Multipurpose solutions are designed to clean, disinfect and maintain contact lenses. For this purpose, they variously include a combination of surfactants, anti-microbial agents (preservatives), a buffer system and agents which control tonicity to the solution. However, a problem may arise due to the inclusion of these very agents, particularly the antibacterial preservatives.
Toxicity of preservative agents
The mechanism of action of preservatives is based on the disruption of the life cycles of individual microorganisms so preventing their proliferation. Therefore, the use of preservatives or disinfectants in solutions that come into contact with the eye (for example in multipurpose solutions and lubricating drops) is not without risk, especially regarding the corneal-conjunctival surface where they may induce a toxic response.
All existing care products have undergone rigorous pre-clinical and clinical trials that demonstrate reduced ocular toxicity. However, it is not uncommon for patients who maintain prolonged use of care systems with preservatives to complain of discomfort or eye irritation.
Moreover, it has been demonstrated in vitro that the cytotoxicity of preservatives varies for different types of cells in culture representing different structures of the anterior eye: epithelial cells of the cornea or conjunctiva, corneal keratocytes,1,2,3 corneal endothelial cells,3 Tenon capsule fibroblasts, trabecular cells,3 and cells of the crystalline lens.5,6
The cytotoxicity of preservatives particularly affects in vitro cell viability (through impaired plasma membrane integrity and mitochondrial energy metabolism)2,7-9 cell proliferation10-12 and cellular lineage.4 These cytotoxic effects increase with the concentration of preservative and, especially, with exposure time.
However, it is necessary to note that the basal tear volume dilutes the concentration of the preservative solution upon contact with the eye, so that the concentration actually contacting corneal-conjunctival cells is much lower in vivo. For this reason, the cytotoxic effect of preservatives is not revealed immediately. A long period of exposure to a multipurpose solution is usually required before any cytotoxicity effects manifest.
Aloe Vera: Ocular surface protection
Alvera is a new multipurpose contact lens solution that includes aloe vera as a method of protecting the corneal epithelium against irritation caused by other component chemicals such as preservatives and surfactants. Alvera is an aqueous, sterile, isotonic and buffered solution which contains:
- boric acid
- sodium tetraborate decahydrate
- sodium chloride
- EDTA 0,1%
- Polyvinylpyrrolidone
- protein remover agent
- aloe vera
- polyhexanide 0.0002%
The inclusion of aloe vera is because it may have a protective effect against epithelial aggression of potentially toxins. Furthermore, it has been shown that aloe vera itself has bactericidal and bacteriostatic properties, and also capacity for the absorption of UV radiation. These properties are ideal for a multipurpose solution.
Aloe vera has been tested in vitro by looking at its ability to protect cells which are deliberately exposed to the irritant dimethyl sulphoxide (DMSO). This cytotoxicity study consisted in two phases.
- The first phase was to evaluate if the presence of aloe vera (and at which concentrations) in a multipurpose solution had any effect the corneal epithelium. The test involved exposing epithelial cells in cell culture growth medium (DMEM) to DMSO at 1% and evaluating the cell integrity with a marker. Other cells in DMEM without irritant acted as control. To evaluate if aloe vera had an effect on cellular viability, multipurpose solutions with and without aloe vera were tested at different concentrations. As a result, we found that the inclusion of aloe vera offered protection against irritation to the corneal epithelial cells, and this effect was optimal at the C3 concentration, which is the concentration of aloe vera in Alvera. The results are shown in figure 1.
- The second phase involved comparison of the Alvera with C3 concentration aloe vera with other available multipurpose solutions. Results are shown in figure 2.
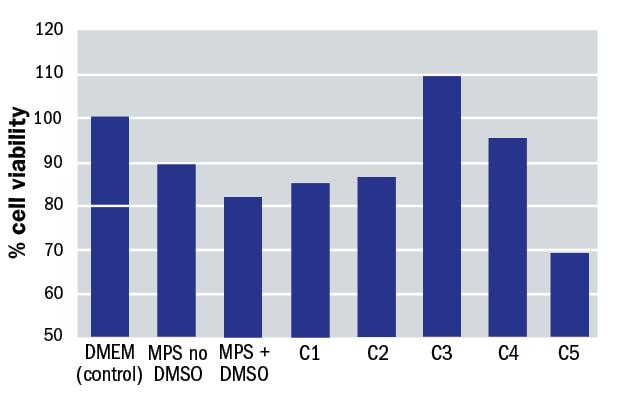
Figure 1: Impact upon cell viability with different solution constituents
Properties of Alvera
The pH of Alvera is adjusted to 7.2, similar to that of the tear film. Numerous studies have shown that, to maximize tolerability, solutions instilled into the eye should be neutral or slightly alkaline.13 Buffering is provided by boric acid and sodium tetraborate decahydrate.
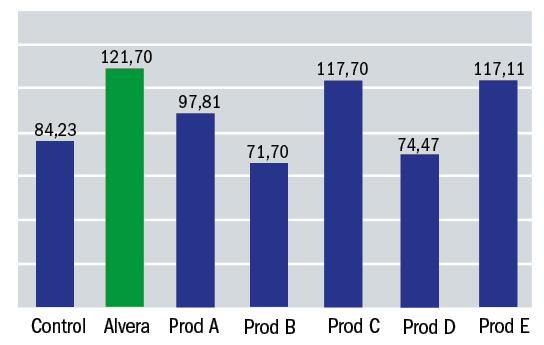
Figure 2: Alvera performance compared with other multipurpose solutions available (% cell viability)
The osmolarity of the tear film is usually about 300-310 mOsm/L. Alvera includes an adequate concentration of sodium chloride which reproduces the tear film osmolarity. A non-isotonic ocular solution can produce disequilibrium in the osmotic pressure between the ocular surface and a solution.14
Cleaning by Alvera is made possible by the inclusion of a poloxamer surfactant agent. Poloxamers are polymers of the type ABA, consisting of a central, hydrophobic block of polypropylene oxide, which is edged by two hydrophilic blocks of polyethylene oxide. The polymers are derived from the sequential polymerization of propylene oxide and ethylene oxide. The general formula is shown in figure 3.
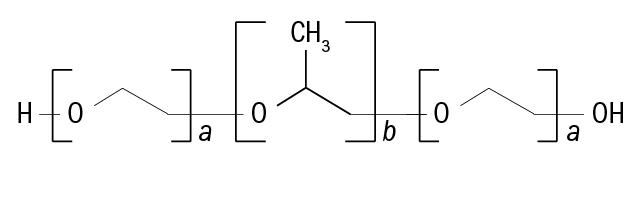
Figure 3: Molecular structure of a poloxamer
Poloxamers vary considerably in molecular weight depending on the nature of the individual monomers they include. In the case of Alvera, the poloxamer has an average molecular weight of about 12.200 D.
The polyoxyethylene units represent about 73% of the molecular weight whereas that one of polyoxypropylene stands for about 27%. The purpose of a cleaner is to remove loosely held lens deposits on the lens surface. A surfactant lowers the surface tension of the solution to facilitate removal of loosely held lens deposits on the lens surface in conjunction with mechanical means (blinking) (see figure 4).
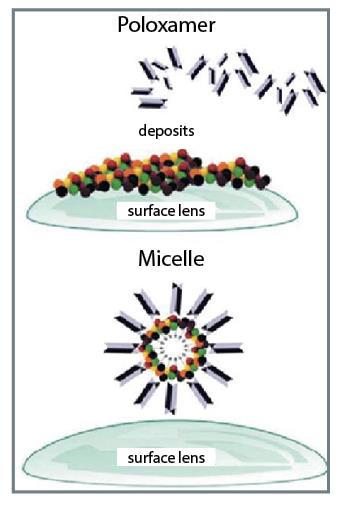
Figure 4: Mechanism of action of a poloxamer
In an aqueous solution of a surfactant, the surfactant is dispersed as molecules at low concentrations. At higher concentrations, however, when a certain critical concentration is reached, the molecules form micelles. These micelles are in equilibrium with the free surfactant molecules.
The concentration that must be reached in order that micelles are formed is called the critical micelle concentration (CMC). So, the cleaning effectiveness of Alvera can be gauged by determining that the concentration of poloxamer in the solution is higher than the critical micelle concentration of poloxamer.
Many physical properties of a surfactant solution when plotted against the concentration show more or less sudden changes at the CMC. By measuring the surface tension as a function of the concentration of poloxamer, the CMC is determined as the concentration at which the surface tension versus concentration curve shows a change in slope (figure 5).15
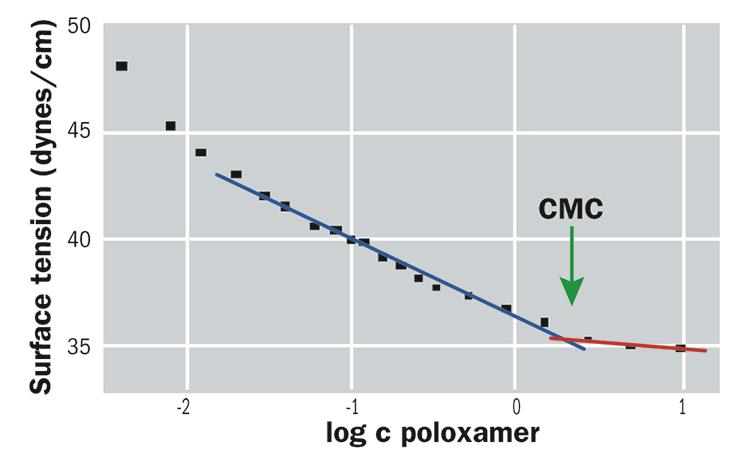
Figure 5: Determination of the critical micelle concentration (CMC)
The determination of the CMC of poloxamer in Alvera was carried out by the Physical and Chemical Department of the University of Alcala. The result obtained showed a CMC of 1.8 mg/ml. The concentration of poloxamer in Alvera is 2.5 mg/ml and so one would predict micelle formation and therefore effective removal of lens surface deposits.
An in vitro study was undertaken to simulate the effectiveness of Alvera in removing surface lipids from silicone hydrogel contact lenses. This study consisted in depositing different contact lenses with a lipid which is present in the tears; cholesterol. After that, the lenses were cleaned by soaking in solution for four hours and finally the amount of cholesterol remaining on the lens was measured. A treatment with phosphate buffered saline was used as a control. The contact lenses included in the study were Biofinity (CooperVision), Acuvue Oasys (J&J) and PureVision (Bausch+Lomb). The results for each are shown in figures 6, 7 and 8.
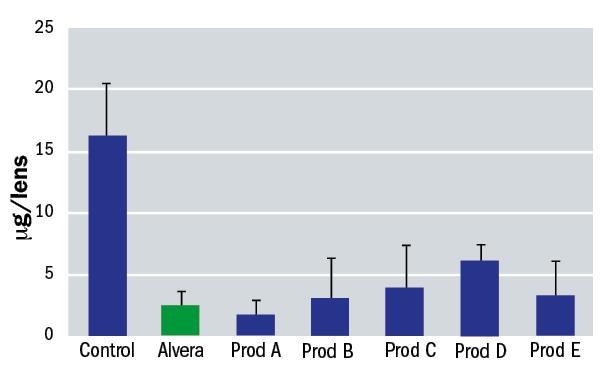
Figure 6: Residual surface lipid on Biofinity contact lenses after soaking with various solutions
Prevention of calcific deposits is promoted by the inclusion in Alvera of a chelating agent (figure 9). Ethylenediaminetetra-acetic acid (EDTA) acts in combination with the surfactant agent and has the ability to trap divalent cations, as Ca2+. This cationic sequestering action prevents the adhesion of calcium deposits on the contact lens.
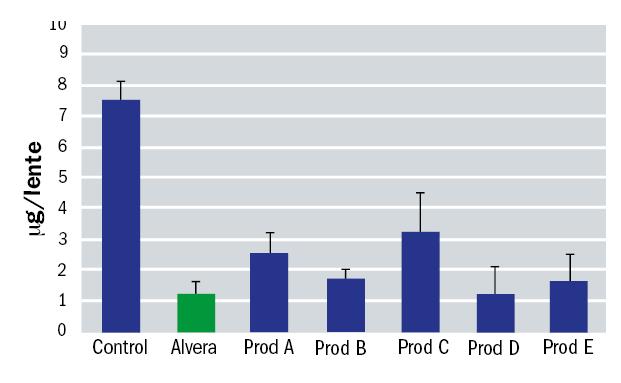
Figure 7: Residual surface lipid on Acuvue Oasys contact lenses after soaking with various solutions
For effective contact lens wetting, the surface tension of any solution must be lower than the surface energy of the lens. Because water has a relatively high surface tension (72.8 dynes/cm), the wettability of the lens is increased by lowering the surface tension of surrounding water with surfactants or viscolysing agents These substances are water soluble and have elongated molecular structures with one extremity hydrophilic and the other hydrophobic.
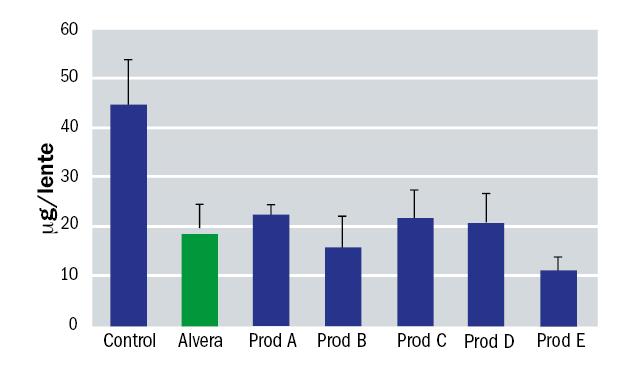
Figure 8: Residual surface lipid on PureVision contact lenses after soaking with various solutions
When a detergent is dissolved in water, its molecules tend to accumulate on the surface resulting in lowering of the surface tension of the water. When the surface tension of an aqueous solution is less than the surface energy of a lens material, the solution spreads spontaneously on the surface. In such a case, the solution wets the material perfectly and, as a result, the contact angle between the tear film and the material decreases when the user put the lens on the eye.4
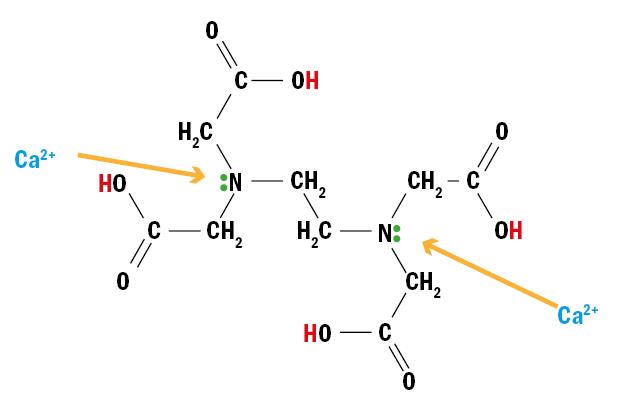
Figure 9: EDTA molecular structure allows sequestration of Ca2+ ions
Taking in count there are many materials used to manufacture contact lenses (HEMA, silicone hydrogel, etc), it has been calculated that for good wetting of contact lenses, a solution has to have a surface tension lower than 45 dynes/cm. Alvera includes a lubricant commonly used in pharmaceutical compositions, polyvinylpyrrolidone, and a surfactant agent, poloxamer. Both components combine to meet the required lowering of surface tension. It has been measured by the Physical and Chemical Department of the University of Alcala to reduce to 38 dynes/cm (20ºC) with use of Alvera.
But what of the anti-microbial performance of Alvera? Ocular infection associated with microbiological contamination of products for the maintenance of contact lenses is the most important problem for contact lens users. The efficacy of Alvera against bacteria and fungi has been evaluated according to a Stand Alone Test procedure described in the ISO norm 14729 (Ophthalmic optics. Contact lens care products – Microbiological requirements and test methods for products and regimens for hygienic management of contact lenses). The results are shown in a comparison form in figure 10.
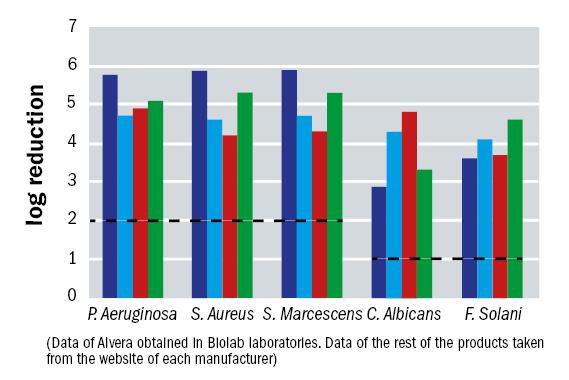
Figure 10: Biolab results for Alvera (dark blue) and comparison solutions on the market (pale blue, red and green) in antimicrobial activity against five individual species
Alvera has also been found to be effective against Acanthamoeba. At present there is no ISO norm to set the guidelines to demonstrate that a product for the maintenance of contact lenses is effective against this microorganism. However, based on the ISO norm 14729, a study was conducted at the University of Leicester in Stand Alone Test regime where Alvera efficacy was evaluated at different times.
Acanthamoeba species included in this study included both the feeding and dividing trophozoite stage and the resilient latent cyst stage. Activity against both forms was tested. Furthermore, it is known that the species A. castellanii and A. polyphaga are most commonly the cause of amoeboid keratitis. Results are shown in figure 11.
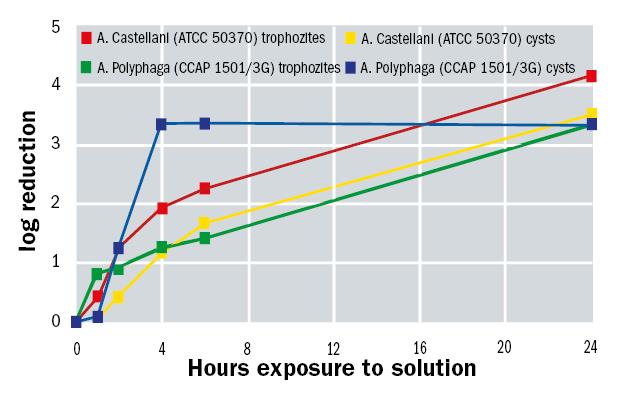
Figure 11: Acanthamoeba spp activity over time exposed to Alvera solution
Clinical performance data
To evaluate the efficacy and safety of Alvera, a single-centre, controlled, randomised, double-masked and crossover clinical trial was undertaken (n=20). The study was conducted according to the UNE EN ISO 11980 ‘Ophthalmic optics – Contact lenses and contact lens care products – Guidance for clinical investigations (ISO 11980).’
Each subject used for one month either Alvera or another multipurpose solution as a control. Before and between treatments, a washout period of one week was undertaken where subjects used a hydrogen peroxide system. The lenses used in the study were Biofinity (comfilcon A, hydration 48%).
To determine the safety of Alvera, the main variable assessed was corneal staining. Subjects showed fluorescein corneal staining values of grade 0 or 1, with the exception of four patients who showed grade 2 after first month. With respect to efficacy, deposits on the surface of contact lens were assessed. It was found that in the majority of subjects grade of 0 or 1 deposition was found, with the exception of two subjects with grade 2.
Satisfaction and comfort were graded by subjective scoring (obtaining 8.8 and 8.2 respectively in a scale of 0 to 10). Wettability of the anterior surface of the cornea was measured by non-invasive break up time and evaluated at 15 and 30 days. At these times there was also a comprehensive assessment of the ocular surface (grading limbal hyperaemia, bulbar hyperaemia, conjunctival staining, stromal oedema and corneal vascularization). In all cases, grades 0 or grade 1 were found.
These results support the view that Alvera multipurpose solution is effective and safe.
Elena Ruiz works at the Research and Development Department, Avizor, Spain.
References
1 Lapalus P, Ettaïche M, Fredj-Reygrobellet D, Jambou D, Elena PP. ‘Cytotoxicity studies in ophthalmology’ Lens Eye Tox Res 1990; 7: 231-42.
2 Parnigotto PP, Bassani V, Montesi F, Conconi MT. ‘Bovine corneal stroma and epithelium reconstructed in vitro: characterisation and response to surfactants’. Eye 1998; 12: 304-10.
3 Samples JR, Binder PS, Nayak S. ‘The effect of epinephrine and benzalkonium chloride on cultured corneal endothelial and trabecular meshwork cells’ Exp Eye Res 1989; 49: 1-12.
4 Williams DE, Nguyen KD, Shapourifar-Tehrani S, Kitada S, Lee DA. ‘Effects of timolol, betaxolol, and levobunolol on human tenon’s fibroblasts in tissue culture’ Invest Ophtalmol Vis Sci 1992; 33: 2233-41
5 Hamard P, Blondin C, Debbasch C, Warnet JM, Baudouin C, Brignole F. ‘In vitro effects of preserved and unpreserved antiglaucoma drugs on apoptotic marker expression by human trabecular cells’ Graefes Arch Clin Exp Ophthalmol 2003; 241: 1037-43.
6 Goto Y, Ibaraki N, Miyake K. ‘Human lens epithelial cell damage and stimulation of their secretion of chemical mediators by benzalkonium chloride rather than latanoprost and timolol’ Arch Ophthalmol 2003; 121: 835-9
7 De Saint-Jean M, Brignole F, Bringuier AF, Bauchet A, Feldmann G, Baudouin ‘Effects of benzalkonium chloride on growth and survival of Chang conjonctival cells’ Invest Ophthalmol Vis Sci 1999; 40: 619-30.
8 Debbasch C, Rat P, Warnet JM, De Saint Jean M, Baudouin C, Pisella PJ. ‘Evaluation of the toxicity of benzalkonium chloride on the ocular surface’ Toxicol Cut Ocul Toxicol 2000; 19: 105-15
9 Saarinen-Savolainen P, Järvinen T, Araki-Sasaki K, Watanabe H, Urtii A. ‘Evaluation of cytotoxicity of various ophthalmic drugs, eye drop excipients and cyclodextrins in an immortalized human corneal epithelial cell line’ Pharm Res 1998; 15: 1275-80
10 Imperia PS, Lazarus HM, Botti RE, Lass JH. ‘An in vitro method for measuring ophthalmic preservative cytotoxicity’ J Toxicol Cut Ocular Toxicol 1986; 5: 309-17
11 Lazarus HM, Imperia PS, Botti RE, Mack RJ, Lass JH. ‘An in vitro method which assesses corneal epithelial toxicity due to antineoplastic, preservative and antimicrobial agents’ Lens Eye Toxic Res 1989; 6: 59-85
12 Mencucci R, Scrivanti M, Crisa A, Salvi G. ‘La culture d’épithélium cornéen humain et les conservateurs pour solution à usage ophtalmologique’. Ophtalmologie 1996; 10: 13-5.
13 Gangrede NK, Gaddipati NB, Ganesan MG, Reddy IK ‘Topical ophthalmic formulations: basic considerations’ Op. Cit.; 377-405
14 Gilbard, Jeffrey P M.D. ‘Human Tear Film Electrolyte Concentrations in Health and Dry-Eye Disease’ International Ophthalmology Clinics 1994; 34: 27-36
15 1997 FDA Guidelines’ Premarket Notification (510(k)) Guidance Document for Contact Lens Care Products’ Micro-Appendix B, Part 2. Regimen Procedure for Disinfecting
16 ISO 14729 ‘Ophthalmic optics – Contact lens care products- Microbiological requirements and test methods for products and regimens for hygienic management of contact lenses’
