
Choroidal melanomas are a rare occurrence in general practice. Choroidal naevi, on the other hand, are common observations and it is important that we keep a very close watch on these to ensure that they do not develop into choroidal melanomas. In this article, I will run through the various aids that we utilise in our practice to gain insight into the development of choroidal melanomas. I will also show that melanomas can suddenly appear.
Case
54-year-old female, JN, was seen by my colleague for a routine eye examination. Her last eye examination had been some 5 years earlier (elsewhere) and she was managing with off-the-shelf +1.00 ready readers. She had an amblyopic left eye but no other history of note.
Clinical Findings:
- Anterior eye; no abnormalities
- Binocular status; 10 to 12Δ left esotropia
- Visual acuity; R. 6/6, L. 6/12 (+1.75DS addition; R. N5 , L. N6)
- Fundus examination
- Right; no abnormalities
- Left; retinal imaging revealed a large pigmented naevus inferior to the optic disc. The naevus had blurred borders and possible serous changes around the lesion especially temporal and superior aspect. There were no obvious drusen over the naevus.
- OCT examination; a 6mm diameter mass pushing the retina up and serous changes within the outer retina.
Management:
The appearance of hazy margins, with subretinal fluid surrounding the pigmented lesion, were suggestive of a choroidal melanoma. The patient was immediately sent to the general practitioner with the stipulation that she should be referred to an ocular oncology department within 2 weeks. The general practitioner did not refer the patient to the oncology department but, instead, to the local eye clinic.
The conclusion of the eye clinic was that the patient had a flat choroidal naevus measuring 4 disc diameters, with ‘no lipofuscin and no sub-retinal fluid.’ They were going to monitor her on an annual basis.
Annual Follow-up
The patient attended our practice for an annual follow-up. The lesion showed some changes (figure 1).
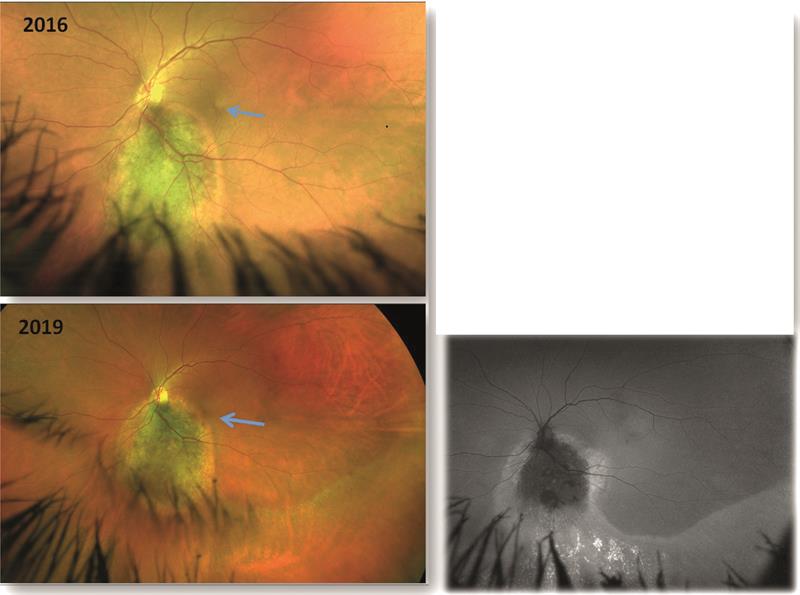
Figure 1. Fundus appearance in 2016 (top) showing a large naevus inferior and adjacent to the disc. The naevus has blurry edges and pigmentation on its surface. There appears a band of subretinal fluid, but macula is unaffected. Appearance in 2019 (bottom left) shows darkening of the naevus due to increase pigmentation. Fluid is now affecting the macula causing a drop in acuity. Also seen is an arc of fluid inferotemporal to the naevus. Autofluorescence imaging (bottom right) shows fluorescence of the subretinal fluid and hyperfluorescence suggesting retinal pigment epithelium damage
The main clinical findings were as follows;
- Visual acuity; left eye 6/18 (N10)
- Visual fields (figure 2);
- Left; superior scotoma with full field 120 degree visual field test seen in 2016
- No change in in 2019
- OCT; slight increase in number of sub-retinal fluid cysts (figure 3)
- Daytona SLO imaging; a push of fluid towards the macula with fluorescence of subretinal fluid and hyperfluorescence suggesting retinal pigment epithelium changes
- Pattern ERG; no dysfunction of the retinal ganglion cells in the affected left eye.
- Flicker ERG; no losses of cone cells in either eye. The phase showed asymmetry in the left eye compared to the right eye indicating that there was slight dysfunction of the cone cells in the left eye compared to the right eye (figure 4)
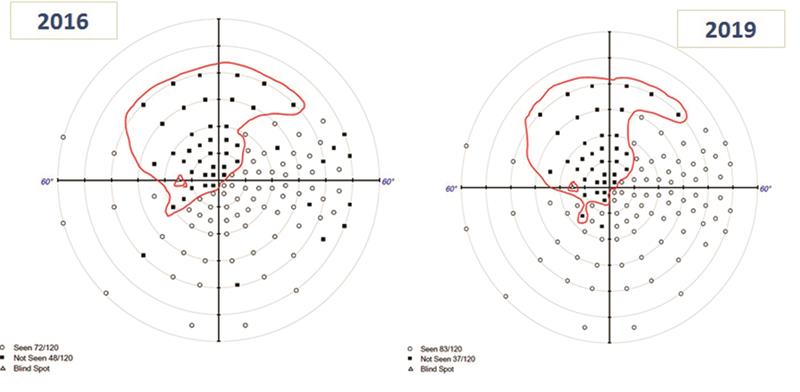 Figure 2. Left scotoma showing little progression
Figure 2. Left scotoma showing little progression
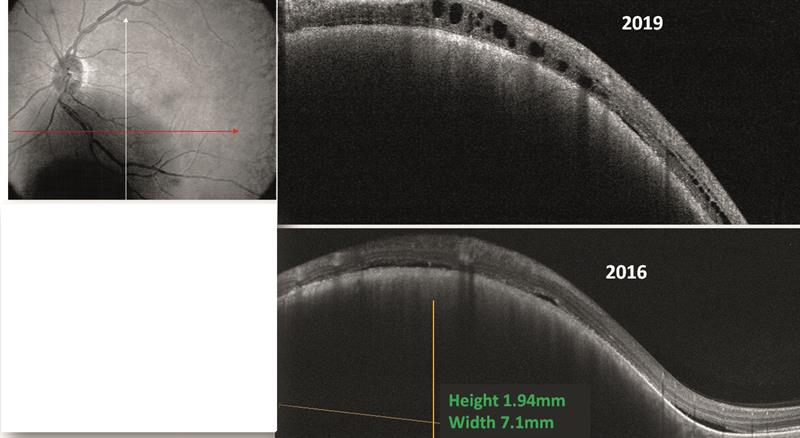 Figure 3. OCT image reveals increased subretinal fluid, but no change in height and width of the naevus. (constant at 7.1mm diameter and 1.94mm height)
Figure 3. OCT image reveals increased subretinal fluid, but no change in height and width of the naevus. (constant at 7.1mm diameter and 1.94mm height)
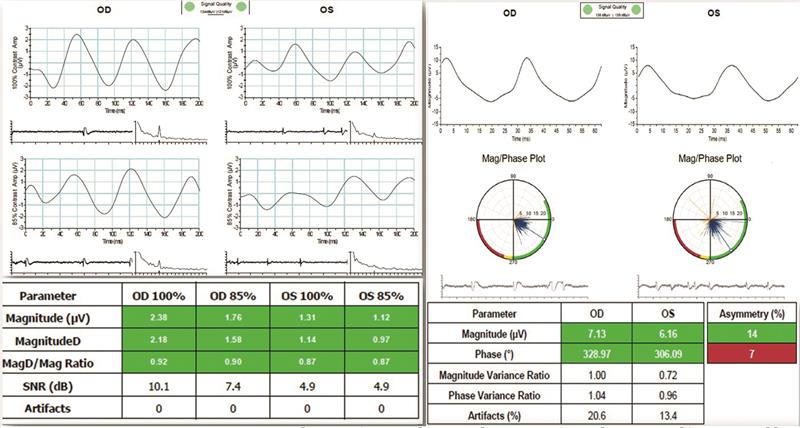 Figure 4. Pattern ERG (left) revealing healthy retinal ganglion cells as shown by their MagD to Mag ratio of 0.87 microvolts in the left and 0.90 in the right. Flicker ERG (right) shows good magnitude for both eyes indicating no cell death. The phase shows early dysfunction of cone cells and in the left eye there is slight asymmetry in phase
Figure 4. Pattern ERG (left) revealing healthy retinal ganglion cells as shown by their MagD to Mag ratio of 0.87 microvolts in the left and 0.90 in the right. Flicker ERG (right) shows good magnitude for both eyes indicating no cell death. The phase shows early dysfunction of cone cells and in the left eye there is slight asymmetry in phase
Management
The signs again pointed towards a suspect choroidal melanoma, but we could not over-ride the monitoring by the local eye clinic where they still maintained that the lesion a naevus measuring 4 disc diameters with no sub-retinal fluid. It is standard practice for tumours with elevation of less than 2.25mm and diameter of less than 10mm to be observed annually for growth.
Discussion
Primary choroidal melanomas are thought to develop from pre-existing melanocytic naevi. It is therefore very important to monitor any naevi observed on the retina. Choroidal melanomas are essentially a primary malignant intraocular tumour affecting mainly Caucasians, with the highest incidence after the age of 55. In Scandinavian countries, there are 7.5 cases per million a year. Rarely, choroidal melanomas can occur due to metastasis to the choroid from melanoma elsewhere in the body. These patients typically exhibit symptoms of weight loss, marked fatigue, cough or change in bowel or bladder habits.
Choroidal melanomas remain asymptomatic for long periods and usually are found incidentally during ophthalmoscopy or retinal imaging. Patients may present with symptoms of blurred vision, visual field loss, floaters and flashes and occasionally severe ocular pain.
Pathophysiology
Choroidal melanomas vary in appearance, and may range from amelanotic to dark pigmented (figure 5). Other points of note include;
- Light coloured melanomas exhibit abnormal vascularisation
- Choroidal melanomas can cause deprivation of choroidal circulation to the RPE as they grow and consequently lead to RPE detachment, drusen formation and atrophy
- The RPE plays a role in phagocytosis of the rod and cone discs and, when this is compromised by the choroidal melanoma, orange-coloured areas containing melanin and lipofuscin may appear. Lipofuscin build up can be picked up by autofluorescence imaging and seen as hyperfluorescent
- There is a risk of retinal hypoxia and choroidal neovascularisation. Choroidal melanomas can create exudative retinal detachment as well as sub-retinal haemorrhage or vitreous haemorrhage
- Anterior choroidal melanomas might show dilated episcleral blood vessels on the conjunctiva (called sentinel vessels) that feed the metabolically active tumour
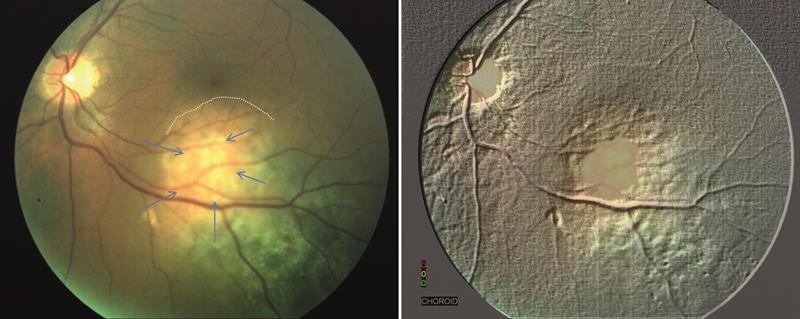 Figure 5. Amelanotic choroidal melanoma indicated by arrows. Note, there is a pigmented naevus to the right of this malignant lesion. Also seen is a cuff of subretinal fluid superiorly which is pushing the fovea upwards. This patients had symptoms of photopsia and blurred vision (6/12 and N10). The image on the right uses software to highlight the choroid and reveals deprivation of choroidal circulation to the RPE resulting in its atrophy. Note the raised retinal vessels over the melanoma.
Figure 5. Amelanotic choroidal melanoma indicated by arrows. Note, there is a pigmented naevus to the right of this malignant lesion. Also seen is a cuff of subretinal fluid superiorly which is pushing the fovea upwards. This patients had symptoms of photopsia and blurred vision (6/12 and N10). The image on the right uses software to highlight the choroid and reveals deprivation of choroidal circulation to the RPE resulting in its atrophy. Note the raised retinal vessels over the melanoma.
Diagnosis may be confirmed by the following;
- Choroidal melanomas most commonly metastasise to the liver so liver enzyme level tests are always necessary in uveal melanoma patients
- B-scan ultrasonography of the eye is useful in tumours thicker than 2-3 mm. B-scan ultrasonography is used in patients with posterior segment mass and especially important in patients with media opacities. B-scan ultrasonography will enable the clinician to establish tumour size, possible extra ocular extension and to plan therapeutic intervention
- Chest X-ray may help rule out possible lung metastasis
Management typically involves the following;
- Observation of tumours less than 2.25mm in elevation and less than 10mm in diameter until growth is confirmed
- Enucleation of large tumours (diameter >15mm and elevation >10mm) is deemed necessary, especially if the tumour compromises vision or where other therapies fail
- Plaque brachytherapy is routinely used for tumours < 15mm in diameter and <10mm in elevation. Radiotherapy seeds are placed inside a gold plaque and this focuses radiation to the site of the tumour. Ruthenium-106 or iodine-125 radioactive isotopes are used
- External beam irradiation (with proton or helium ions) is used for medium sized choroidal melanomas. Proton beams can be channelled into a very tiny beam, so it is possible to deliver radiation precisely without scatter and also may be stopped within <1mm, so there is no effect on the surrounding healthy tissue
Choroidal melanomas can lead to either partial or total visual loss due to destruction of the affected eye’s ocular structures, either by the tumour or as a consequence of radiation treatment (figure 6). A study undertaken by National institute of Health found that, over a 5 year period following radiation therapy, there was a 64% risk of radiation maculopathy, 35% risk of radiation papillopathy and 68% risk of vision loss (figure 7). The study considered moderate-sized tumours (15mm in diameter and less than 5mm in height, and roughly 4 disc diameters of the macula or the optic disc).
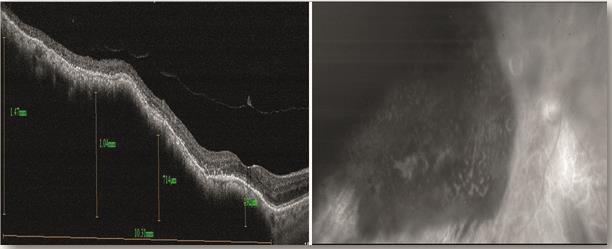 Figure 6. The green free image (right) shows a choroidal melanoma after recent plaque radiotherapy treatment. The left image reveals the height of the melanoma from the fovea to the temporal retina. The melanoma is elevated at 1.47mm on the temporal aspect and 0.192mm at the fovea. Note the perifoveal hyaloid vitreous detachment.
Figure 6. The green free image (right) shows a choroidal melanoma after recent plaque radiotherapy treatment. The left image reveals the height of the melanoma from the fovea to the temporal retina. The melanoma is elevated at 1.47mm on the temporal aspect and 0.192mm at the fovea. Note the perifoveal hyaloid vitreous detachment.
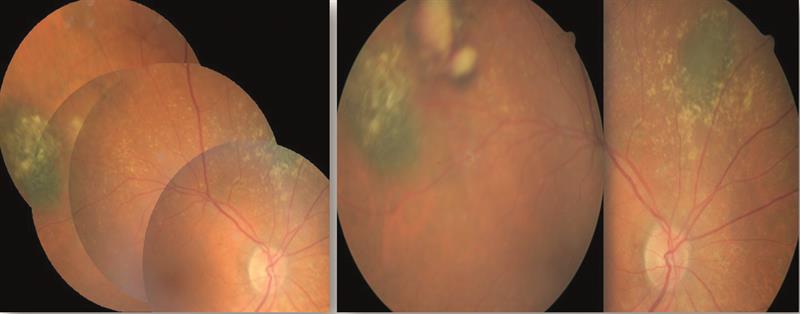 Figure 7. Fundus appearance in 2001 (left) taken with a Canon 45 degree camera revealing two new naevi. The appearance in 2005 (right) showed an amelanotic melanoma appearing on the superior temporal aspect of the right eye.
Figure 7. Fundus appearance in 2001 (left) taken with a Canon 45 degree camera revealing two new naevi. The appearance in 2005 (right) showed an amelanotic melanoma appearing on the superior temporal aspect of the right eye.
Patients with choroidal melanomas have a high mortality rate with 42% of patients dying within 10 years from diagnosis and treatment. Death is usually secondary to distant metastasis and the risk is greater with larger tumours.
Kirit Patel is an optometrist in independent practice in Radlett, Hertfordshire.
