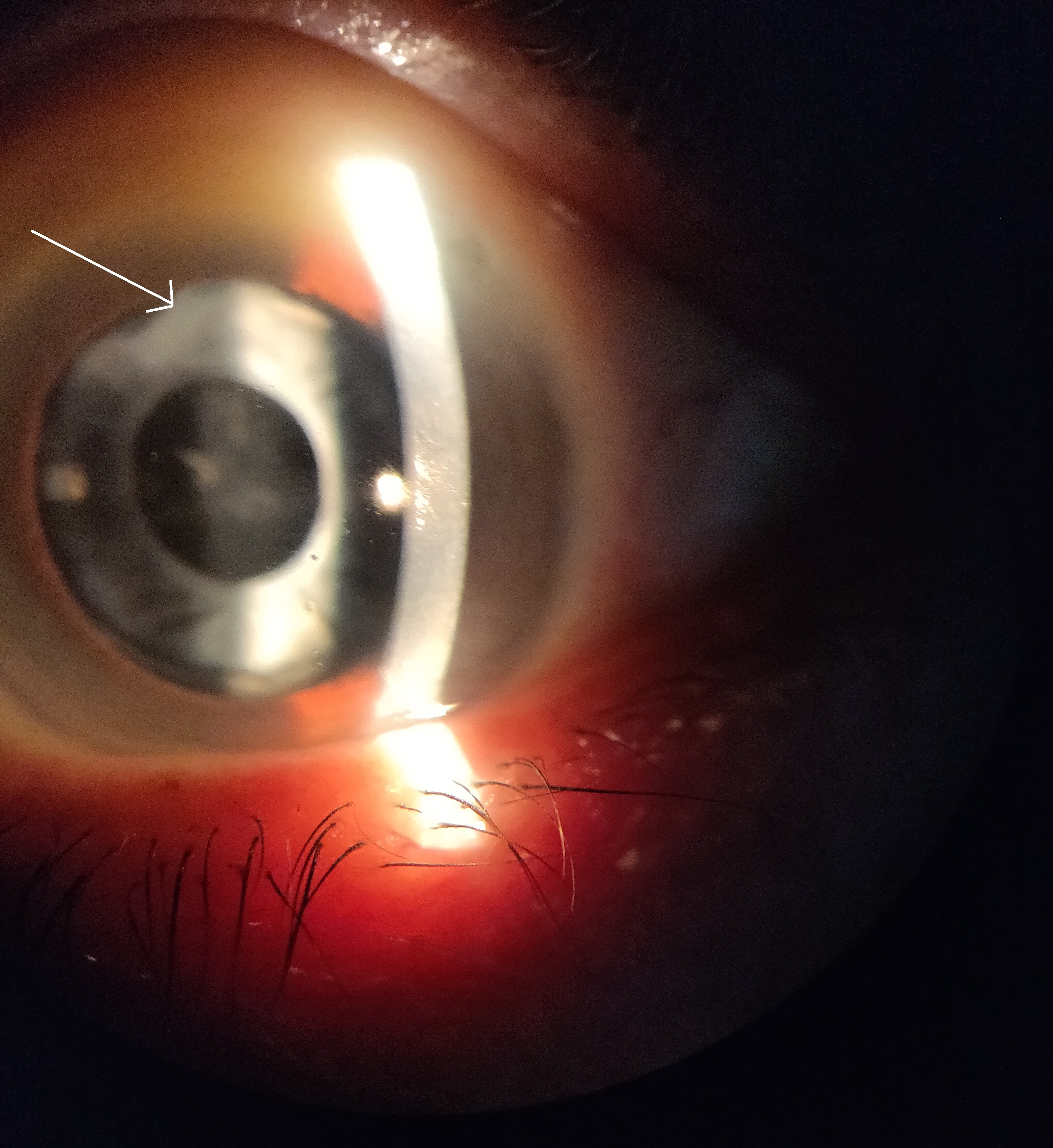
Soemmering’s ring is caused by an insult to the anterior capsule which results in extruded cortical material. The equatorial LECs proliferate and migrate to the posterior and anterior capsule to form Elschnig’s pearls and give the lesion its characteristic donut shape.12
Referral Criteria
Patients suffering from PCO often experience reduced VA, misty vision and/or glare. Visual acuity should be considered alongside other independent causes of visual disability such as contrast sensitivity, glare and stereoacuity31 as these can have a significant impact on quality of life despite good visual acuity.32
This approach, coupled with a consideration of the clinical signs, is supported by the Royal College of Ophthalmologists, since the severity of PCO correlates poorly with high contrast VA.33
The referral guidelines for PCO vary between different integrated care systems, so it is important to be aware of local thresholds. Typically, an onward referral is made if PCO has caused a reduction in VA to 6/9 or less, or if there is patient-reported significant blurring of vision affecting quality of life.34
Treatment
Nd:YAG laser capsulotomy is the mainstay of treatment for adult PCO, whilst a posterior capsulotomy may be preferred in young children or children with developmental delay.35
The incidence of Nd:YAG capsulotomy ranges between 2.4 to 12.6% at 3 years and 5.8 to 19.3% at 5 years after cataract surgery.29 After topical anaesthesia of the eye, a contact lens is fitted to help with laser focusing, to magnify the view and to prevent blinking, while individual laser bursts are applied to the central posterior capsule in a circular or cruciate pattern to create an opening in the visual axis.36
Complications of Nd:YAG capsulotomy include the following:
- Elevated intraocular pressure
- Iritis
- Corneal damage
- IOL pitting
- Cystoid macular oedema
- Disruption of the anterior hyaloid surface
- Increased risk of retinal detachment
- IOL movement or dislocation36, 37
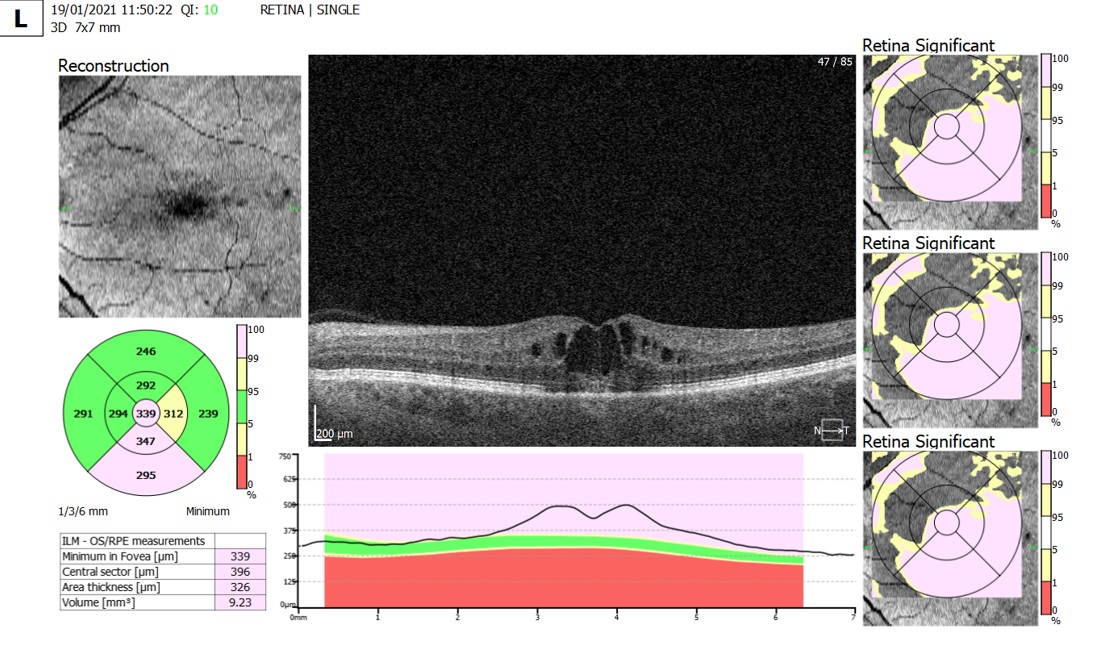
Figure 2: OCT scan depicting post cataract operation macular oedema six weeks after cataract surgery
There is an increasing backlog of patients in the hospital eye services (HES) who require YAG capsulotomy, especially so following the Covid-19 pandemic.38 Community ophthalmology clinics, such as Evolutio Ophthalmology, are supporting the HES with their backlogs. More recently, and with the appropriate guidance and training,39 these procedures are being safely performed by a growing number of trained optometrists.
Post-Operative Cystoid Macular Oedema (CMO)
Pathophysiology
Histologically, post-operative cystoid macular oedema (CMO) occurs when clear fluid displaces retinal cells, forming ‘cyst-like spaces’ in the macula.40 The perifoveal vessels dilate and leak, first causing macular thickening, then causing cystoid spaces in the outer plexiform layer and inner nuclear layer.41
Post-op CMO typically occurs four to six weeks after cataract surgery,42 but can present at any time from days to months post-op. The exact pathogenesis of this condition is unclear. Some studies suggest that it occurs because of inflammation caused by the surgery which in turn disrupts the blood-aqueous barrier.43, 44 Others suggest that inflammatory markers disrupt the blood-retinal barrier causing fluid to leak from the retinal pigment epithelium into the intra-retinal layers.45
Incidence
A literature review conducted by Flach et al. found the incidence of CMO to range from 0.2% to 20%.41 A more recent study found the incidence to be 1.17%, with an increased incidence in patients with existing macular pathology.46 The reason for this variation in reported incidence could be due to the diagnostic tools used in each study (for example whether an angiogram or OCT was used); the incidence might be lower in the more recent studies due to prophylactic measure taken and more modern surgical techniques which are less invasive than intracapsular extraction.47,41
Risk factors
Complications during surgery can increase the risk of CMO. One of those complications is posterior capsular rupture. If this occurs, an increased number of inflammatory markers reach the posterior segment, which in turn can lead to CMO.48 Furthermore, any retained lens fragments or nuclear drop will also increase the risk of CMO49 and result in poorer visual outcomes.49 For this reason, surgeons should make every effort to preserve the posterior capsule.50
The iris produces inflammatory markers when traumatised.51 Therefore, iris trauma during surgery will significantly increase the risk of CMO.52 The risk of CMO also increases when there is significant anterior chamber inflammation, which can also be exacerbated by iris trauma.52 Anterior chamber inflammation seems to impact upon the integrity of the blood-aqueous barrier, the breakdown of which can cause CMO.41
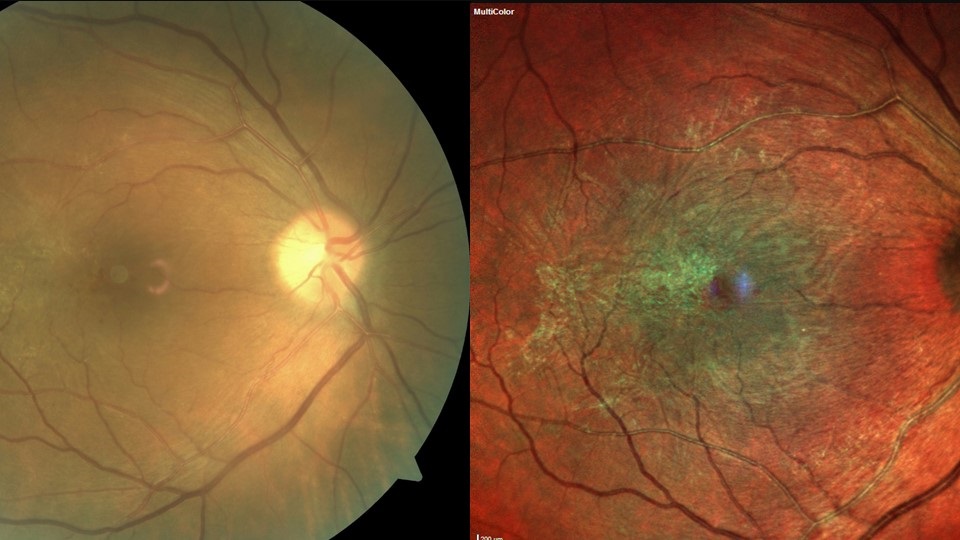
Pre-existing and previous retinal pathology, such as retinal vein occlusion, previous retinal detachment, AMD and diabetic retinopathy (figure 3), are known to increase the risk of post-op CMO. In diabetic patients, epiretinal membrane (ERM) presents the most significant risk of CMO and reduced post-operative VA (figure 4).53, 54 Patients with a significant ERM sometimes undergo combined membrane peel and cataract surgery to reduce the risk of post-op. Diabetes itself can increase the risk of post op CMO, even if there is no pre-existing retinopathy.46
Prostaglandin analogues (PGA) are commonly used to treat raised intraocular pressure. These drugs disrupt the blood aqueous barrier and act as inflammatory mediators, thus increasing the risk of post-op CMO.51
Figure 3: Risk factors for post-operative CMO: (a) Retinal vein occlusion, (b) previous retinal detachment, (c) age-related macular degeneration, and (d) diabetic retinopathy
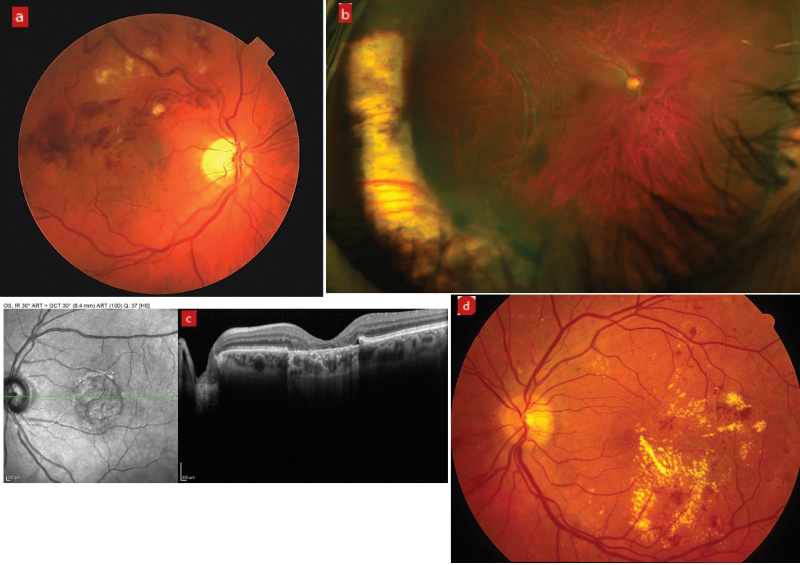
Figure 4: Epiretinal membrane presents the most significant risk of CMO and reduced post-operative VA for diabetic patients
Prophylaxis
Although it is agreed that steroids and non-steroidal anti-inflammatory drugs (NSAIDs) should be used to prevent post-op macular oedema, there seems to be no consensus on a specific prophylactic post-op regime, so different independent care boards (ICBs) implement their own policy on post-op management.48 Some studies have suggested that NSAIDs are more effective than steroids,55 whereas other studies show NSAIDs are better at improving the immediate post-op vision when compared to placebo or steroids. However, 3 months after the operation, the vision with and without NSAIDs is the same.56 Few trials have found that prescribing NSAIDs and steroids together is more effective for prophylaxis than just NSAIDs or steroids alone.57
NICE guidance recommends prescribing NSAIDs and steroids together for patients who are at greater risk of developing post-op CMO, such as diabetics, and to prescribe either NSAIDs or steroids for those who are at low risk.58
Treatment
Topical steroid, topical NSAID or a combination of both can be used for treating post-op macular oedema, although acute CMO may self-resolve without intervention.59 Some studies found that prescribing NSAIDs results in a better visual outcome when compared with placebo.60 One literature review concluded that NSAIDs used with or without steroids are able to treat significant post-op CMO successfully.59 NSAIDs inhibit the enzyme cyclooxygenase which reduces the production of prostaglandins, the inflammatory mediators.61
Other studies have found that using NSAIDs and steroids together to treat macular oedema results in a better visual outcome than just using one of these drugs.62 NICE also recommend a combination of NSAIDs and steroids for post-op CMO.63
There are other treatments that can be considered if the macular oedema is chronic and non-responsive. These include:
- Dexamethasone implants64
- Intravitreal anti-VEGF65, 66
- Retrobulbar and posterior sub-Tenon corticosteroid injections67
- Specific micropulse lasers68
While individual case reports and small trials are promising, larger, controlled, and randomised trials are needed to establish their true efficacy.
Chronic Post-Operative Endophthalmitis (POE)
Pathophysiology
Post-operative endophthalmitis (POE) is a rare, but severe, sight-threatening complication of cataract surgery. It describes an intraocular inflammation that involves the vitreous cavity and the anterior chamber and can also affect the choroid, retina, sclera and cornea.69
Acute-onset POE is defined as an infection within 1 to 6 weeks of surgery which presents as a single episode of rapidly progressing severe inflammation. Acute POE is an ophthalmic emergency and the College of Optometrists’ guidance recommends a same day referral to eye casualty.70
Chronic or delayed-onset postoperative endophthalmitis is defined as insidious inflammation 6 weeks after surgery which is milder than acute cases and may persist for months.69 Community optometrists typically encounter patients at 4 to 6 weeks after surgery.71 Therefore, it is likely that they will encounter the chronic rather than the acute form of endophthalmitis. Hence, this article will focus on chronic POE.
The overall incidence of POE is low in the UK, reported to be 0.03% to 0.14% after cataract surgery.72, 73 The exact incidence of chronic POE is unclear. However, one study recorded rates of 0.05% over a 5-year period.74
Low pathogenicity bacteria are the most common infectious agent, typically arising from intraoperative irrigation fluids, surgical instruments, and inadvertent contact of the intraocular lens on external ocular surfaces.1 Propionibacterium acnes, which (as the name suggests) is related to chronic skin infections, accounts for most cases, with coagulase-negative Staphylococcus spp. and fungal species as other notable causes.75
The organisms that cause acute POE tend to be more virulent and include Gram-positive, coagulase-negative Staphylococcus spp. in 70% of cases.76 In acute POE symptoms occur within 1 week post-operatively with severe ocular inflammation. VA is reduced, the eye is red and 75% of cases report pain. Retinal vessels are often difficult to visualise due to vitreous inflammation.77
Figure 5: White blood cells in the anterior chamber
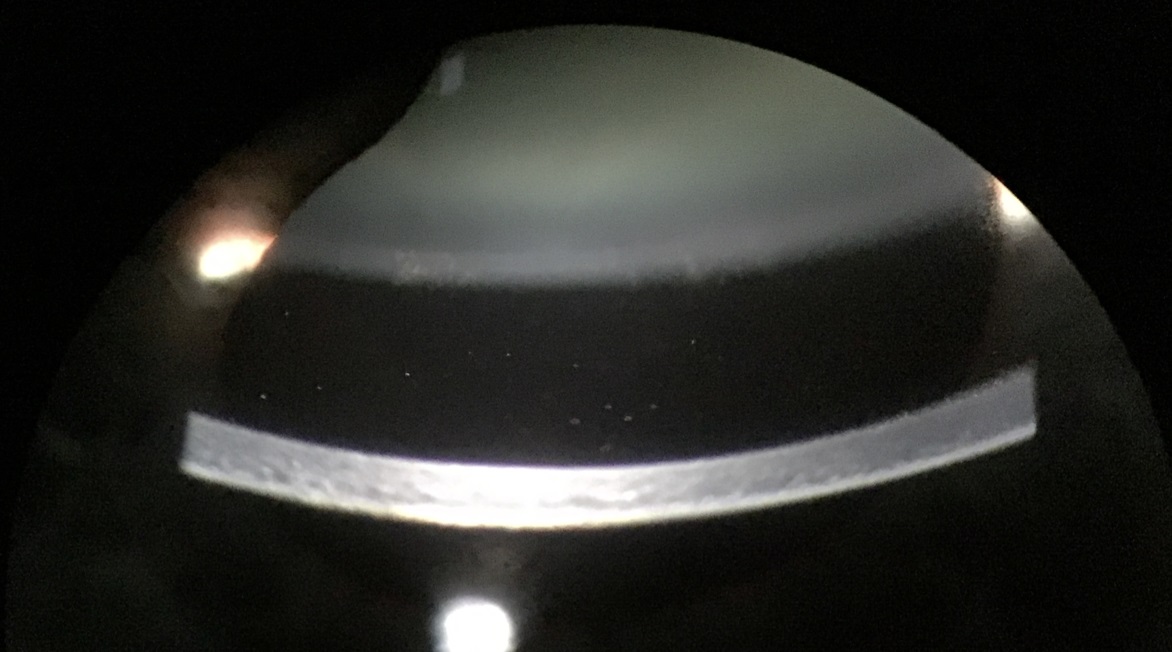
Chronic POE typically presents as a relatively low-grade but progressive uveitis with symptoms of reduced vision with or without mild eye pain. On slit lamp examination, white blood cells are visible in the aqueous humour. The uveitis may be granulomatous with large precipitates on the corneal endothelium or intraocular lens; a micro-hypopyon may be visible during gonioscopy. A white plaque on the posterior lens capsule is more commonly observed in Propionibacterium spp. infections whereas stringy white infiltrates and ‘fluff balls’ or ‘pearls-on-a-string’ near the capsular remnant are often characteristic but not pathognomonic for fungal infection. Vitreous activity is usually mild but can be dense and diffuse particularly with Staphylococcus epidermidis infections.69
Risk factors and prophylaxis
Surgical-specific risk factors include longer operating times, silicone intraocular lenses and intraoperative complications, such as posterior capsular rupture or wound leakage. There is strong evidence to suggest that prophylaxis with perioperative intracameral antibiotics lowers the chance of POE after surgery and this is considered usual practice, despite the lack of any randomised controlled trials to support this.71, 78, 79, 80
Patient-specific factors include conditions that increase atypical organisms in the eyelid and ocular surface flora. The Royal College of Ophthalmologists’ Preoperative Guidance is to manage chronic bacterial blepharitis, active conjunctivitis and infections or obstruction of the lacrimal drainage system prior to surgery.73, 81 Referring optometrists are pivotal in diagnosing and managing these conditions while patients await pre-operative assessment. Systemic conditions that lower infection resistance, such as diabetes, immunosuppression and HIV infection, also carry an increased risk of POE.
Differential Diagnosis
In any case of suspected chronic POE, consider non-infectious causes of uveitis (such as lens-induced inflammation secondary to retained cortical material), IOL-induced uveitis (secondary to IOL malposition causing iris chafing and chronic inflammation) and other causes of uveitis unrelated to surgery.69
Management and Treatment
Chronic POE has a more favourable visual prognosis than acute POE.82 The College of Optometrists’ Clinical Guidelines suggest emergency referral to an ophthalmologist, although this doesn’t differentiate between acute and chronic POE.70 Indeed, it is hard to justify such urgency for chronic POE.
Typically, acute endophthalmitis is a clinical diagnosis supported by vitreous and/or aqueous culture to isolate the organism involved. Therefore, emergency, same-day referral is mandatory. Negative cultures do not exclude the diagnosis, since 20 to 30% of endophthalmitis cases are culture negative; molecular diagnostic techniques (such as a PCR test) have demonstrated a pathogen in many culture-negative cases, and these techniques may play a larger role in endophthalmitis diagnosis in the future.83
Although there is no standardised treatment for chronic POE, most cases are mild and respond to topical steroid and antibiotics, although recurrence is common and drops may be required long term.77 Where active intervention is needed, a combination of removal or exchange of the IOL, total capsulectomy, vitrectomy and intravitreal antibiotics has been the most successful approach. Fungal infections carry a poorer visual prognosis and additionally, systemic antifungals can be indicated.
- Jasraj Bhangra, Naima Babar and Kishan Patel are clinical optometrists working in the Evolutio Community Ophthalmology clinics. Christian Dutton is Evolutio’s Professional Services Manager. Lyn Price is Evolutio’s Clinical Lead (Optometry) and Simon Hardman-Lea is Evolutio’s Clinical Lead (Ophthalmology).
References
- Gonzalez-Salinas R, Guarnieri A, Guirao Navarro MC, Saenz-de-Viteri M. Patient considerations in cataract surgery – the role of combined therapy using phenylephrine and ketorolac. Patient Preference Adherence, 2016;10:1795-1801
- Desai P, Minassian DC, Reidy A. National cataract surgery survey 1997-8: a report of the results of the clinical outcomes. British Journal of Ophthalmology, 1999;83(12):1336-1340.
- National Health Service. Cataract Surgery: Overview. Updated February 09, 2021. www.nhs.uk/conditions/cataract-surgery (Accessed March 07, 2022)
- Davis G. The Evolution of Cataract Surgery. Molecular Medicine, 2016;113(1):58-62.
- Raj SM, Vasavada AR, Johar SRK, Vasavada VA, Vasavada VA. Post-operative capsular opacification: a review. International Journal of Biomedical Science, 2007;3(4):237-250.
- Ursell PG, Dhariwal M, Majirska K, et al. Three-year incidence of Nd:YAG capsulotomy and posterior capsule opacification and its relationship to monofocal acrylic IOL biomaterial: a UK Real World Evidence study. Eye, 2018;32(10):1579-1589
- Iongh RU de, Wederell E, Lovicu FJ, McAvoy JW. Transforming growth factor-β-induced epithelial-mesenchymal transition in the lens: a model for cataract formation. CTO, 2005;179(1-2):43-55
- Wormstone IM, Wang L, Liu CSC. Posterior capsule opacification. Experimental Eye Research, 2009;88(2):257-269.
- Aslam TM, Dhillon B, Werghi N, Taguri A, Wadood A. Systems of analysis of posterior capsule opacification. British Journal of Ophthalmology, 2002;86(10):1181-1186
- Kruger AJ, Schauersberger J, Abela C, Schild G, Amon M. Two year results: sharp versus rounded optic edges on silicone lenses. Journal of Cataract and Refractive Surgery, 2000;26(4):566-570
- Sellman TR, Lindstrom RL. Effect of a plano-convex posterior chamber lens on capsular opacification from Elschnig pearl formation. Journal of Cataract and Refractive Surgery, 1988;14(1):68-72
- Pandey SK, Apple DJ, Werner L, Maloof AJ, Milverton EJ. Posterior capsule opacification: a review of the aetiopathogenesis, experimental and clinical studies and factors for prevention. Indian Journal of Ophthalmology, 2004;52(2):99-112.
- Vasavada AR, Singh R, Apple DJ, Trivedi RH, Pandey SK, Werner L. Effect of hydrodissection on intraoperative performance: randomized study. Journal of Cataract and Refractive Surgery, 2002;28(9):1623-1628
- Vasavada AR, Dholakia SA, Raj SM, Singh R. Effect of cortical cleaving hydrodissection on posterior capsule opacification in age-related nuclear cataract. Journal of Cataract and Refractive Surgery, 2006;32(7):1196-1200
- Packer M, Teuma EV, Glasser A, Bott S. Defining the ideal femtosecond laser capsulotomy. British Journal of Ophthalmology, 2015;99(8):1137-1142
- Smith SR, Daynes T, Hinckley M, Wallin TR, Olson RJ. The effect of lens edge design versus anterior capsule overlap on posterior capsule opacification. American Journal of Ophthalmology, 2004;138(4):521-526
- Nagy Z, Takacs A, Filkorn T, Sarayba M. Initial clinical evaluation of an intraocular femtosecond laser in cataract surgery. Journal of Refractive Surgery, 2009;25(12):1053-1060
- Reddy KP, Kandulla J, Auffarth GU. Effectiveness and safety of femtosecond laser-assisted lens fragmentation and anterior capsulotomy versus the manual technique in cataract surgery. Journal of Refractive Surgery, 2013;39(9):1297-1306
- Kovács I, Kránitz K, Sándor GL, et al. The effect of femtosecond laser capsulotomy on the development of posterior capsule opacification. Journal of Refractive Surgery, 2014;30(3):154-158
- Kolb CM, Shajari M, Mathys L, et al. Comparison of femtosecond laser-assisted cataract surgery and conventional cataract surgery: a meta-analysis and systematic review. Journal of Cataract and Refractive Surgery, 2020;46(8):1075-1085
- Verdina T, Peppoloni C, Barbieri L, et al. Long-term evaluation of capsulotomy shape and posterior capsule opacification after low-energy bimanual femtosecond laser-assisted cataract surgery. Journal of Ophthalmology, 2020; 2020: e6431314
- Wertheimer C, Kreutzer TC, Dirisamer M, et al. Effect of femtosecond laser-assisted lens surgery on posterior capsule opacification in the human capsular bag in vitro. Acta Ophthalmologica, 2017;95(2):e85-e88
- Rostami B, Tian J, Jackson N, Karanjia R, Lu K. High rate of early posterior capsule opacification following femtosecond laser-assisted cataract surgery. COP, 2016;7(3):491-495
- Berk TA, Schlenker MB, Campos-Möller X, Pereira AM, Ahmed IIK. Visual and refractive outcomes in manual versus femtosecond laser–assisted cataract surgery: A single-center retrospective cohort analysis of 1838 eyes. Ophthalmology, 2018;125(8):1172-1180
- Pérez-Vives C. Biomaterial Influence on Intraocular Lens Performance: An Overview. Journal of Ophthalmology, 2018;2018:e2687385
- Meacock WR, Spalton DJ, Stanford MR. Role of cytokines in the pathogenesis of posterior capsule opacification. British Journal of Ophthalmology, 2000;84(3):332-336
- Zhao Y, Yang K, Li J, Huang Y, Zhu S. Comparison of hydrophobic and hydrophilic intraocular lens in preventing posterior capsule opacification after cataract surgery: An updated meta-analysis. Medicine (Baltimore), 2017;96(44):e8301
- Thom H, Ender F, Samavedam S, et al. Effect of AcrySof versus other intraocular lens properties on the risk of Nd:YAG capsulotomy after cataract surgery: A systematic literature review and network meta-analysis. PLOS ONE. 2019;14(8):e0220498. doi:10.1371/journal.pone.0220498
- Ursell PG, Dhariwal M, O’Boyle D, Khan J, Venerus A. 5-year incidence of YAG capsulotomy and PCO after cataract surgery with single-piece monofocal intraocular lenses: a real-world evidence study of 20,763 eyes. Eye, 2020;34(5):960-968
- Hartman M, Rauser M, Brucks M, Chalam K. Evaluation of anterior capsular contraction syndrome after cataract surgery with commonly used intraocular lenses. Clinical Ophthalmology, 2018;12:1399-1403
- Rubin GS, Bandeen-Roche K, Huang GH, et al. The association of multiple visual impairments with self-reported visual disability: SEE project. Investigative Ophthalmology & Vision Science, 2001;42(1):64-72.
- Lu B, Zhu W, Fan Y, Shi D, Ma L. Utility of the optical quality analysis system for decision-making in Nd: YAG laser posterior capsulotomy in patients with light posterior capsule opacity. BMC Ophthalmology, 2021;21(1):7
- Hollingworth W et al. Chapter 6, Case study 2: interventions for treating posterior capsule opacification – a rapid systematic review. In: Hollingworth W et al. Using clinical practice variations as a method for commissioners and clinicians to identify and prioritise opportunities for disinvestment in health care: a cross-sectional study, systematic reviews and qualitative study. Volume 3, Issue 13. Southampton (UK): NIHR Journals Library; 2015:51
- Norfolk and Norwich University Hospitals NHS Foundation Trust. Posterior capsular opacification direct referral form. Published October 14, 2021. www.nnuh.nhs.uk/publication/posterior-capsular-opacification-direct-referral-form-electronic-v1 (Accessed March 28, 2022)
- Shihan MH, Novo SG, Duncan MK. Cataract surgeon viewpoints on the need for novel preventative anti-inflammatory and anti-posterior capsular opacification therapies. Current Medical Research Opinion, 2019;35(11):1971-1981
- Vella M, Wickremasinghe S, Gupta N, Andreou P, Sinha A. YAG laser capsulotomy, an unusual complication. Eye, 2004;18(2):193-194
- Khambhiphant B, Liumsirijarern C, Saehout P. The effect of Nd:YAG laser treatment of posterior capsule opacification on anterior chamber depth and refraction in pseudophakic eyes. Ophthalmology, 2015;9:557-561. doi:10.2147/OPTH.S80220
- Rahman I. Viewpoint: Reducing the burden. OPTICIAN. Published February 11, 2022. www.opticianonline.net/opinion/viewpoint-reducing-the-burden (Accessed March 28, 2022)
- The Royal College of Ophthalmologist. Workforce Census 2018. Published 2018. www.rcophth.ac.uk/wp-content/uploads/2020/05/RCOphth-Workforce-Census-2018.pdf (Accessed March 28, 2022)
- Rotsos TG, Moschos MM. Cystoid macular edema. Clinical Ophthalmology (Auckland NZ), 2008;2(4):919-930.
- Flach AJ. The incidence, pathogenesis and treatment of cystoid macular edema following cataract surgery. Transactions of the American Ophthalmology Society, 1998;96:557-634.
- Erikitola OO, Siempis T, Foot B, Lockington D. The incidence and management of persistent cystoid macular oedema following uncomplicated cataract surgery—a Scottish Ophthalmological Surveillance Unit study. Eye, 2021;35(2):584-591
- Arcieri ES, Santana A, Rocha FN, Guapo GL, Costa VP. Blood-aqueous barrier changes after the use of prostaglandin analogues in patients with pseudophakia and aphakia: A 6-month randomized trial. Archives of Ophthalmology, 2005;123(2):186-192
- Miyake K, Ota I, Maekubo K, Ichihashi S, Miyake S. Latanoprost accelerates disruption of the blood-aqueous barrier and the incidence of angiographic cystoid macular edema in early postoperative pseudophakias. Archives of Ophthalmology, 1999;117(1):34-40
- Daruich A, Matet A, Moulin A, et al. Mechanisms of macular edema: Beyond the surface. Progress in Retinal Eye Research, 2018;63:20-68
- Chu CJ, Johnston RL, Buscombe C, Sallam AB, Mohamed Q, Yang YC. Risk Factors and incidence of macular edema after cataract surgery. Ophthalmology, 2016;123(2):316-323
- Intracapsular cataract extraction - an overview, ScienceDirect Topics. www.sciencedirect.com/topics/nursing-and-health-professions/intracapsular-cataract-extraction (Accessed May 13, 2022)
- Han JV, Patel DV, Squirrell D, McGhee CNJ. Cystoid macular oedema following cataract surgery: A review. Clinical & Experimental Ophthalmology, 2019;47(3):346-356
- Ionides A. Visual outcome following posterior capsule rupture during cataract surgery. British Journal of Ophthalmology, 2001;85(2):222-224
- Menchini U, Bandello F, Brancato R, Camesasca FI, Galdini M. Cystoid macular oedema after extracapsular cataract extraction and intraocular lens implantation in diabetic patients without retinopathy. British Journal of Ophthalmology, 1993;77(4):208-211
- Lobo C. Pseudophakic Cystoid Macular Edema. Ophthalmologica, 2012;227(2):61-67
- Gulkilik G, Kocabora S, Taskapili M, Engin G. Cystoid macular edema after phacoemulsification: risk factors and effect on visual acuity. Canadian Journal of Ophthalmology, 2006;41(6):699-703
- Clinical pseudophakic cystoid macular edema. https://oce.ovid.com/article/02158034-200709000-00019/HTML (Accessed May 13, 2022)
- Hardin JS, Gauldin DW, Soliman MK, Chu CJ, Yang YC, Sallam AB. Cataract surgery outcomes in eyes with primary epiretinal membrane. JAMA Ophthalmology, 2018;136(2):148-154
- Kessel L, Tendal B, Jørgensen KJ, et al. Post-cataract prevention of inflammation and macular edema by steroid and nonsteroidal anti-inflammatory eye drops. Ophthalmology, 2014;121(10):1915-1924
- Kim SJ, Schoenberger SD, Thorne JE, Ehlers JP, Yeh S, Bakri SJ. Topical nonsteroidal anti-inflammatory drugs and cataract surgery. Ophthalmology, 2015;122(11):2159-2168
- Wielders LHP, Schouten JSAG, Winkens B, et al. European multicenter trial of the prevention of cystoid macular edema after cataract surgery in nondiabetics: ESCRS PREMED study report 1. Journal of Cataract and Refractive Surgery, 2018;44(4):429-439
- Recommendations: Cataracts in adults: management, guidance, NICE. www.nice.org.uk/guidance/ng77/chapter/Recommendations#preventing-and-managing-complications (Accessed May 13, 2022)
- Guo S, Patel S, Baumrind B, et al. Management of pseudophakic cystoid macular edema. Surveys in Ophthalmology, 2015;60(2):123-137
- Flach AJ, Dolan BJ, Irvine AR. Effectiveness of ketorolac tromethamine 0.5% ophthalmic solution for chronic aphakic and pseudophakic cystoid macular edema. American Journal of Ophthalmology, 1987;103(4):479-486
- Russo A, Costagliola C, Delcassi L, et al. Topical nonsteroidal anti-inflammatory drugs for macular edema. Mediators of Inflammation, 2013;2013:e476525
- Heier JS, Topping TM, Baumann W, Dirks MS, Chern S. Ketorolac versus prednisolone versus combination therapy in the treatment of acute pseudophakic cystoid macular edema. Ophthalmology, 2000;107(11):2034-2038
- Recommendations: Cataracts in adults: management, guidance, NICE. www.nice.org.uk/guidance/ng77/chapter/Recommendations (Accessed May 13, 2022)
- Dutra Medeiros M, Navarro R, Garcia-Arumí J, Mateo C, Corcóstegui B. Dexamethasone intravitreal implant for treatment of patients with recalcitrant macular edema resulting from Irvine-Gass Syndrome. Investigative Ophthalmology & Vision Science, 2013;54(5):3320-3324
- Demirel S, Batioglu F, Özmert E. Intravitreal ranibizumab for the treatment of cystoid macular edema in Irvine-Gass Syndrome. Journal of Ocular Pharmacology and Therapeutics, 2012;28(6):636-639
- Lin CJ, Tsai YY. Use of aflibercept for the management of refractory pseudophakic macular edema in Irvine-Gass syndrome and literature review. Retina Cases and Brief Reports, 2018;12(1):59-62
- Thach AB, Dugel PU, Flindall RJ, Sipperley JO, Sneed SR. A comparison of retrobulbar versus sub-Tenon’s corticosteroid therapy for cystoid macular edema refractory to topical medications. Ophthalmology, 1997;104(12):2003-2008
- Verdina T, D’Aloisio R, Lazzerini A, et al. The role of subthreshold micropulse yellow laser as an alternative option for the treatment of refractory postoperative cystoid macular edema. Journal of Clinical Medicine, 2020;9(4):1066
- Maalouf F, Abdulaal M, Hamam R. Chronic postoperative endophthalmitis: a review of clinical characteristics, microbiology, treatment strategies, and outcomes. International Journal of Inflammation, vol. 2012, Article ID 313248, 6 pages
- College of Optometrist clinical management guidelines. Endophthalmitis (post-operative) (Exogenous endophthalmitis). www.college-optometrists.org/clinical-guidance/clinical-management-guidelines/endophthalmitis_post-operative_exogenousendophthal. (Published 2022, accessed May 11, 2022)
- Cataracts in adults: management. London: National Institute for Health and Care Excellence (NICE); 2017 Oct. (NICE Guideline, No. 77.) Appendix E, Evidence tables.
- Day A, Donachie P, Sparrow J, Johnston R. The Royal College of Ophthalmologists’ National Ophthalmology database study of cataract surgery: report 1, visual outcomes and complications. Eye, 2015;29:552-560
- The Royal College of Ophthalmologists Ophthalmic Service Guidance. Managing an outbreak of postoperative endophthalmitis - The Royal College of Ophthalmologists. The Royal College of Ophthalmologists. www.rcophth.ac.uk/resources-listing/managing-an-outbreak-of-postoperative-endophthalmitis-2016 (Published 2022, accessed May 11, 2022)
- Nowak MS, Grzybowski A, Michalska-Małecka K, et al. Incidence and Characteristics of Endophthalmitis after Cataract Surgery in Poland, during 2010-2015. International Journal of Environmental Research & Public Health, 2019;16(12):2188 (Published 2019 Jun 20)
- Shirodkar AR, Pathengay A, Flynn HW Jr, Albini TA, Berrocal AM, Davis JL, Lalwani GA, Murray TG, Smiddy WE, Miller D. Delayed- versus acute-onset endophthalmitis after cataract surgery. American Journal of Ophthalmology, 2012 Mar;153(3):391-398.e2
- Results of the Endophthalmitis Vitrectomy Study. A randomized trial of immediate vitrectomy and of intravenous antibiotics for the treatment of postoperative bacterial endophthalmitis. Endophthalmitis Vitrectomy Study Group. Archives of Ophthalmology, 1995;113(12):1479-1496
- Durand ML. Endophthalmitis. Clinical Microbiology and Infection, 2013;19(3):227-234
- Kessel L, Flesner P, Andresen J, Erngaard D, Tendal B, Hjortdal J. Antibiotic prevention of postcataract endophthalmitis: A systematic review and meta-analysis. Acta Ophthalmologica, 2015 Jun; 93(4):303-17
- Endophthalmitis Study Group, European Society of Cataract and Refractive Surgeons. Prophylaxis of postoperative endophthalmitis following cataract surgery: results of the ESCRS multicenter study and identification of risk factors. Journal of Cataract and Refractive Surgery, 2007;33(6):978-88
- Gower EW, Lindsley K, Tulenko SE, Nanji AA, Leyngold I, McDonnell PJ. Perioperative antibiotics for prevention of acute endophthalmitis after cataract surgery. Cochrane Database Systematic Review, 2017;2(2):CD006364 (Published 2017 Feb 13)
- Verma L, Chakravarti A. Prevention and management of postoperative endophthalmitis: A case-based approach. Indian Journal of Ophthalmology, 2017;65(12):1396-1402
- Fox GM, Joondeph BC, Flynn HW Jr, Pflugfelder SC, Roussel TJ. Delayed-onset pseudophakic endophthalmitis. American Journal of Ophthalmology, 1991, Feb 15;111(2):163-73
- Durand ML. Bacterial and Fungal Endophthalmitis. Clinical Microbiology Review, 2017;30(3):597-613
