
he prevalence of myopia has increased in recent decades to affect approximately 30% of the world’s population and it has been estimated to significantly increase to affect about 50% of the world’s population by 2050.1 Of particular concern is that even relatively low degrees of myopia may be associated with an increased risk of sight-threatening ocular complications, with the risk increasing substantially with higher levels of myopia.2-8
Concerned with the growing incidence of myopia and its health consequences worldwide, Menicon Co Ltd has dedicated significant resources to develop the Menicon Bloom Myopia Control Management System, a holistic approach for myopia control management. This system features two components:
- Menicon Bloom Day; an extended depth of focus daily disposable soft contact lens with CE-approval specifically for myopia progression control.
- Menicon Bloom Night; the first and only CE-approved orthokeratology contact lens for myopia control management in Europe.
With this comprehensive myopia care therapy, Menicon is the only company in the world offering both soft and orthokeratology contact lens devices specifically approved for myopia control in Europe. The rationale for the launch and the details of this myopia control management system are explained in this article.
What Is Myopia?
Our understanding of myopia has increased substantially with the recent publication of a number of white papers providing global consensus on different aspects related to myopia.9-15 The International Myopia Institute has defined myopia, also known as near or short-sightedness, as a ‘refractive error in which rays of light entering the eye parallel to the optic axis are brought to a focus in front of the retina when ocular accommodation is relaxed. This usually results from the eyeball being too long from front to back, but can be caused by an overly curved cornea and/or a lens with increased optical power’ (figure 1).9
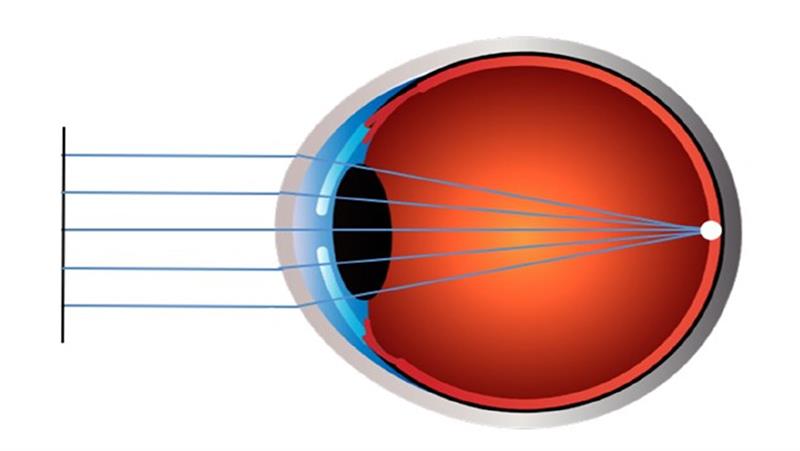
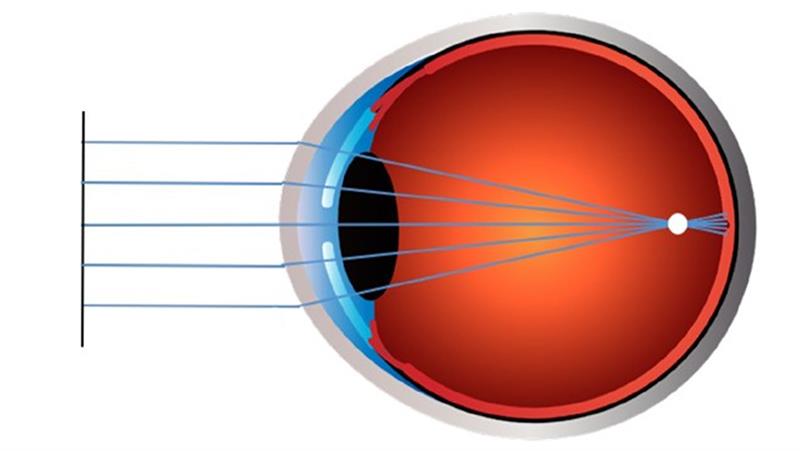 Figure 1: The image on the top left shows the refractive status of an emmetropic eye where light rays entering the eye focus on the retina, whereas the image on the bottom left represents a myopic eye where light rays entering the eye focus in front of the retina causing blurred distance vision
Figure 1: The image on the top left shows the refractive status of an emmetropic eye where light rays entering the eye focus on the retina, whereas the image on the bottom left represents a myopic eye where light rays entering the eye focus in front of the retina causing blurred distance vision
Myopia typically causes blurred distance vision while objects at near may appear clear. It normally develops during childhood and progresses until the mid to late teenage years, with younger children and females showing greater annual rates of myopia progression.16,17 Myopia is the most common refractive error and the major cause of vision impairment worldwide.1,18 Globally, it is recognised as a significant public health concern associated with increased ocular-related morbidity and considerable healthcare costs.2,8,9,18-20 Of particular concern is the association of increasing levels of myopia with a higher risk of potentially blinding ocular pathologies such as glaucoma, myopic maculopathy, and vitreous and retinal detachments.2-8 It affects approximately 30% of the world’s population and its prevalence has been forecast to affect about 50% of the world’s population by 2050.1 The prevalence of myopia in young adolescents has been increasing in recent decades to about 30% in industrialized societies of the West and epidemic levels of over 90% in some parts of Far East Asia.1,21-26
What Causes Myopia?
The underlying cause behind the onset and progression of myopia is believed to be a combination of genetic and environmental factors.11 Risk factors include the following: 10,27-34
- Ethnicity
- Number of myopic parents
- Time engaged in close work
- Lack of time spent outdoors
- Country of residence
- Environmental setting of residence; urban or rural
Furthermore, high myopia has also been found to contribute to a general degradation of quality of life due to psychological, cosmetic and practical reasons.35
How Can Myopia Be Detected?
Qualified eye care professionals can diagnose myopia through an eye examination. While the refractive state of myopic individuals can be successfully corrected to achieve acceptable distance vision by conventional spectacles or contact lenses, these remedies are not intended to control myopia progression. On the contrary, in some cases these optical devices may exacerbate the progression of myopia.10,15,36
There is evidence indicating myopia can be mitigated by having children spend more time outdoors and through the use of specialised optical devices and medicines.10,15 There is strong scientific evidence from case reports, retrospective studies, prospective clinical trials, systematic reviews and meta-analyses that centre-distance multifocal soft contact lens wear and overnight orthokeratology contact lens wear are effective treatment options for myopia control in children and young adults.10,15,36-39 However, there are currently very limited treatment options that have official regulatory approval for myopia control.14
Menicon Bloom Myopia Control Management System – How Does It Work?
The Menicon Bloom Myopia Control Management System has been carefully developed to provide eye care professionals worldwide with a variety of high quality, officially approved (by licence) tools to tackle the myopia epidemic. This myopia control management system encompasses both soft and orthokeratology contact lens devices, which can be conveniently worn either during the day or night (Menicon Bloom Day and Menicon Bloom Night, respectively), thus making it the most comprehensive regulatory approved myopia care therapy available in Europe today.
Menicon Bloom Day
Taking inspiration from the design of optics in advanced modern camera systems, the Menicon Bloom Day design uses an extended depth of focus technology to provide a smooth transition in refractive power from a central zone that provides sharp distance vision to a peripheral zone that generates relative plus power. The gradual and continuous change in lens power from the centre to the periphery of the lens has been carefully engineered to impose myopic defocus on the peripheral retina. This provides a putative stimulus to slow eye growth, while preventing simultaneous image formation on the retina and allowing for sharp, crisp vision at all distances (figure 2).40-42
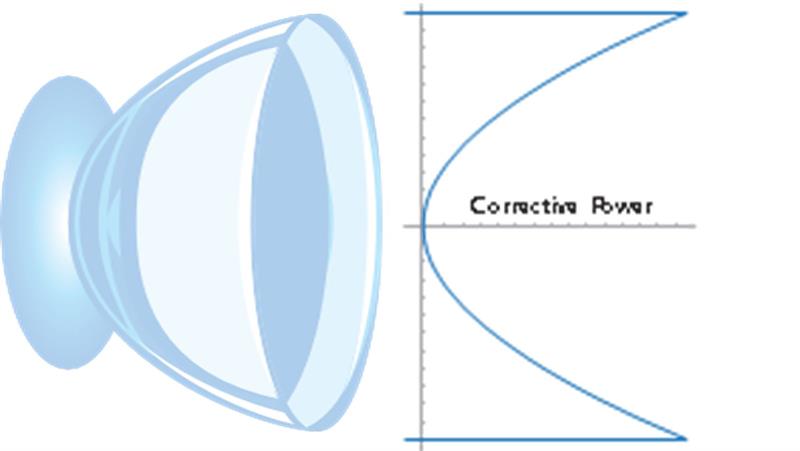 Figure 2: Menicon Bloom Day exclusive design for myopia progression control featuring extended depth of focus technology to provide a smooth transition in refractive power from a central zone that provides sharp distance vision to a peripheral zone that generates relative plus power
Figure 2: Menicon Bloom Day exclusive design for myopia progression control featuring extended depth of focus technology to provide a smooth transition in refractive power from a central zone that provides sharp distance vision to a peripheral zone that generates relative plus power
Menicon Bloom Day features one universal lens design for optimal fit efficiency. The lens design generates an extended depth of focus that encompasses add power requirements ranging from +0.75 to +3.00D, making it an easy-to-fit daily disposable soft contact lens for myopia progression control in children. The higher active add power of up to +3.00D has been designed to create a significant peripheral myopic defocus that provides for clinically relevant levels of myopia control efficacy when compared to other centre-distance multifocal contact lens designs.10,37,41 Add powers of +3.00D or more have been shown to create a significant relative myopic shift in peripheral refraction relative to central refraction.43
Menicon Bloom Day daily disposable soft contact lenses are made from etafilcon A and are indicated for daily wear for the correction of refractive myopia and for myopia progression control. The lens may be worn by persons who exhibit astigmatism of up to 1.00DC where mean sphere has no impact on subjective acuity.
Menicon Bloom Night
Menicon Bloom Night therapy involves the overnight wear of a specially designed orthokeratology contact lens manufactured in the hyperpermeable Menicon Z material. This ensures optimal corneal oxygenation for comfortable and safe overnight wear.44-48 The treatment temporarily changes the shape of the cornea by flattening and steepening the central and mid-peripheral corneal curvatures, respectively. These corneal changes occur overnight and reduce refractive error, thus eliminating the need to wear contact lenses throughout the waking hours after lenses are removed.49 The modified corneal shape provides a particular optical path for incoming light that counters the ocular growth response associated with myopia development (figure 3).37 Through this mechanism, Menicon Bloom Night is indicated for the correction of refractive myopia and for control of myopia when prescribed and managed by a qualified eye care professional.
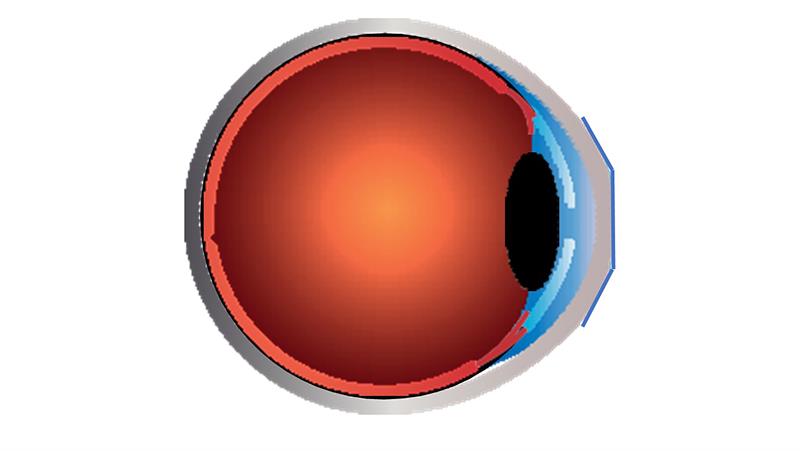
Figure 3: Menicon Bloom Night innovative orthokeratology contact lens design for myopia control fitted on an eye. Upon lens removal, the treatment provides a new corneal shape that counters the ocular growth response associated with myopia development
Menicon Bloom Night myopia control therapy is currently available in two different contact lens designs:
- Menicon Bloom Night
- Menicon Bloom Night Toric
Both lens types can correct up to -4.00D of myopia, with Menicon Bloom Night Toric providing additional options for correcting higher levels of corneal and refractive astigmatism.
The recommended care solution for Menicon Bloom Night contact lenses is Menicon Bloom Care, a multipurpose solution with a demonstrated wide spectrum of disinfection against pathogenic microorganisms.50-52 The solution comes in a bottle designed for improved handling by children. Menicon Bloom Care is also manufactured with significantly less plastic consistent with Menicon’s commitment towards sustainable development goals (www.menicon.com/corporate/sdg). Additionally, to ensure safe and comfortable lens wear the use of Menicon Progent (a combined protein remover, disinfectant and intensive cleaner) is recommended in combination with Menicon Bloom Care.
Is Menicon Bloom Efficacious, Safe & Acceptable?
Comprehensive scientific evidence collected over the years has supported the efficacy, safety and acceptance of Menicon Bloom Day and Menicon Bloom Night as successful treatment options for myopia control. The latter has been independently confirmed by notified bodies in Europe, ultimately granting the treatments CE-approval for the specific indication of myopia control.
Efficacy
A retrospective case series analysis from ten practice locations in the US, which analysed data from 32 patients (ages six to 19 years), has shown Menicon Bloom Day to significantly slow myopia progression in children.53 Additionally, the extended depth of focus design has been shown to correct peripheral hyperopia, the putative stimulus responsible for myopia progression,54,55 and improve amplitude and lag of accommodation by 1.00D and 0.50D, respectively.56
Several peer-reviewed studies conducted with Menicon Bloom Night for myopia control treatment have demonstrated significant levels of efficacy.57-59 Furthermore, recent results demonstrate Menicon Bloom Night can reduce myopia progression in children over long periods of contact lens wear.60
Safety
Like any other treatment, contact lens wear can be associated with the development of adverse events and complications. However, recent large studies, including systematic reviews and meta-analyses, have demonstrated Menicon Bloom Day and Menicon Bloom Night type contact lenses, if fitted correctly by an eye care professional according to the manufacturer’s instructions, are safe to use in paediatric populations.61-65
A review of nine prospective studies, conducted in children aged from seven to 19 years and wearing soft contact lenses (representing 1800 patient years of wear) reported the incidence of corneal infiltrative events in children to be no higher than that found in adults. The incidence was found to be markedly lower in the youngest age range of eight to 11 years in comparison with adults.64 A more recent data analysis from six randomised myopia control trials conducted with daily disposable hydrogel (etafilcon A) contact lenses in 663 myopic children (aged seven to 15 years at baseline) reported no significant or serious ocular adverse events, including corneal infections or serious corneal infiltrative events indicating daily disposable etafilcon A hydrogel contact lenses are safe for use in children.65 All together, these results support the safety of Menicon Bloom Day daily disposable soft contact lenses for the purposes of myopia progression control in children.
Specific studies performed with Menicon Bloom Night for myopia control management have shown that the complications associated with the use of the device have the following characteristics:57,59,66
- Not usually considered to be serious.
- Similar to those reported with other contact lens types.
- Can be managed straightforwardly in clinical practice.
Additionally, post-marketing surveillance and complaint trend data from the manufacturer as well as potential adverse events reported with Menicon Bloom Night orthokeratology contact lenses in external databases have been reviewed. Analysis of all this data has provided conclusive evidence supporting Menicon Bloom Night as a safe, viable myopia control treatment option.67
Acceptance
The fitting of contact lenses on children and young adolescents has been met with considerable resistance from many eye care professionals over the years. This is likely due to misplaced perceptions of a decreased capacity for minors to care for contact lenses, a requirement for more fitting and training time, and an inferior risk-to-benefit ratio compared to adults. The latter might explain why children and teenagers with refractive errors have traditionally been corrected with spectacles, despite reports of successful contact lens wear in minors with different types of contact lenses, including soft, rigid gas-permeable and
orthokeratology contact lenses.68-74 In fact, many studies have shown that minors are fully capable of using and caring for soft and orthokeratology contact lenses.57,58,60,71,75-80
Studies have also shown that certain soft and orthokeratology contact lenses are becoming popular forms of optical correction and myopia control management for children and young adolescents.81-84 The use of contact lenses has been found to dramatically improve how children and teenagers feel about their appearance and participation in activities, leading to greater satisfaction with their refractive error correction.70,74 Children can achieve long wearing times71,73 and also benefit from contact lenses through an enhancement of a variety of self-perceptions such as physical appearance, athletic competence and social acceptance.72
Menicon Bloom Day has been shown to perform comparably to a single vision spherical lens by providing excellent distance, intermediate and near vision at high and low contrasts, with subjective overall vision rated ‘remarkably high’.56 Additionally, Menicon Bloom Day provided stereoacuity identical to that found with spherical soft contact lens and a slight improvement in reading rates for smaller text.56 Similarly, Menicon Bloom Night therapy has shown to be well-accepted by parents and to improve children’s overall vision, far distance vision, symptoms, appearance, satisfaction, activities, academic performance, handling and peer perceptions in comparison to single vision spectacle lens wear.85
Collectively, the above studies indicate that the benefits of prescribing the Menicon Bloom Myopia Control Management System to children with progressive myopia would outweigh any potential risks associated with the treatment. Menicon Bloom Day and Menicon Bloom Night both have met the highest standards of safety, efficacy and quality required to grant the treatments CE approval for myopia progression control management in Europe. As such, if used correctly in accordance with the instructions for use, Menicon Bloom Day and Menicon Bloom Night provide excellent benefits for myopia control with very limited risks in children.86
Choosing the Right Treatment for Your Patient
In implementing a myopia management strategy, it is important to choose the right treatment type for your patient while also considering the best time to start and stop treatment. Research indicates that lower levels of hypermetropia at a young age is a strong risk factor for future myopia development.87,88 Furthermore, the major factor contributing to faster myopia progression is myopia onset at a younger age, with this factor being independent of gender, ethnicity, school, time spent reading and parental myopia.10
Myopia progresses at much faster rates in children compared to teenagers, with faster progression rates typically being observed in children between seven to 12 years of age,89 thus supporting the need for earlier intervention in myopic children.17 To maximise the myopia control effect and to minimise potential rebound effects upon cessation of treatment,90 eye care professionals are recommended to continue myopia control treatment until myopia progression stabilises. This has been reported to occur sometime between 16 to 21 years of age in early-onset myopes.17
Eye care professionals prescribing the Menicon Bloom Myopia Control Management System are able to choose between Menicon Bloom Day and Menicon Bloom Night for myopia control management. Factors affecting this decision will include the child’s refractive and biometric status, as well as on visual, handling and lifestyle demands.
How to Prescribe Menicon Bloom Day & Night?
Menicon Bloom Day features a single easy-to-fit lens design for optimal fit efficiency that provides an 88% initial on-eye fitting success rate.91 The fitting of Menicon Bloom Night is optimised by the use of a corneal topographer to precisely measure corneal shape. This is used in conjunction with Menicon’s Easyfit software, a user-friendly tool which accurately guides the practitioner through the fitting process. Following the first night of overnight wear, Menicon Bloom Night has demonstrated a 90% first fit success rate in children.92 Additionally, a specially designed mobile phone application, Menicon’s Virtual Doctor, has been developed to enhance the monitoring and communication process between eye care professionals and patients.
Why CE Approval Matters
Marketing a medical device in Europe requires a marketing authorisation (‘product license’) for specified indications under specified conditions (eg target population, indication, specific use), regulated by the country’s medicines and health care products regulatory agency.93,94 This process is employed to ensure that medical products meet the highest standards of safety, efficacy and quality before being issued a marketing authorisation, which allows the medical device to become available to the general public.
In Europe, products that hold a marketing authorisation are designated a ‘CE’ marking. Prescribing a licensed product outside the approved scope of use is called ‘off-label’ prescribing. An example of off-label prescribing occurs when a regular soft multifocal contact lens, which has an indication for vision correction for patients >40 years who experience reading difficulties, is fitted for myopia control purposes to a child where both the indication (ie reading difficulties vs reducing myopia progression) and target group (ie adults vs children) are different from those for which the product has been approved for. Similarly, off-label prescribing also occurs when an orthokeratology contact lens, which is approved for the correction of manifest myopia in adults, is prescribed for reducing myopia progression in children, where again both the indication (ie correcting manifest myopia vs reducing myopia progression) and target group (ie adults vs children) are different from those for which the product has been approved for.
When prescribing a treatment for myopia control, the eye care professional should ideally start by considering all on-label products that may be available and only contemplate off-label prescribing if there are no on-label options or if approved products are not effective or appropriate.14 In off-label prescribing, the patient must be adequately informed about the lack of product authorisation and the possible existence of unknown risks.95-99 Parents and legal guardians should be informed of all options and associated risks in order to decide whether the child should be treated with a tested and approved on-label treatment or with an off-label treatment that might give a successful result, but has unknown risks.
With the official marketing authorisation for myopia control management, Menicon Bloom Day and Menicon Bloom Night have both met the required standards of safety, efficacy and quality for CE approval for myopia control management in Europe. With such approval, eye care professionals can now have peace of mind with the on-label prescription of this myopia control therapy. •
Neil Retallic is Professional Services Director for Europe at Menicon, a GOC registered Optometrist and College of Optometrist Scheme for Registration Assessor and Examiner.
Josie Barlow is Professional Services Manager at Menicon UK Limited and a GOC registered Contact Lens Optician
References
- Holden BA, Fricke TR, Wilson DA, et al. Global prevalence of myopia and high myopia and temporal trends from 2000 through 2050. Ophthalmology 2016;123(5):1036-42.
- Flitcroft DI. The complex interactions of retinal, optical and environmental factors in myopia aetiology. Prog Retin Eye Res. 2012;31(6):622-60.
- Tano Y. Pathologic myopia: where are we now? Am J Ophthalmol 2002;134(5):645-60.
- Vongphanit J, Mitchell P, Wang JJ. Prevalence and progression of myopic retinopathy in an older population. Ophthalmology 2002;109(4):704-11.
- Wong TY, Klein BE, Klein R, Knudtson M, Lee KE. Refractive errors, intraocular pressure, and glaucoma in a white population. Ophthalmology 2003;110(1):211-7.
- Saw SM, Gazzard G, Shih-Yen EC, Chua WH. Myopia and associated pathological complications. Ophthalmic Physiol Opt 2005;25(5):381-91.
- Ikuno Y, Jo Y, Hamasaki T, Tano Y. Ocular risk factors for choroidal neovascularization in pathologic myopia. Invest Ophthalmol Vis Sci 2010;51(7):3721-5.
- Tideman JWL, Snabel MCC, Tedja MS, et al. Association of axial length with risk of uncorrectable visual impairment for europeans with myopia. JAMA Ophthalmol 2016; 134(12):1355-63.
- Flitcroft DI, He M, Jonas JB, et al. IMI - Defining and classifying myopia: a proposed set of standards for clinical and epidemiologic studies. Invest Ophthalmol Vis Sci 2019;60(3):M20-M30.
- Gifford KL, Richdale K, Kang P, et al. IMI - Clinical Management Guidelines Report. Invest Ophthalmol Vis Sci 2019;60(3):M184-M203.
- Tedja MS, Haarman AEG, Meester-Smoor MA, et al. IMI - Myopia genetics report. Invest Ophthalmol Vis Sci 2019;60(3):M89-M105.
- Troilo D, Smith 3rd EL, Nickla DL, et al. IMI - Report on experimental models of emmetropization and myopia. Invest Ophthalmol Vis Sci 2019;60(3):M31-M88.
- Wolffsohn JS, Kollbaum PS, Berntsen DA, et al. IMI – Clinical myopia control trials and instrumentation report. Investig Ophthalmol Vis Sci 2019; 60(3):M132-M160.
- Jones L, Drobe B, González-Méijome JM, et al. IMI – Industry guidelines and ethical considerations for Myopia control report. Investig Ophthalmol Vis Sci 2019;60(3):M161-M183.
- Wildsoet CF, Chia A, Cho P, et al. IMI - Interventions for controlling myopia onset and progression report. Invest Ophthalmol Vis Sci 2019;60(3):M106-M131.
- Goss DA, Winkler RL. Progression of myopia in youth: age of cessation. Am J Optom Physiol Opt 1983;60(8):651-8.
- Group C. Myopia stabilization and associated factors among participants in the Correction of Myopia Evaluation Trial (COMET). Invest Ophthalmol Vis Sci 2013;54(13):7871-84.
- Flaxman SR, Bourne RRA, Resnikoff S, et al. Global causes of blindness and distance vision impairment 1990-2020: a systematic review and meta-analysis. Lancet Glob Heal 2017;5(12):e1221-e34.
- Vitale S, Cotch MF, Sperduto R, Ellwein L. Costs of refractive correction of distance vision impairment in the United States, 1999-2002. Ophthalmology 2006;113(12):2163-70.
- Lim MC, Gazzard G, Sim EL, Tong L, Saw SM. Direct costs of myopia in Singapore. Eye 2009;23(5):1086- 9.
- Pan CW, Dirani M, Cheng CY, Wong TY, Saw SM. The age-specific prevalence of myopia in Asia: a meta-analysis. Optom Vis Sci 2015;92(3):258-66.
- Koh V, Yang A, Saw SM, et al. Differences in prevalence of refractive errors in young Asian males in Singapore between 1996-1997 and 2009-2010. Ophthalmic Epidemiol 2014;21(4):247-55.
- Pan CW, Ramamurthy D, Saw SM. Worldwide prevalence and risk factors for myopia. Ophthalmic Physiol Opt 2012;32(1):3-16.
- Wang TJ, Chiang TH, Wang TH, Lin LL, Shih YF. Changes of the ocular refraction among freshmen in National Taiwan University between 1988 and 2005. Eye 2009;23(5):1168-9.
- Vitale S, Sperduto RD, Ferris 3rd FL. Increased prevalence of myopia in the United States between 1971-1972 and 1999-2004. Arch Ophthalmol 2009;127(12):1632-9.
- Gilmartin B. Myopia: precedents for research in the twenty-first century. Clin Exp Ophthalmol 2004;32(3):305-24.
- Rose KA, Morgan IG, Smith W, Burlutsky G, Mitchell P, Saw SM. Myopia, lifestyle, and schooling in students of Chinese ethnicity in Singapore and Sydney. Arch Ophthalmol 2008;126(4):527-30.
- French AN, Morgan IG, Mitchell P, Rose KA. Risk factors for incident myopia in Australian schoolchildren: the Sydney adolescent vascular and eye study. Ophthalmology 2013;120(10):2100-8.
- Mutti DO, Hayes JR, Mitchell GL, et al. Refractive error, axial length, and relative peripheral refractive error before and after the onset of myopia. Invest Ophthalmol Vis Sci 2007;48(6):2510-9.
- He M, Zheng Y, Xiang F. Prevalence of myopia in urban and rural children in mainland China. Optom Vis Sci 2009;86(1):40-4.
- Gwiazda J, Hyman L, Dong LM, et al. Factors associated with high myopia after 7 years of follow-up in the Correction of Myopia Evaluation Trial (COMET) Cohort. Ophthalmic Epidemiol 2007;14(4):230-7.
- Pacella R, McLellan J, Grice K, Del Bono EA, Wiggs JL, Gwiazda JE. Role of genetic factors in the etiology of juvenile-onset myopia based on a longitudinal study of refractive error. Optom Vis Sci 1999;76(6):381-6.
- Wu MM, Edwards MH. The effect of having myopic parents: an analysis of myopia in three generations. Optom Vis Sci 1999;76(6):387-92.
- Rosenfield M, Gilmartin B. Myopia and Nearwork. Oxford, UK.: Butterworth-Heinemann; 1998.
- Rose K, Harper R, Tromans C, et al. Quality of life in myopia. Br J Ophthalmol 2000;84(9):1031-4.
- Huang J, Wen D, Wang Q, et al. Efficacy comparison of 16 interventions for myopia control in children: a network meta-analysis. Ophthalmology 2016;123(4):697-708.
- Smith 3rd EL. Optical treatment strategies to slow myopia progression: effects of the visual extent of the optical treatment zone. Exp Eye Res 2013;114:77-88.
- Wen D, Huang J, Chen H, et al. Efficacy and acceptability of orthokeratology for slowing myopic progression in children: a systematic review and meta-analysis. J Ophthalmol 2015;2015:360806.
- Li SM, Kang MT, Wu SS, et al. Studies using concentric ring bifocal and peripheral add multifocal contact lenses to slow myopia progression in school-aged children: a meta-analysis. Ophthalmic Physiol Opt 2017;37(1):51-9.
- Hair LA, Steffensen EM, Benoit JS, Berntsen DA. Changes in peripheral defocus with center-distance and center-near multifocal contact lenses. Optom Vis Sci 2019:E-abstract 195209.
- Lee R, Achenbach P, Schwiegerling J. Instantaneous power profiles vs sagittal power profiles of various center distance multifocal lenses. Optom Vis Sci 2019;96:E-abstract 195249.
- Nti AN, Gregory HR, Ritchley ER, Berntsen DA. Contrast sensitivity with center-distance multifocal contact lenses. Optom Vis Sci 2019;96:E-abstract 190033.
- Lopes-Ferreira D, Ribeiro C, Neves H, et al. Peripheral refraction with dominant design multifocal contact lenses in young myopes. J Optom 2013;6(2):85-94
- Ladage PM, Yamamoto K, Ren DH, et al. Effects of rigid and soft contact lens daily wear on corneal epithelium, tear lactate dehydrogenase, and bacterial binding to exfoliated epithelial cells. Ophthalmology 2001;108(7):1279-88.
- Morgan PB, Maldonado-Codina C, Efron N. Comfort response to rigid and soft hyper-transmissible contact lenses used for continuous wear. Eye Contact Lens 2003;29(1 Suppl):S127-30;
- Maldonado-Codina C, Morgan PB, Efron N, Efron S. Comparative clinical performance of rigid versus soft hyper Dk contact lenses used for continuous wear. Optom Vis Sci 2005;82(6):536-48.
- Morgan PB, Efron N, Maldonado-Codina C, Efron S. Adverse events and discontinuations with rigid and soft hyper Dk contact lenses used for continuous wear. Optom Vis Sci 2005;82(6):528-35.
- Albright RA, Venuti BD, Ichijima H, Nyunt AK, Cavanagh HD. Postmarket surveillance of Menicon Z rigid gas-permeable contact lenses for up to 30 days continuous wear in the United States. Eye Contact Lens 2010;36(5):241-4.
- Swarbrick HA. Orthokeratology review and update. Clin Exp Optom 2006;89(3):124-43.
- Boost M, Cho P, Lai S. Efficacy of multipurpose solutions for rigid gas permeable lenses. Ophthalmic Physiol Opt 2006;26(5):468-75.
- Choy CKM, Cho P, Boost M V. Cytotoxicity of rigid gas-permeable lens care solutions. Clin Exp Optom 2013;96(5):467-71.
- Shi G Sen, Boost M V, Cho P. Does the presence of QAC genes in staphylococci affect the efficacy of disinfecting solutions used by orthokeratology lens wearers? Br J Ophthalmol 2016;100(5):708-12.
- Cooper J, O’Connor B, Watanabe R, et al. Case Series Analysis of Myopic Progression Control With a Unique Extended Depth of Focus Multifocal Contact Lens. Eye Contact Lens 2018;44(5):e16-e24.
- R. Payor, J. Woods, D. Fonn, P. Situ, S. Dillehay, R. Griffin, M. Tyson LJ. Feasibility Testing of a Novel Soft Contact Lens Optical Design to Reduce Suspected Risk Factors for the Progression of Juvenile Onset Myopia. Invest Ophthalmol Vis Sci 2014;55:E-abstract 3638.
- S. Dillehay, J. Woods, P. Situ , S. Guthrie, R. Paynor, R. Griffin, M. Tyson, L. Jones. Comparison of Three Power Levels of A Novel Soft Contact Lens Optical Design to Reduce Suspected Risk Factors for the Progression of Juvenile Onset Myopia. Invest Ophthalmol Vis Sci 2014;55:E-abstract 3637.
- Miller J, Long B, Dillehay S. Children’s evaluation of a unique myopia progression control lens design. Optom Vis Sci 2013;88:E-abstract 115896.
- Cho P, Cheung SW. Retardation of myopia in Orthokeratology (ROMIO) study: a 2-year randomized clinical trial. Invest Ophthalmol Vis Sci 2012;53(11):7077-85.
- Santodomingo-Rubido J, Villa-Collar C, Gilmartin B, Gutiérrez-Ortega R. Myopia control with orthokeratology contact lenses in Spain: Refractive and biometric changes. Invest Ophthalmol Vis Sci 2012;53(8):5060-5
- Chen C, Cheung SW, Cho P. Myopia control using toric orthokeratology (TO-SEE study). Invest Ophthalmol Vis Sci 2013;54(10):6510-7.
- Santodomingo-Rubido J, Villa-Collar C, Gilmartin B, Gutiérrez-Ortega R, Sugimoto K. Long-term efficacy of orthokeratology contact lens wear in controlling the progression of childhood myopia. Curr Eye Res 2017;42(5):713-20
- Bullimore MA, Sinnott LT, Jones-Jordan LA. The risk of microbial keratitis with overnight corneal reshaping lenses. Optom Vis Sci 2013;90(9):937-44.
- Li SM, Kang MT, Wu SS, et al. Efficacy, safety and acceptability of orthokeratology on slowing axial elongation in myopic children by meta-analysis. Curr Eye Res 2016;41(5):600-8.
- Liu YM, Xie P. The Safety of Orthokeratology--A Systematic Review. Eye Contact Lens 2016;42(1):35- 42.
- Bullimore MA. The Safety of Soft Contact Lenses in Children. Optom Vis Sci 2017;94(6):638-46.
- Brennan NA, Toubouti Y, Greenaway N, Cheng X. Safety of contact lenses in pediatrics: data analysis from six randomized myopia control trials. Optom Vis Sci 2019;96:E-abstract: 195208.
- Santodomingo-Rubido J, Villa-Collar C, Gilmartin B, Gutiérrez-Ortega R. Orthokeratology vs. spectacles: adverse events and discontinuations. Optom Vis Sci 2012;89:1133-9
- Clinical Evaluation Report for Menicon Z Night Orthokeratology Contact Lenses. 2017.
- Perrigin J, Perrigin D, Quintero S, Grosvenor T. Silicone-acrylate contact lenses for myopia control: 3-year results. Optom Vis Sci 1990;67(10):764-9.
- Katz J, Schein OD, Levy B, et al. A randomized trial of rigid gas permeable contact lenses to reduce progression of children’s myopia. Am J Ophthalmol 2003;136(1):82-90.
- Walline JJ, Gaume A, Jones LA, et al. Benefits of contact lens wear for children and teens. Eye Contact Lens 2007;33(6):317-21.
- Lipson MJ. Long-term clinical outcomes for overnight corneal reshaping in children and adults. Eye Contact Lens 2008;34(2):94-9.
- Walline JJ, Jones LA, Sinnott L, et al. Randomized trial of the effect of contact lens wear on self- perception in children. Optom Vis Sci 2009;86(3):222-32.
- Jones-Jordan LA, Chitkara M, Coffey B, et al. A comparison of spectacle and contact lens wearing times in the ACHIEVE study. Clin Exp Optom 2010;93(3):157-63.
- Rah MJ, Walline JJ, Jones-Jordan LA, et al. Vision specific quality of life of pediatric contact lens wearers. Optom Vis Sci 2010;87(8):560-6.
- Walline JJ, Jones LA, Sinnott LT. Corneal reshaping and myopia progression. Br J Ophthalmol 2009;93(9):1181-5.
- Hiraoka T, Kakita T, Okamoto F, Takahashi H, Oshika T. Long-term effect of overnight orthokeratology on axial length elongation in childhood myopia: a 5-year follow-up study. Invest Ophthalmol Vis Sci 2012;53(7):3913-9.
- Downie LE, Lowe R. Corneal reshaping influences myopic prescription stability (CRIMPS): an analysis of the effect of orthokeratology on childhood myopic refractive stability. Eye Contact Lens 2013;39(4):303-10.
- Hiraoka T, Kakita T, Okamoto F, Oshika T. Influence of ocular wavefront aberrations on axial length elongation in myopic children treated with overnight orthokeratology. Ophthalmology 2015;122(1):93-100.
- Lee YC, Wang JH, Chiu CJ. Effect of Orthokeratology on myopia progression: twelve-year results of a retrospective cohort study. BMC Ophthalmol 2017;17(1):243.
- Hiraoka T, Sekine Y, Okamoto F, Mihashi T, Oshika T. Safety and efficacy following 10-years of overnight orthokeratology for myopia control. Ophthalmic Physiol Opt 2018;38(3):281-9.
- Efron N, Morgan PB, Woods CA, International contact lens prescribing survey consortium. Survey of contact lens prescribing to infants, children, and teenagers. Optom Vis Sci 2011;88(4):461-8.
- Wolffsohn JS, Calossi A, Cho P, et al. Global trends in myopia management attitudes and strategies in clinical practice. Contact Lens Anterior Eye 2016;39(2):106-16.
- Efron N, Morgan PB, Woods CA, Santodomingo-Rubido J, Nichols JJ. International survey of contact lens fitting for myopia control in children. Contact Lens Anterior Eye 2019 [Epub ahead of print]
- Wolffsohn J, Calossi A, Cho P, et al. Global trends in myopia management attitudes and strategies in clinical practice - 2019 Update. Contact Lens Anterior Eye 2019 [Epub ahead of print]
- Santodomingo-Rubido J, Villa-Collar C, Gilmartin B, Gutiérrez-Ortega R. Myopia control with orthokeratology contact lenses in Spain: A comparison of vision-related quality-of-life measures between orthokeratology contact lenses and single-vision spectacles. Eye Contact Lens 2013;39(2):153-7
- Bullimore M, Ritchey ER. Myopia control: an evidence-based comparison of the benefits and the risks. Optom Vis Sci. 2019;96:E-abstract 190031.
- Mutti DO, Sinnott LT, Mitchell GL, et al. Relative peripheral refractive error and the risk of onset and progression of myopia in children. Invest Ophthalmol Vis Sci.2011;52(1):199-205.
- Zadnik K, Sinnott LT, Cotter SA, et al. Prediction of Juvenile-Onset Myopia. JAMA Ophthalmol. 2015;133(6):683-689.
- Donovan L, Sankaridurg P, Ho A, Naduvilath T, Smith 3rd EL, Holden BA. Myopia progression rates in urban children wearing single-vision spectacles. Optom Vis Sci 2012;89(1):27-32.
- Cho P, Cheung SW. Discontinuation of orthokeratology on eyeball elongation (DOEE). Contact Lens Anterior Eye 2017;40(2):82-7.
- Company Data on File, 2015.
- Chan KY, Cheung SW, Cho P. Clinical performance of an orthokeratology lens fitted with the aid of a computer software in Chinese children. Cont Lens Anterior Eye 2012;35(4):180-4.
- Wittich CM, Burkle CM, Lanier WL. Ten common questions (and their answers) about off-label drug use. Mayo Clin Proc 2012;87(10):982-90.
- Aronson JK, Ferner RE. Unlicensed and off-label uses of medicines: definitions and clarification of terminology. Br J Clin Pharmacol 2017; 83(12):2615-25.
- Riley Jr. JB, Basilius PA. Physicians’ liability for off-label prescriptions. Nephrol News Issues 2007;21(7):43-4,46-7.
- Wilkes M, Johns M. Informed consent and shared decision-making: a requirement to disclose to patients off-label prescriptions. PLoS Med 2008;5(11):e223.
- Lenk C, Koch P, Zappel H, Wiesemann C. Off-label, off-limits? Parental awareness and attitudes towards off-label use in paediatrics. Eur J Pediatr 2009;168(12):1473-8.
