Photochromic materials have the property of variable light transmittance as a function of incident light intensity and wavelength. Over time, the responsiveness of such materials has been developed for inclusion in a range of lens products widely used in eye care practice.
Nature’s solution to changing light conditions is, of course, the pupillary response (figure 1), which provides a mechanism of alteration of pupil diameter between a nominal 2mm diameter in bright conditions to a pupil diameter of up to 8mm in low light conditions. The pupil response to a light stimulus is relatively rapid,1 with pupil constriction taking place in around three seconds in a healthy adult eye. Recovery of pupil diameter, after removal of a light stimulus, is slightly slower and where recovery takes longer for blue light and is described by Markwell et al as the post-illumination pupil response.2
Figure 1: The direct reflex pupil response to light takes just two to three seconds
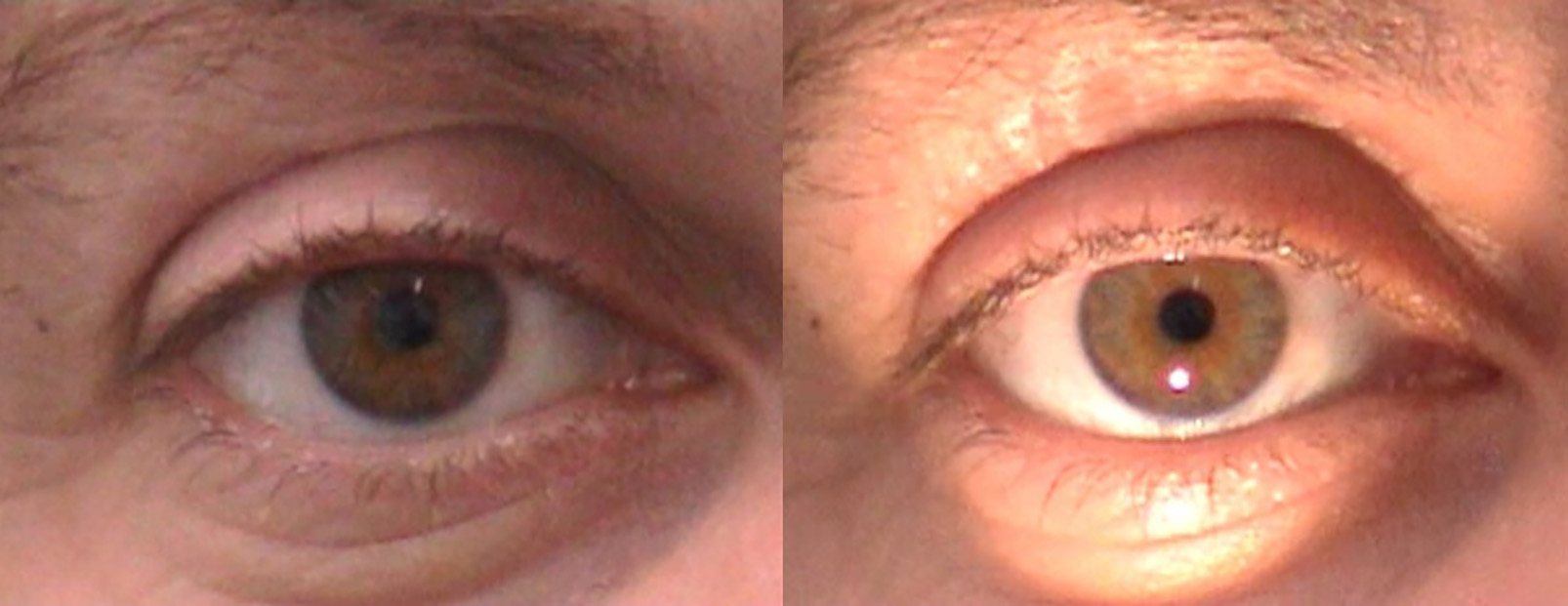
It is important to note that pupil constriction is also linked with improved visual acuity, constricting with accommodation to aid near vision. Also, in moving from photopic to scotopic conditions, the wider pupil improves vision by collecting more light on the retina.
Photochromic Glass
A key feature of photochromic glass is the incorporation of tiny crystals (or crystallites) of a silver halide, such as silver bromide or chloride, within a matrix of inert glass and was initially reported by Arnistead and Stookey,3 and subsequently described by Megla.4
Incident ultraviolet photons have sufficient energy to release an electron from a chlorine ion within the ionic matrix set within the crystallites. The free electron can attach itself to various silver ions, but finds greater stability in silver atoms that exist as defects within the ionic matrix. These negatively charged sites subsequently encourage the migration of neighbouring silver ions, resulting in the dispersal of metallic silver specks within the ionic matrix with a consequent reduction of light transmission. The presence of singly charged ‘impurity’ copper ions within the ionic matrix of crystallites leads to the creation of doubly charged copper ions in the presence of light. With a reduction in light intensity, these migrate to the sites of silver deposition and, in so doing, cause the silver atoms to revert back to ions with a resulting increase in light transmission (figure 2). This mechanism contrasts with that of silver halide photographic film, where the creation of silver particles is an irreversible and permanent event.
Figure 2: Schematic representation of the mechanism of photochromic glass

The Challenge of Plastic Lenses
While it was estimated that around 10 million pairs of glass/halide photochromic lenses were dispensed in the US in 1989, this was at a time when 70% of ophthalmic lenses were plastic.5 The focus of developers to create new photochromic lens technologies was firmly set on incorporating photochromic compounds within plastic lenses.
While the transition to plastic lenses is often described as a trend in the market, this hides a tale of epic struggle by a handful of lens manufacturers aiming to perfect production processes, largely involving the use of the CR-39 monomer that had initially been developed around 1940 by PPG Industries (known as Pittsburgh Plate Glass until 1965). An initial patent application for the CR-39 monomer (in around 1940) had been as a bonding resin used in the fabrication of lightweight fuel tanks of the B-17 bomber. This bonding property of the CR-39 monomer would later present extreme difficulties for numerous plastic lens manufacturers in releasing cured elements from lens moulds. This phase of plastic lens evolution, linked with developments of photochromic technologies, has been carefully documented by PPG Industries.6
Photochromic Dyes
The mainstream technology of photochromic materials in optical systems relates to organic dye compounds, where a given molecule can exist in at least two energy states:
- A; unenergised
- B; energised
The energy exchange between the two states takes place via photon energy exchange, with the activating energy typically within the ultraviolet wavelength range of 300 to 400nm, but could also occur within the visible range (400 to 700nm).
The reverse reaction of B to A is typically triggered by thermal mechanisms as demonstrated by photochromic compounds such as spiropyrans, spiroxamines and chromenes. It is important, however, to recognise the importance of the material into which the photochromic compound is infused since this can significantly affect the rate of the decay of the activated molecule state and also dictate the colours of the energised and unenergised states. Compounds can also be included to prevent degradation of photochromic dye material.
One of the most investigated family of photochromic compounds is that of the spiropyrans family of compounds, within which the specific indolinospiropyrans has been extensively studied. A notable feature of the spiroxamines family of photochromic compounds, which behave in a similar way to spiropyrans, is their superior fatigue-resistance under conditions of long-term light exposure; a property particularly suited to that of ophthalmic lenses. More recently, the benzo and naphthopyrans (or chromenes) have also been developed7 and found application within ophthalmic lenses.
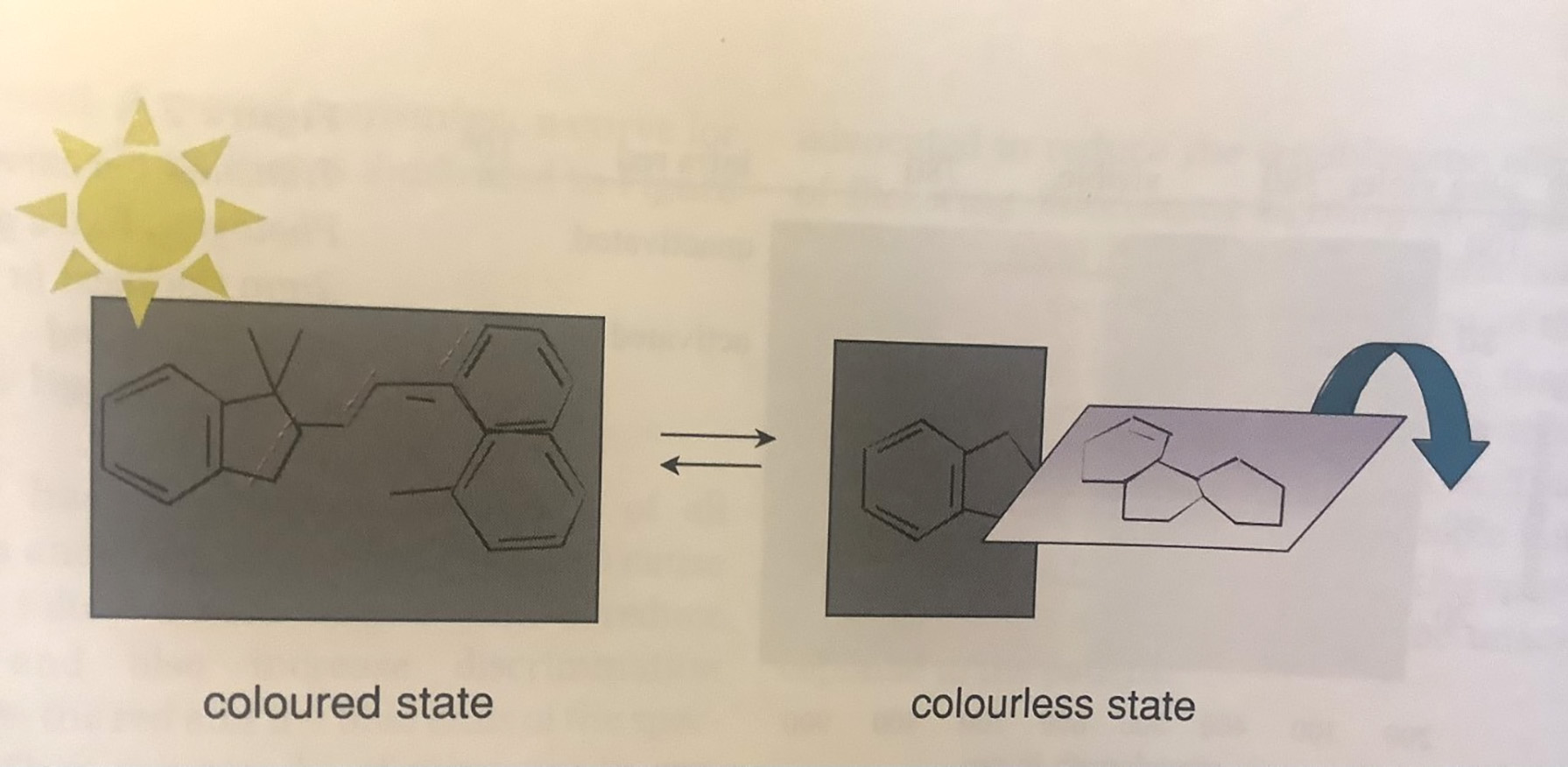
When the molecules of the photochromic material are exposed to ultraviolet radiation, a portion of the molecule rotates, causing the material to darken and absorb strongly in the UV and blue visible wavelength range. Removal of the exciting wavelengths of radiation allows the molecule to flip back to their original orientation and cause the lens to fade back to its original transmittance (figure 3).
Figure 3: Mechanism of photochromatic response by spiropyrans
The basic essentials of an early generation of plastic photochromic lenses were described by Crano and Elias in a 1991 publication,5 at a time when various technical issues relating to the incorporation of photochromic compounds into plastic lenses had at last been successfully overcome. In reality, PPG Industries had been mindful of developing plastic photochromic lenses since around 1973, though activity was increased by the development in 1981 of the Photolite product by American Optical.8 This product, however, would not prove to be a commercial success. The subsequent increased activity at PPG Industries would, however, lead to the development of a new family of photochromic compounds (the pyridobenzoxazines) and also development of the process of imbibition, whereby photochromic dyes are absorbed at depth onto polymers and co-polymers derived from CR-39 monomer.
Crano and Elias also describe how a significant development in photochromic compounds was that of the indolinospiroxazines family, which demonstrated improved fatigue-resistance compared with, for example, spiropyrans. Reference was also made to the issue of relatively narrow absorption bands of activated photochromic compounds, where it is implied that a ‘cocktail’ of matched compounds provides improved photochromic performance. This may, in fact, be an indicator that, in general, photochromic lens technologies use a suitable ‘blend’ of photochromic dyes rather than a single compound.
The imbibition process (developed by PPG Industries) is described as a one where, through heat treatment, the photochromic dyes can penetrate up to 150 microns into the plastic lens surface. This compares with a surface film of around one micron thickness achieved through dip-coating, which would be insufficient to develop the required photochromic properties. Subsequently, the lens was sealed by a hard coating to prevent oxidation of the dye material.
Additional structural options for photochromic plastic lens fabrication by other manufacturers would include embedding the photochromic materials throughout the lens material and various methods of surrounding an inner layer of photochromic material with clear lens material. In the first option, however, thicker areas of such lenses would tend to appear darker when activated by light.
The 1991 publication by Cruno and Elias5 also marked the formation of Transitions Optical as a joint venture between PPG Industries and Essilor International for the manufacture of plastic photochromic lenses. It was a significant loss to the photochromic lens industry, however, when John Cruno (of PPG Industries) and his wife were killed in a traffic accident in 1998. The publication of Organic Photochromic and Thermochromic Compounds,9 with John Cruno as co-editor, provides an insight into the complexities of photochromic dye technology around the time of his death.
Assessing Photochromic Lens Performance
Measurements of the light-reactive characteristics of photochromic lenses made by independent observers provide a valuable insight into their properties. A review by Eppig et al,10 using a common light path for activation and calculation of luminous transmittance, illustrates the basic characteristics, although subsequent standards were to define a more demanding measurement framework. Similar observations are described by Ross.11
Relevant measurement parameters include:
- τ0; the luminous transmittance in the faded state
- τ1; the luminous transmittance in the darkened state
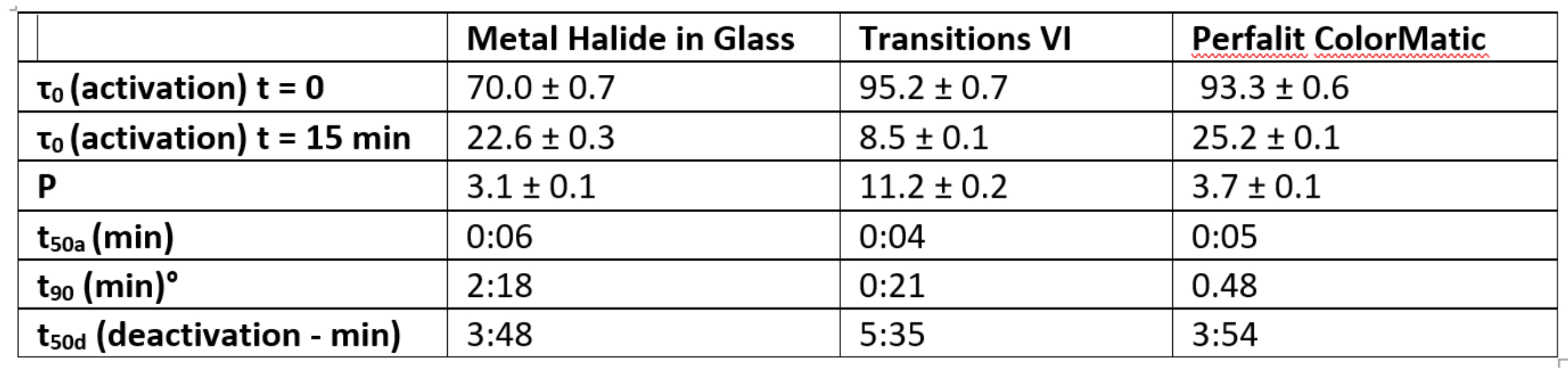
The photochromic response given by the ratio τ0/ τ1 provides an indication of the relative transmission of the faded state and the darkened state. A measure t50a is described as the time to achieve a photochromic response of 0.5 p, and a comparable value of t90 as the time to achieve a photochromic response of 0.9 p when activated by light. In deactivating mode, when the photochromic lens is exposed to interior light levels of 500 lux, a term t50d corresponding to the time to recover to a transmission level of 0.5(τ0 - τ1), provides an indication of how well a darkened lens recovers its transmission levels.
Table 1 shows the specific values derived from observed measurements and demonstrates how activation typically takes place faster than deactivation and how the values of t50a are comparable.
Table 1: Activation properties of photochromic materials10
While measurements were made at 26°C, decreasing temperature would decrease parameters referenced by the value of the value of τ1. Figure 4 shows the typical responses of three photochromic materials. Subsequently, a range of standards have been developed which provide a more formal basis for the assessment of photochromic lens performance.12-14
Figure 4: Response of three photochromic materials10

While no formal standard as yet exists for photochromic contact lenses, Alabi et al describe a system for testing photochromic contact lenses where a novel component is a contact lens holder to establish reference temperatures for measurement procedures.15 The basic system utilises a 50kLux Xenon solar simulator to switch the transmission characteristics of the photochromic lens sample. A separate low intensity beam is used to detect the transmission characteristics of the sample using a spectroradiometer system. The authors indicate that it is only relevant to check photochromic contact lenses at 35°C, rather than at 23°C and 35°C. It was observed that the lenses exhibited maximum darkening after about 15 minutes of exposure to the solar simulator output. In the absence of incident solar radiation, the transmission recovers in a comparable time of 15 to 20 minutes. In comparison with lens characteristics at 23°C and 35°C, transmission levels were consistently higher at the 35°C setting.
The BPC300 photochromic lens spectrophotometer (manufactured by Bentham Instruments in the UK) offers a highly automated testing facility for photochromic lenses where the BPC300-C system is adapted for photochromic contact lens measurements (figure 5).
Figure 5: The Bentham BPC300-C System

In meeting the measurement requirements of the various standards, the Bentham Systems include a spectrophotometer system capable of measuring from 280nm to 780nm in the presence of solar simulated light and, with the inclusion of a monochromatic beam of light of sufficiently low irradiance that does not contribute to the lens photochromic properties. Controlled temperature of the sample under simulation is achieved using a water bath, where standard testing is at 26°C, although determination of lens properties can be undertaken at anything between 5°C and 35°C to verify specific performance claims.
A specific challenge in reference measurement procedures is the ability to maintain even illumination of the activating light beam across the test sample. It has also been observed that the speeds of the reaction process in darkening and bleaching do not feature prominently in reported photochromic lens performance statistics. Furthermore, there is no reference to uniformity of the transmittance values across a photochromic lens surface. It is relevant to note that measurements of ultraviolet exposure of photochromic lenses relate to transmission through the front face of such lenses, with no reference to possible reflected components from the rear of the lens surface.
The use of a solar simulator set at air mass 2 (AM2) references the level of direct solar radiation present for light to travel through the atmosphere for twice the distance of that for vertical delivery at the equator, air mass 1 (AM1). Due to variability atmospheric absorption, as described by Riordan et al, the percentage of incident ultraviolet radiation (which is largely responsible for photochromic lens response) is around 4 to 5% for air mass 1 (AM1), 2% for air mass 2 (AM2) and 0.5 % for air mass 4 (AM4).16 The test conditions also relate to those of direct sunlight. This implies that, even in an outdoors environment, the condition of activation referenced in standards may be infrequently encountered due to factors of latitude, time of day, season of the year and prevailing weather conditions.
Miscellaneous Developments
Intraocular lens implants have been developed with photochromic properties. An example of such is the Photochromic IOL Aurium (from Medennium),17 which enhances blue light absorbance when sunlight is detected. Such a lens was developed to maximise blue light absorbance without the disadvantage of the reduced colour discrimination associated with permanently yellow tinted lens implants.
In the surgical reconstruction of a patient’s damaged iris, consideration has been given to the use of Photopia photochromic material, to replicate a degree of light transmission to the retina. Preliminary proof of concept in vitro studies18 have demonstrated successful encapsulation of photochromic material and useful light control characteristics.
Discussion
The performance characteristics of photochromic lenses are relatively complex, and customer-focused issues such as price and colour tint preference can be key factors of choice. A subjective assessment of the rate of darkening and recovery, and also levels of tint of a range of photochromic lenses, can be conveniently obtained by activation of a sample set of lenses by a hand-held ultraviolet light source.
While the ‘fatigue resistance’ of early photochromic lenses was a limiting factor for their uptake, with the first Transitions lens claiming a 25% loss of function within two years, current offerings across the market offer superior (though often unstated) levels of fatigue resistance. It is certain, however, that manufacturers diligently obtain such information on their photochromic lens products by means of accelerated ultraviolet exposure techniques. For the user, photochromic lens fatigue typically manifests as a reduction of lens darkening when exposed to bright sunlight. Degradation of photochromic lens performance can be considered to ‘creep’ inwards from the lens front where activation levels of ultraviolet light will be higher. The alteration of visible light transmission in the activated state with ‘lens fatigue’ arouses curiosity, however, regarding any change in the associated level of blocking of ultra violet radiation.
- Dr Douglas Clarkson is a research fellow at the department of clinical physics and bio-engineering, Coventry and Warwickshire University Hospital Trust.
References
- Mathôt S. Pupillometry: psychology, physiology, and function. Journal of Cognition, 2018 Feb 21;1(1):16
- Markwell, EL, Feigl, B, & Zele, AJ. (2010). Intrinsically photosensitive melanopsin retinal ganglion cell contributions to the pupillary light reflex and circadian rhythm. Clinical and Experimental Optometry, 93(3), 137–149.
- Arnistead WH and Stookey SD. Photochromic silicate glasses sensitised by Silver Halide. Science, 1964, 144, 250-154.
- Megla GK. Optical properties and applications of photochromic glass. Applied Optics, 1966 Jun 1;5(6):945-60.
- Crano JC, Elias RC, Plastic photochromic eyewear: a status report, Proc. SPIE 1529, Ophthalmic Lens Design and Fabrication, Colin M. Perrott, Editor(s), (1 December 1991);
- CR-39 Celebrating 50 Years -The story behind the development of plastic lenses, PPG Industries, 2639B 25M 0497.
- Sousa CM, Berthet J, Delbaere S, Coelho PJ. Photochromic fused-naphthopyrans without residual color. Journal of Organic Chemicals, 2012 Apr 20;77(8):3959-68
- Young JM. Plastic photochromic lenses. Optical World, March 1983, 17-18.
- Organic Photochromic and Thermochromic Compounds: Main Photochromic Families. Crano JC, Guglielmetti RJ (editors), Springer, New York, 2002
- Eppig T, Speck A, Gillner M, Nagengast D, Langenbucher A. Photochromic dynamics of ophthalmic lenses. Applied Optics, 2012 Jan 10;51(2):133-8.
- Ross DF, Ophthalmic lenses: accurately characterizing transmittance of photochromic and other common lens materials. Applied Optics, 30, 1991, 3673-3677
- ISO 8980-3:2022. Ophthalmic optics - Uncut finished spectacle lenses - Part 3: Transmittance specifications and test methods.
- BS EN ISO 12312-1:2022. Eye and face protection. Sunglasses and related eyewear-Sunglasses for general use.
- ANSI Z80.3-2015. Ophthalmics: non-prescription sunglass and fashion eyewear requirements.
- Alabi EB, Simpson TL, Harris T, Whitten K. Determining the spectral transmittance of photochromic contact lenses. Contact Lens & Anterior Eye, 2021 Oct;44(5):101406
- Riordan CJ Hulstrom HL, Myers DR, Influences of atmospheric conditions and air mass on the ratio of ultra violet to total solar radiation. Solar Energy Research Institute, 1990.
- Shetty V, Haldipurkar SS, Gore R, Dhamankar R, Paik A, Setia MS. A comparison of visual outcomes in three different types of monofocal intraocular lenses. International Journal of Ophthalmology, 2015 Dec 18;8(6):1173-8
- Shareef FJ, Sun S, Kotecha M, Kassem I, Azar D, Cho M. Engineering a light-attenuating artificial iris. Investigative Ophthalmology & Visual Science, 2016 Apr 1;57(4):2195-202.
