It is estimated that around 4.9 million individuals have bilateral blindness and a further 23 million have unilateral blindness due to corneal disease.1 Existing levels of corneal transplantation using human donor tissue are, however, failing to reduce the incidence of blindness due to corneal disease. The review by Gain et al provides a useful assessment of corneal transplant statistics relating to 2012, a year when 184,576 corneal transplants were recorded in 116 countries. However, around 53% of the world’s population had no access to corneal transplantation that year.2
A limiting factor for conventional transplantation techniques the supply of donor material and, even within the developed world, there is considerable variation in the availability of tissue material. The basic statistic that approximately only one donor cornea is available for every 70 patients requiring a transplant is a major incentive for research to discover alternative methods for corneal grafting, such as corneal bioengineering of various types.
Corneal Disease and Management
It is useful, firstly, to review the main indications for corneal transplants, since improved management of eye conditions can reduce their subsequent need. The main indications are as follows.
Fuchs’ endothelial dystrophy
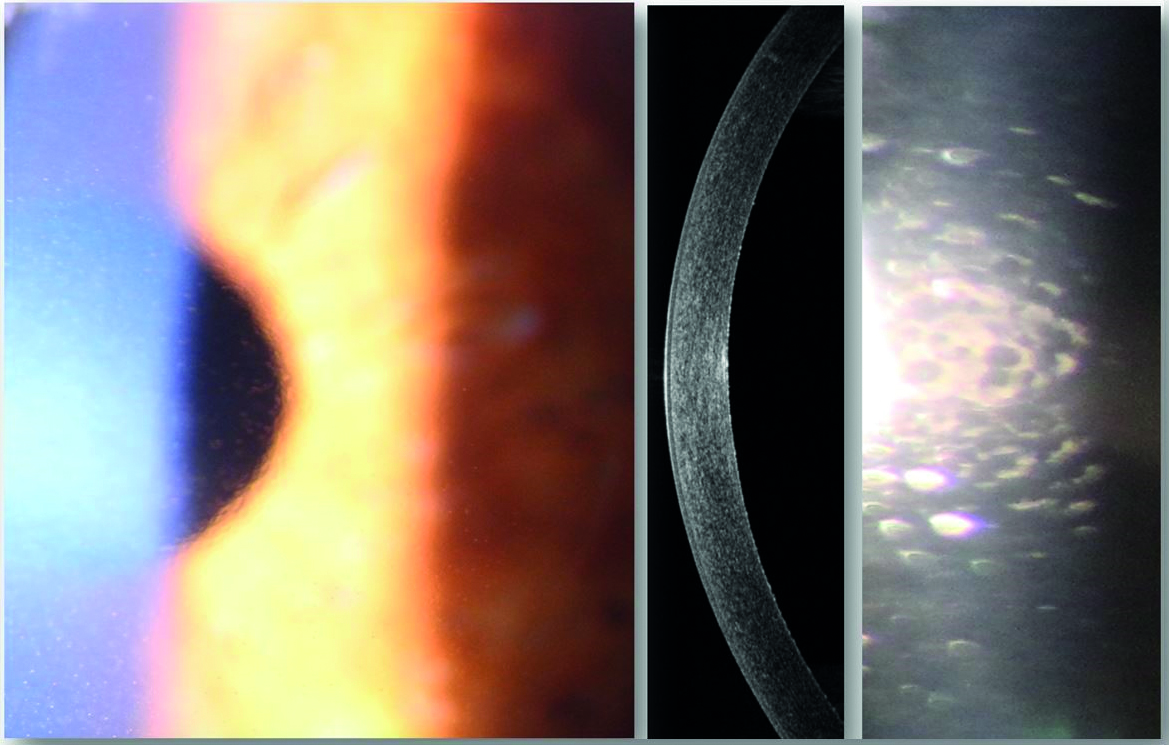
Fuchs’ endothelial dystrophy (figure 1) develops when the density of endothelial cells of the cornea reduces and hence their ability to extract water from the cornea, so maintaining transparency, is also diminished. The consequent oedema causes the cornea to become cloudy or hazy. It is estimated that the number of corneal endothelial cells decreases at around a rate of 0.6% per year.4 A key factor in this reduction is that endothelial cells do not replicate.
Figure 1: Fuchs’ endothelial dystrophy. Retroillumination of the cornea (left) shows low level stromal oedema. Anterior OCT line scan (centre) revealed the central corneal thickness to be 477μ. Endothelial imaging (right) reveals corneal guttata, characterised by the appearance of droplet shaped bulges, and slight disruption to the normal, hexagonal arrangement of the endothelial cells (images from Kirit Patel)
Keratoconus
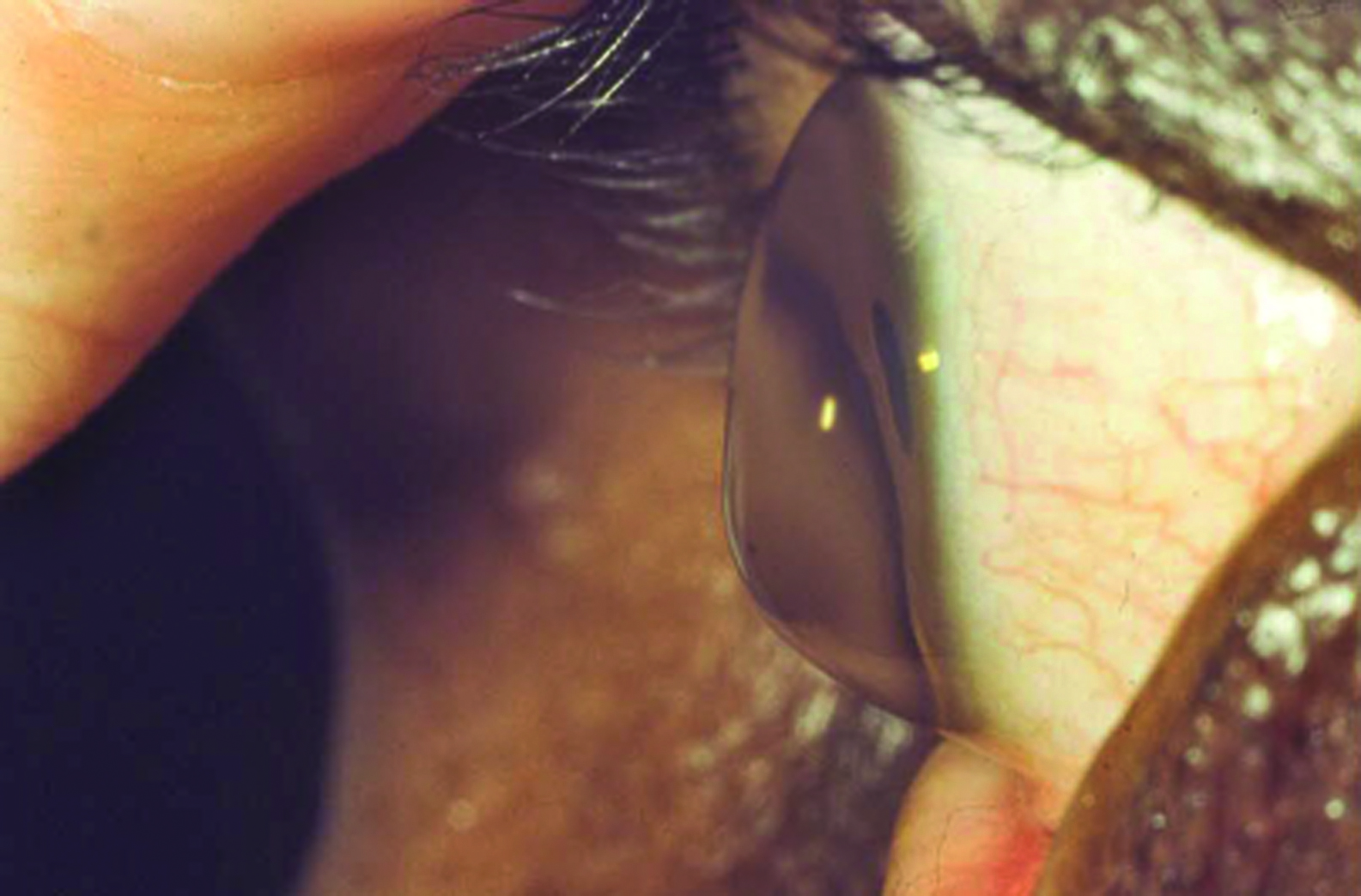
Keratoconus (figure 2) develops as a result of corneal thinning (ectasia) resulting in deformation and forward bowing. This results in unstable and irregular refractive error and subsequent loss of vision. While corneal grafting was at one time the anticipated end stage treatment, the use of topical application of riboflavin drops in association with irradiation of UVA radiation has proven effective to at least stabilise the corneal contour through cross linking of collagen molecules.5 The application of topical riboflavin is undertaken after debridement of epithelium cells and where this procedure can often be undertaken within an ophthalmic theatre environment to ensure appropriate sterile techniques. The use of transglutaminase is being investigated as a possible replacement for the photochemical reactions involving UVA radiation although, as yet, no clinical studies have been reported.6
Figure 2: Late stage keratoconus
Microbial keratitis
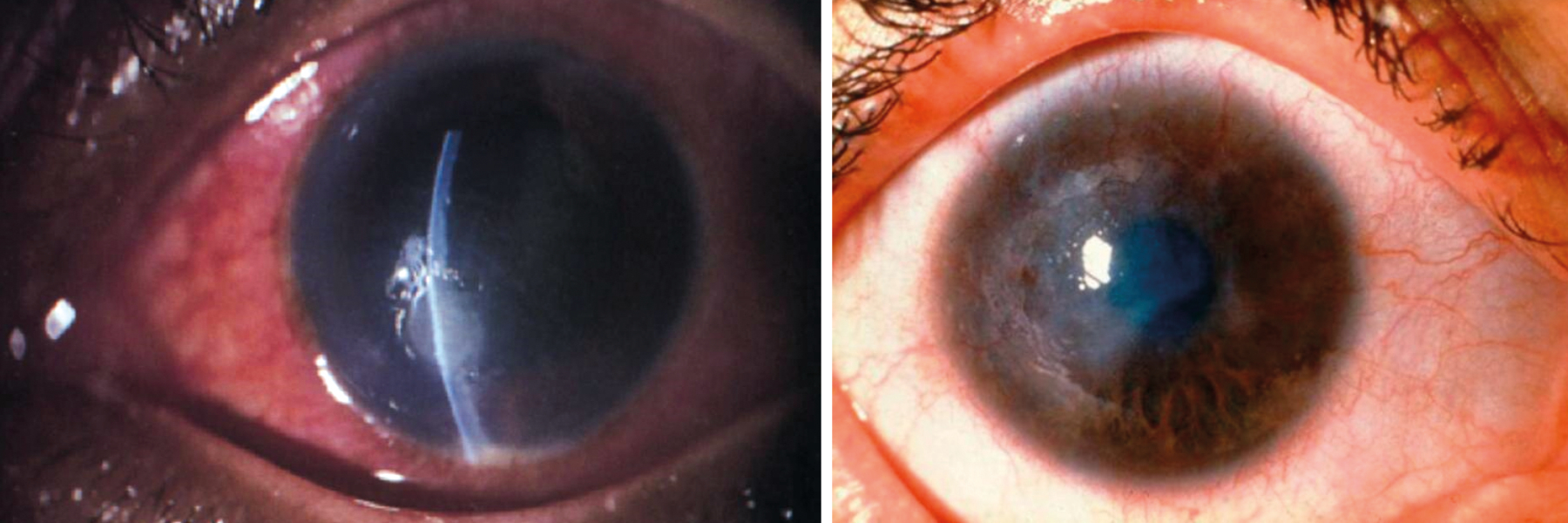
Cabrera-Aguas et al provide a sobering review of both the incidence of microbial keratitis (figure 3) and its management where, in the developed world, a major causative factor is the wearing of contact lenses,3 while in the developing world a major cause is trachoma (figure 4).
Figure 3 & 4: Microbial keratitis; Trachoma
Evolution of Corneal Transplantation
The challenge to improve the availability of corneal transplants is currently being investigated across many different fronts. One line of development is to optimise existing human donor corneal transplantation techniques and the role of eye banks in relation to sample harvesting, screening and storage. In Europe, the European Eye Bank Association,7 founded in 1989, provides an authoritative reference point for eye banks that work within recognised quality frameworks.
Another line of development is the creation of artificial corneas. The greatest challenge here is to replicate the physical structure of the cornea and the features of its cellular metabolism. Such work has endeavoured to unravel the considerable complexities involved and engages the skills of molecular biologists, geneticists, bioengineers, chemists and ophthalmologists.
A concise history of corneal transplantation was published by Crawford et al and describes the first successful full thickness corneal graft, a penetrating keratoplasty (PK), that took place in 1905, undertaken by the Austrian Dr Eduard Zirm.8, 9 Figure 5 shows a clockwork trephine for corneal transplants from this early period. This success was preceded by a prolonged period of speculation and experimentation. It was also not until around 1937 that the use of cadaver corneas was suggested by the Russian ophthalmologist Vladimir Filatov. Initially, PK was the main adopted technique, though others had looked at the technique of lamellar keratoplasty, where deeper layers of the cornea including the endothelial layer were preserved.
Figure 5: Clockwork trephine made by John Weiss of London and invented by corneal transplant pioneer Arthur von Hippel. Credit: Von Hippel-type clockwork trephine, London, England, 1901-19. Science Museum, London with permission

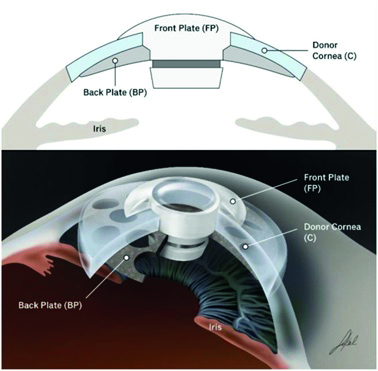
The Boston keratoprosthesis (BKPro) was developed at the Massachusetts Eye and Ear Infirmary as a variation on conventional techniques for use in patients with a history of failed conventional grafts (figure 6).10 It gained initial FDA approval in 1992. The device consists of a central clear optical window of PMMA material acting as a front plate and a back plate of titanium, with the human corneal graft secured between them. The implantation of the device, however, requires significant associated procedures including vitrectomy, lens removal and insertion of a glaucoma tube for management of eye pressures. There is also a considerable level of clinical aftercare.
Figure 6: Schematic representation of the Boston KPro device10. Image reproduced from Clinical Ophthalmology 2020 14 1189-1200, Originally published by and used with permission from Dove Medical Press
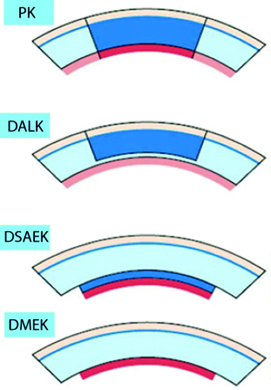
A newer technique, often described as a partial corneal graft, is deep anterior lamellar keratoplasty (DALK). This procedure replaces the main structural stromal layers while preserving the endothelium layer of cells. In a study of over 300 cases of each procedure, Arundhati et al demonstrated improved graft survival rates with DALK compared to PK with, after 10 years, the relative graft survival rates being 93.9% and 72.0% respectively.11 A more comprehensive review of graft survival rates between PK and DALK is described by Liu et al, with a specific focus on corneal grafting within the United Kingdom,12 and suggests the DALK procedure requires a relatively higher level of skill to undertake.
Descemet’s stripping automated endothelial keratoplasty (DSAEK) and Descemet’s membrane endothelial keratoplasty (DMEK) are partial thickness corneal transplants, which are undertaken to replace the endothelium layer. The two procedures are similar, with the primary difference being that, in DMEK, the implanted donor tissue does not include any stromal tissue and is typically around 10 to 15 microns thick. Figure 7 summarises the various surgical techniques described so far.
Figure 7: Diagrammatic representation of different corneal transplant techniques (PK = penetrating keratoplasty; DALK = deep anterior lamellar keratoplasty; DSAEK = Descemet’s stripping automated endothelial keratoplasty; DMEK = Descemet’s membrane endothelial keratoplasty)
In recent years, the use of intra-operative optical coherence tomography (as described by Han et al13) has become a valuable tool for improving the outcome of a wide range of surgical procedures, including DALK, DSAEK and DMEK. Figure 8 shows the Leica EnFocus intra-operative (OCT) imaging system showing a cornea procedure in quad view. Figure 9 shows a view of the compound image system available with intra-operative OCT integrated into operating microscope images of a DMEK procedure.
Figure 8: Leica EnFocus intraoperative OCT imaging system showing a cornea procedure. A quad view presents the microscope image (top left), en face view (bottom left), and the OCT line scan (right). Courtesy of Leica Microsystems
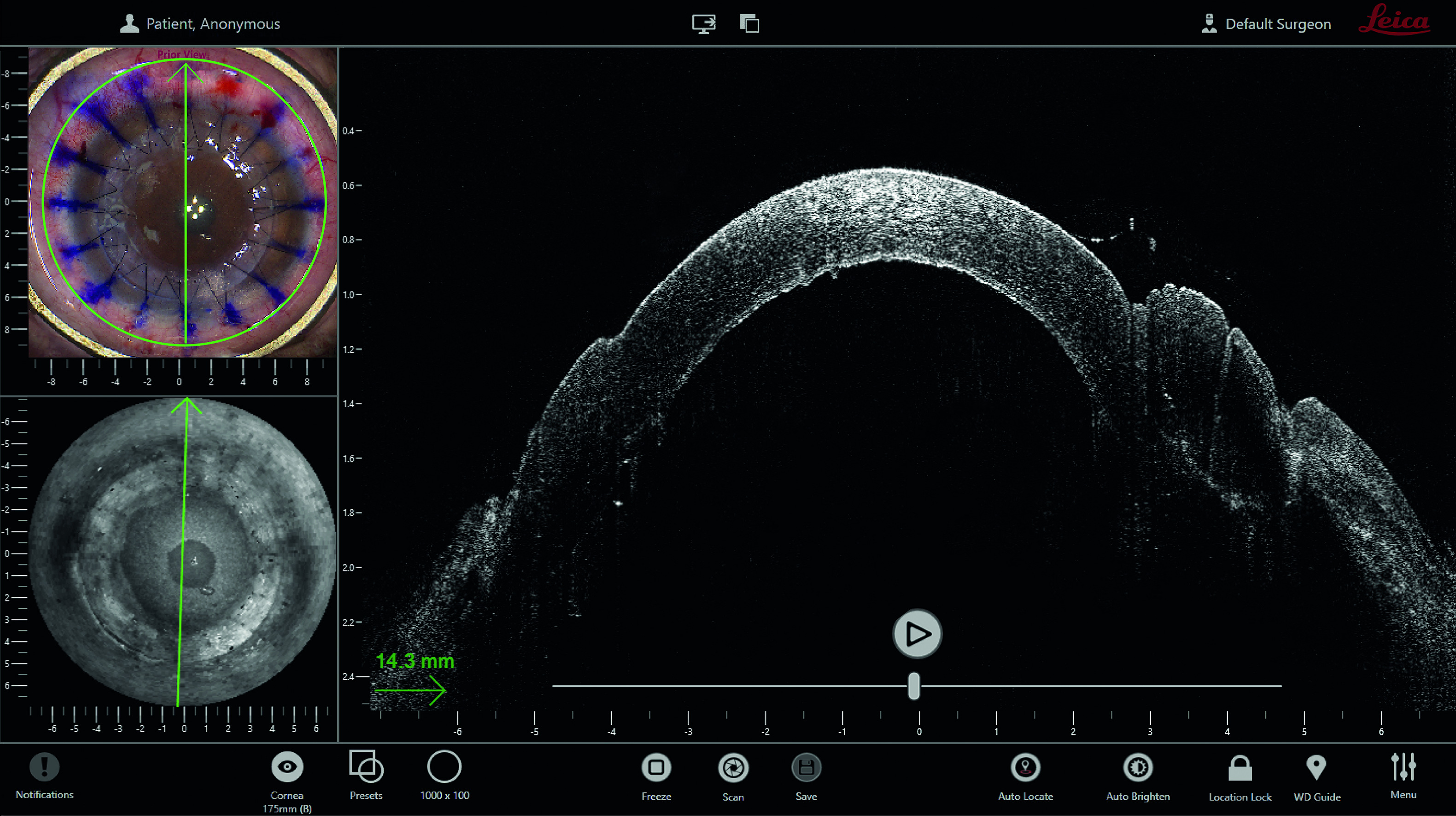
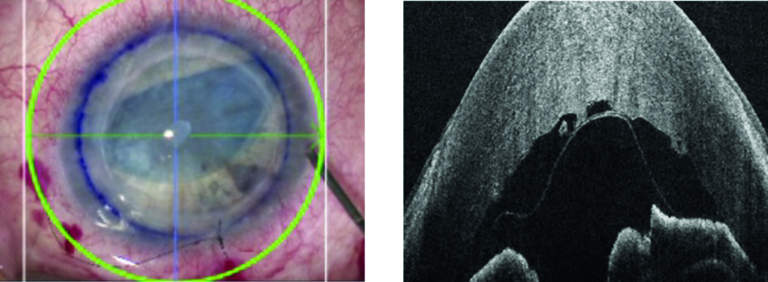
Figure 9: Microscope view during DMEK procedure (left) supplemented with Leica EnFocus OCT (right) revealing scroll orientation of donor membrane. Left microscope image courtesy of Professors Gerd Geerling and Stefan Schraders, Department of Ophthalmology, University Hospital Düsseldorf, Germany
Bio-engineered Corneal Tissue
There is considerable interest in so-called 3D bioprinting of corneal constructs14 using specially adapted 3D printers that can rapidly construct a precisely engineered structure using a slurry of structure-building material containing active cells. This technique anticipates the active process of cell migration and proliferation within a construct that mimics naturally occurring structures. Hurdles to be overcome, however, include ensuring sterility of implant material, integrity and survival of cell implants, and sufficient tensile strength of resultant tissue structures.
While a great many lines of development in terms of bio-engineered corneal tissue are currently being investigated, work undertaken at various centres in Sweden has attracted particular attention on account of the degree of clinical studies undertaken. Fagerholm et al investigated the use of biosynthetic, cell-free bio-reactive tissue, with a component of recombinant human collagen type III, to replace the anterior corneal tissue of a group of 10 patients whose prevailing clinical condition was keratoconus.14, 15 Anterior lamellar keratoplasty was undertaken, which preserved the layer of endothelium cells. Observations at 24 months and 48 months were encouraging, though further optimisation of the technique, such as improved shape retention, was indicated. Epithelial cells regenerated naturally after the procedure. Advantages of the technique were identified as there being no need for steroid immunosuppression and the observation of nerve regeneration. Also, the lack of a component of regenerative medicine would greatly simplify validation of the technique.
Bio-engineered Scleral Implant
As a follow on from this initial Swedish development, a unique collaboration between scientific and clinical teams in Sweden, Iran and India in 2022 reported a significant development in the treatment of keratoconus.16
The Indian cohort included eight patients and the Iranian cohort 12 patients as part of a pilot feasibility study. The basic approach was to insert a strengthening tissue layer based on a cell-free bio-engineered porcine construct, double crosslinked (BPCDX) within existing scleral structures and without disturbance to either epithelium and endothelium. Previous attempts of the research team to use human-derived collagen had not achieved the required structural strength to maintain intraocular pressures.15, 16
Bio-engineered scleral tissue from similar sources such as the Ologen product (developed by ProSys International) has already been adopted within a range of ophthalmic surgical procedures.18, 19 The Ologen product is a type I atelocollagen of animal origin approved for clinical use and has a 3D, porous structure with a pore diameter of between 10 to 300 microns. The material is opaque, providing a dry-form scaffold which biodegrades in approximately three to six months.
The material used in the Swedish based project (BPCDX), however, is specially processed to provide cross-linking strength, transparency, non-biodegradable characteristics and a cell-free structure and where the BCPDX material was manufactured using industry-approved good manufacturing practice. BPCDX material has been validated for storage in sterile packaging for up to two years in addition to meeting international standards of biocompatibility, toxicity and carcinogenicity.
The surgical procedure involves insertion of the implant material within a stromal pocket created by a laser via a single incision for which no suture is subsequently required. The laser technique focuses energy of ultra-short femtosecond pulses within the stromal corneal volume.20 Tissue is vaporised at the focal point to create a zone of separation within an area scanned by the laser beam to allow insertion of the BPCDX implant, although conventional surgical techniques to create the stromal pocket are described as equally effective.20
The thickness of implanted material in the reported study varied according to degree of indicated refractive correction, varying between 280 microns to 440 microns. While the authors indicate further clinical studies are required, the reported technique has the potential for widespread adoption since the actual surgical procedure can be simpler than a routine cataract operation of which there are in excess of 10 million procedures undertaken each year. Currently, a clinical trial is on-going in the European Union where it is planned to include 110 patients in a study to assess safety and efficacy of the BCPDX CorVision bio-engineered corneal inlay (produced by LinkoCare Life Sciences AB of Sweden) for improving uncorrected near vision in presbyopic subjects. In simple terms, therefore, this is a way to ‘sidestep’ an anterior lamellar keratoplasty technique for the treatment of keratoconus.
Regenerative Medicine
The active use of implanted cells is also a current line of research into treatments for corneal disease. Approaches can either employ implanted cells within an introduced construct material or cell sheet engineering, where a layer of cultured cells is placed over a target surface. A significant number of centres are actively engaged in this line of development. Yamato and Okano, in a landmark paper, describe a technique where a cell sample from a patient’s limbal region was cultured to create a viable cell sheet that was subsequently used to treat Salzmann’s nodular degeneration by promoting the regeneration of epithelial cell layers.21
Interest has also been shown in the use of cultured endothelial cells as a means of reversing the reduction in endothelial cell count density resulting from Fuchs’ dystrophy. In another landmark study, Kinoshita et al described such use of human corneal endothelium cells that had been cultured from a donor cornea.22 A bolus of these cells was injected into the anterior chamber of 11 patients who had, effectively, no active corneal endothelial cells. Subsequently, at 24 weeks after the procedure, a cell density in excess of 500 cells per square millimetre was achieved within the group with an associated decrease in corneal thickness of less than 630 microns. The significant developments described in his work include the triggering of replication of human endothelial cells from a donor sample, their satisfactory replication in vitro and the successful selection of stable cell types suitable for transplantation. Sie et al provide a useful overview of the transplantation of endothelial cells.23
The paper by Kinoshita et al has raised widespread interest in a technique which, importantly, does not utilise a bio-engineered scaffold for cell proliferation.22 A key concern, however, as raised by Denny and Mandel,24 relates to the potential risk of donor cells entering systemic host circulation, which could possibly trigger an immune response or lead to seeding and tumour formation. The potential of the technique is, however, highly encouraging.
While there is an extensive programme of regenerative medicine at various centres applied to corneal structures, extremely few products succeed in gaining regulatory approval. Pellegrini et al have described the extensive product validation stages associated with the Holoclar product which, in 2015, was the first stem cell-based medicine to receive authorisation for commercial use within the European Union.25 Limbal cell deficiency can be caused by physical or chemical ocular burns and results in the deterioration of vision arising from proliferation of conjunctival cells. In the Holoclar process, tissue from a patient’s unaffected eye containing corneal epithelial cells and associated stem cells is used to form a graft grown outside the body. This is subsequently transplanted into the injured eye where corneal transparency is restored. Such cell transplantation can often be essential for any subsequent conventional corneal grafting requirement. It is unlikely, however, that regenerative medicine will provide a much greater availability of corneal transplants for the developing world.
Discussion
The studies reviewed in this article are just a small fraction of the extensive clinical research undertaken in corneal transplantation. In order to advance clinical practice, such research must further validate the suitability of implant substrates and bio-engineered cell lines. There is, however, a realistic expectation that scleral implantation as a treatment for keratoconus without the need for human graft material will be successful.
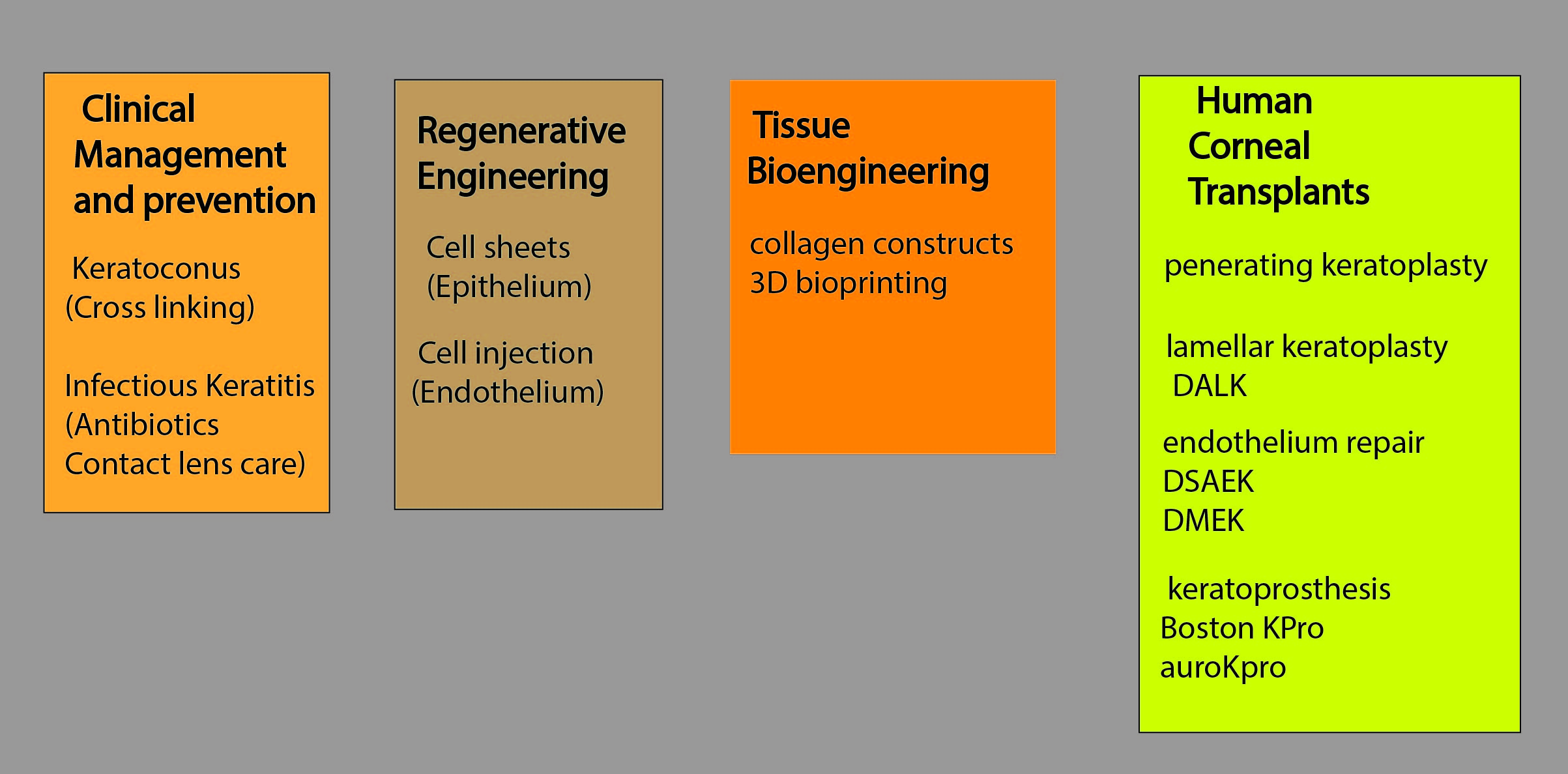
Table 1 outlines the various developments currently being studied to reduce the incidence of blindness due to corneal disease.
The Future
Intriguing questions arise from any review of developments in corneal transplantation. Would a lamellar keratoplasty using BPCDX implant be successful, based on similar earlier work by Farnholm et al?14, 15 What would be the likely outcome of a penetrating keratoplasty procedure using the BPCDX implant material without a functioning endothelial cell layer? Can a nanotechnology-based pump be installed in a scleral pocket to restore corneal transparency after failure of the endothelial cell layer?
Watch this space.
- Dr Douglas Clarkson is a research fellow at the department of clinical physics and bio-engineering, Coventry and Warwickshire University Hospital Trust.
References
- Oliva MS et al. Turning the tide of corneal blindness. Indian Journal of Ophthalmology, 2012 Sep-Oct;60(5):423-7
- Gain P et al. Global survey of corneal transplantation and eye banking. JAMA Ophthalmology, 2016 Feb;134(2):167-73
- Cabrera-Aguas M et al. Infectious keratitis: A review. Clinical & Experimental Ophthalmology, 2022 Jul;50(5):543-562
- Bourne WM et al. Central corneal endothelial cell changes over a ten-year period. Investigative Ophthalmology & Vision Science, 1997 Mar;38(3):779-82
- Seifert FK et al. Long-term outcome of corneal collagen crosslinking with riboflavin and UV-A irradiation for keratoconus. Current Eye Research, 2022 Nov;47(11):1472-1478
- Wu Y et al. Efficacy and safety of transglutaminase-induced corneal stiffening in rabbits. Translational Vision Science & Technology, 2019 Dec 12;8(6):27
- Jones GLA et al. European eye bank association. Developments in Ophthalmology, 2009;43:15-21
- Crawford AZ et al. A brief history of corneal transplantation: From ancient to modern. Oman Journal of Ophthalmology, 2013 Sep;6(Suppl 1):S12-7
- Armitage WJ et al. The first successful full-thickness corneal transplant: a commentary on Eduard Zirm’s landmark paper of 1906. British Journal of Ophthalmology, 2006 Oct;90(10): 1222-3
- Nonpassopon M et al. Boston Type 1 Keratoprosthesis: Updated Perspectives. Clinical Ophthalmology, 2020 Apr 29;14:1189-1200
- Arundhati A et al. Comparative study of long-term graft survival between penetrating keratoplasty and deep anterior lamellar keratoplasty. American Journal of Ophthalmology, 2021 Apr;224:207-216
- Liu S et al. Current perspectives on corneal transplantation. Clinical Ophthalmology, 2022 Mar 4;16:631-646
- Han SB et al. Application of intraoperative optical coherence tomography technology in anterior segment surgery. Journal of Ophthalmology, 2022 Apr 8;2022:1568406
- Isaacson A et al. 3D bioprinting of a corneal stroma equivalent. Experimental Eye Research, 2018 Aug;173:188-193
- Fagerholm P et al. A biosynthetic alternative to human donor tissue for inducing corneal regeneration: 24-month follow-up of a phase 1 clinical study. Science and Translational Medicine, 2010 Aug 25;2(46):46ra61
- Fagerholm P et al. Stable corneal regeneration four years after implantation of a cell-free recombinant human collagen scaffold. Biomaterials, 2014 Mar;35(8):2420-7
- Rafat M et al. Bio-engineered corneal tissue for minimally invasive vision restoration in advanced keratoconus in two clinical cohorts. Nature Biotechnology, 2023 Jan;41(1):70-81
- Gad AAM et al. Combined phacoemulsification and viscocanalostomy with Ologen implant versus combined phacoemulsification and viscocanalostomy. BMC Ophthalmology, 2019 Feb 6;19(1):45
- Jacobson A et al. Ologen augmentation of Ahmed glaucoma drainage devices in pediatric glaucomas. BMC Ophthalmology, 2021;21(1):72
- Lagali N et al. Femtosecond laser-assisted surgery for implantation of bio-engineered corneal stroma to promote corneal regeneration. Methods in Molecular Biology, 2020;2145:197-214
- Yamato M et al. Cell sheet engineering. Materials Today, 2004, 7(5), 42-47
- Kinoshita S et al. Injection of cultured cells with a ROCK inhibitor for bullous keratopathy. New England Journal of Medicine, 2018 Mar 15;378(11):995-1003
- Sie NM et al. Regenerative capacity of the corneal transition zone for endothelial cell therapy. Stem Cell Research and Therapeutics, 2020 Dec 4;11(1):523
- Denny MR et al. New treatment for corneal endothelial dysfunction rocks the boat. Surveys in Ophthalmology, 2018 Nov-Dec;63(6):884-885
- Pellegrini G et al. Navigating market authorization: The path holoclar took to become the first stem cell product approved in the European Union. Stem Cells and Translational Medicine, 2018 Jan;7(1):146-154
