The annual conference of the Scottish Contact Lens and Ocular Surface Society (SCLOSS) is now established as one of the important dates in the diary for live CET. A packed, indeed at times almost too packed, agenda offered a full range of lectures (figure 1), discussion seminars and workshops. The popularity of the event is now being discussed by the organisers so that next year the programme will be spread to offer greater access to individual sessions as competition for places at each was very high.
The second workshop I will focus on in these two review features (first part in Optician 20.01.17) was designed to look at how imaging technology has developed to better visualise retinal structures. Delegates were asked to complete a number of questions at each station and it is these which this article will now clarify.
Station 1 True Colour Scanning (supported by Haag Streit UK)
This station centred around the new Eidon AF (figure 2 and 3). The instrument is a confocal scanning ophthalmoscope that uses white light instead of monochromatic lasers, hence providing true colour imaging and offering major benefits in terms of fidelity to real retinal appearance, no distortion and dilation-free operation. The AF signifies the instrument now also includes autofluorescence.

Figure 2
Figure 3
The first question addressed was why a white light source might have any advantage over monochromatic stimulation. Typically, confocal scanning laser ophthalmoscopy systems use incident light in narrow wavelength bands with red and green and sometimes blue channels. These narrow bands when reflected from the object at the confocal plane are then combined and an artificially full colour (or pseudocolour) image produced. The argument is that, by having an image with no artificial construct, there is a better clarity. Figure 4 shows a large atrophic area surrounded by drusen, the true colour image on the left and the artificial construct on the right. There is clearly better definition in the left hand image.
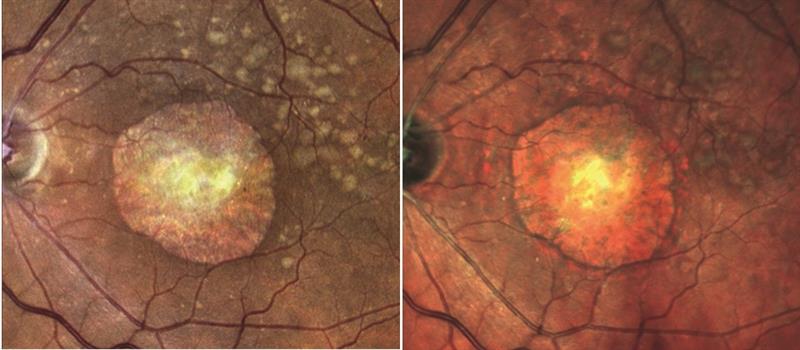
Figure 4
Next, delegates were asked about field of view of an image. The Eidon is able to capture a 60º field in one image without the need for dilation. By use of a mosaic function, images taken at different positions of gaze may be mashed together to produce a simulated wider field – 120º of lateral field is now possible, see figure 5.
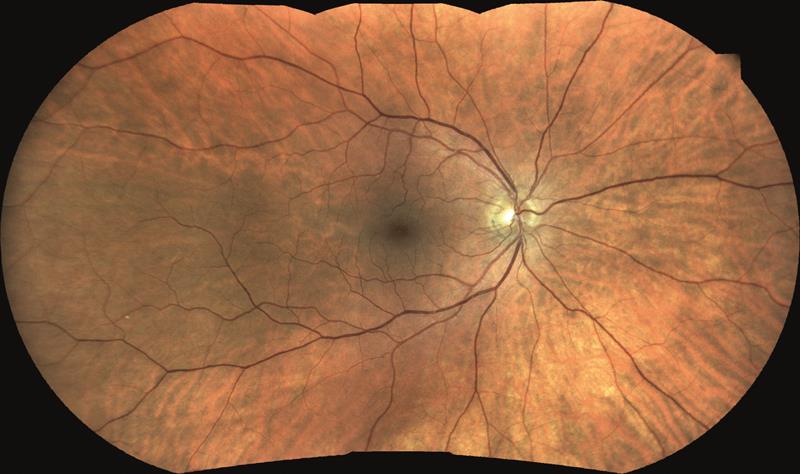
Figure 5
Next, autofluorescence was considered. Autofluorescence is a technique that exploits the fact that lipofuscin, the byproduct of photoreceptor phagocytosis that accumulates with age and is integral to the age-related degenerative process, is excited by short to medium wavelength light and infrared and will be seen to fluoresce. Therefore, any disease process resulting in the degeneration of the retina may be seen clearly by autofluorescence. Drusen are seen as bright (figure 6 left), while atrophic or dead areas are black (figure 6 right). However, ‘sick’ areas, often seen just outside an atrophic area (look again at the margins of the atrophic area on the right hand image of figure 6) will also fluoresce as there is an aggregation of lipofuscin here.
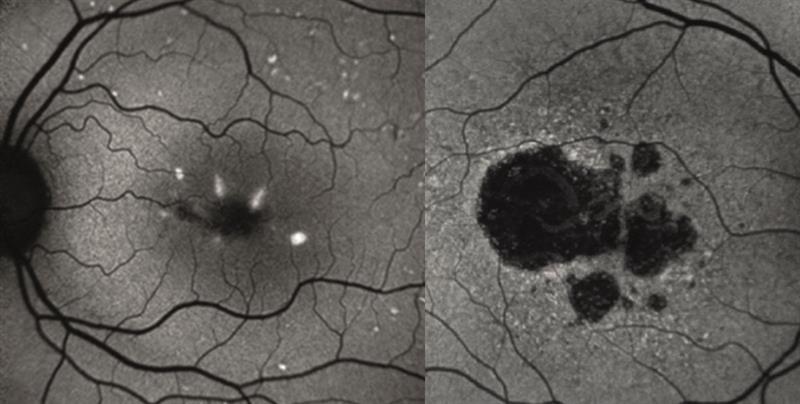
Figure 6
Retinal digital cameras with autofluorescence use AF excitation light tending towards the green while SLO systems (other than the Optos) use bluer light. The latter are better able to detect macular pigment absorption.
Finally, when displaying images where there may have been some minor changes over time, the Eidon images may be displayed on a linked website where they may be viewed in a flicker presentation. Can you see the difference between the left and right image in figure 7? If the two are shown in repeated rapid succession, then the appearance and disappearance of the king’s beard is obvious.

Figure 7
Station 2 Ultrawide field capture (supported by Optos)
This station centred around the Optos Daytona (figure 8 and 9). This very stylish scanning laser ophthalmoscopy system employs the famous Optos ellipsoid mirror enabling it to achieve up to 200º of view (82.5 % of retinal surface area) of the ocular fundus (figure 10). The Optos system simultaneously uses two excitation wavelengths of red (633nm) and green (532nm) light with an emission filter of >540nm. Similar to the fundus camera, the longer wavelength spectra of this system reduces absorption by macular pigment and allows for a clear image after fluorescein angiography. The Daytona also includes autofluorescence and such images differ from the other SLO systems in that the Optos uses a longer wavelength excitation light.

Figure 8
Selection of the different channels for viewing offers some indication of the level of any particular lesion. A choroidal lesion such as a naevus would show up well in the red channel, which would focus further back than the green channel. A retinal pigmentary change might show up better on the green channel.

Figure 9
In recent months there has been some excitement around the ability for SLO scans to detect drusen-like changes as indicative of central nervous system degenerative changes (figure 11).
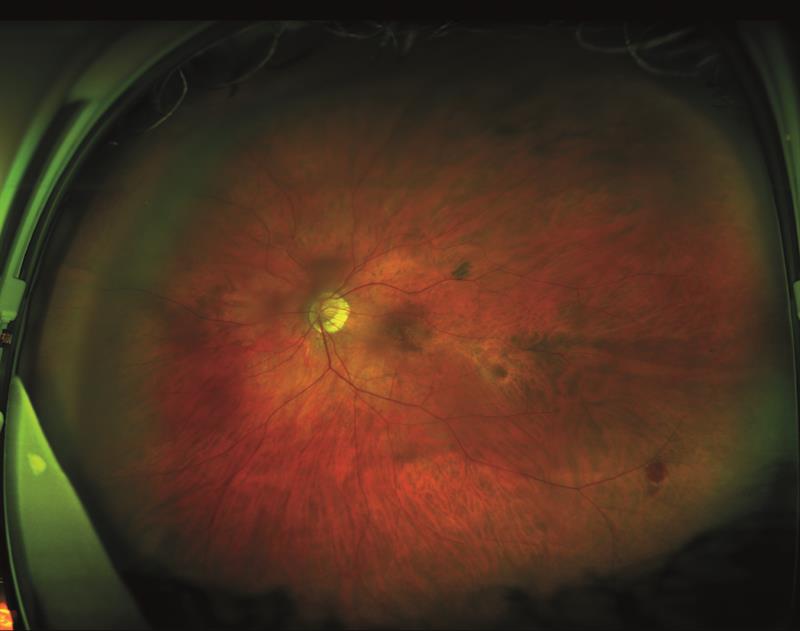
Figure 10
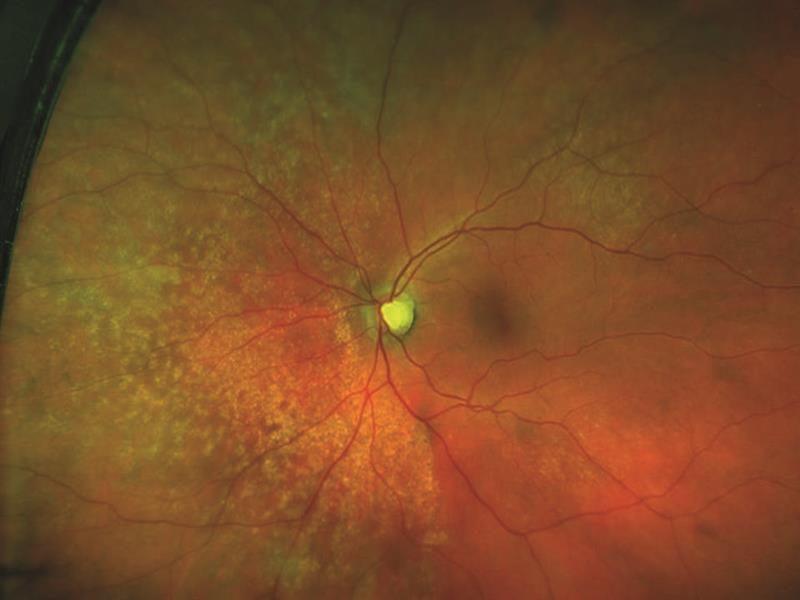
Figure 11
Station 3 Swept-Source OCT (supported by Topcon)
This station centred around the Topcon Triton OCT system (figure 12).

Figure 12
Swept source OCT is able to use faster scanning so providing high resolution images, and also uses a longer wavelength of incident light which allows better penetration of tissues.
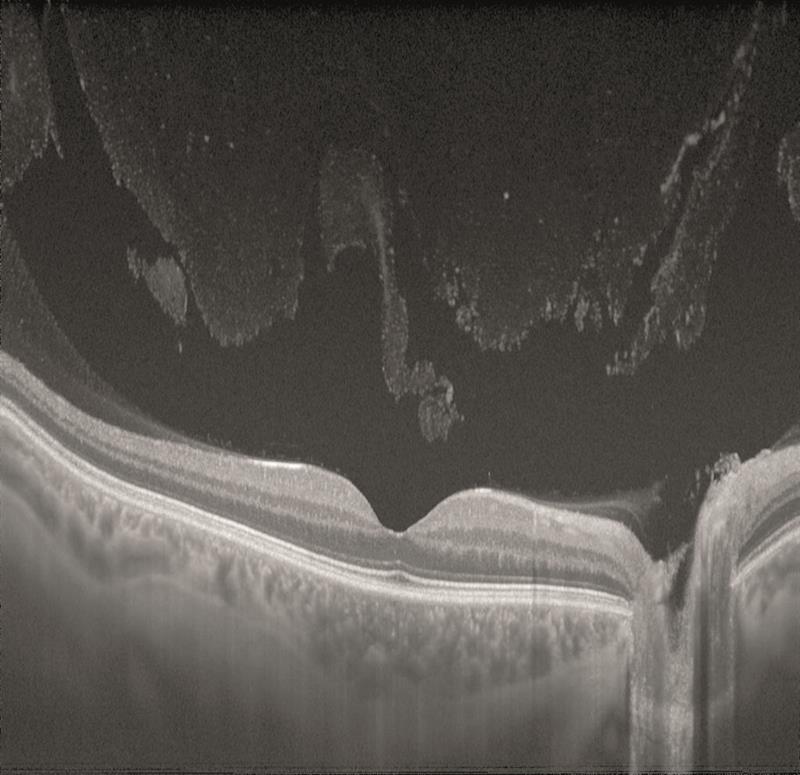
Figure 13
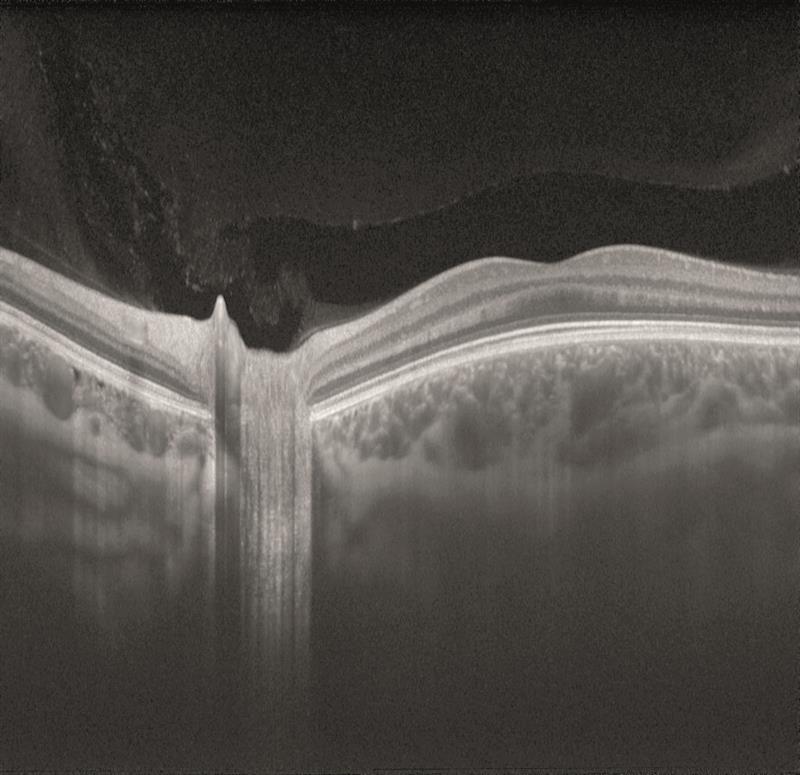
Figure 14
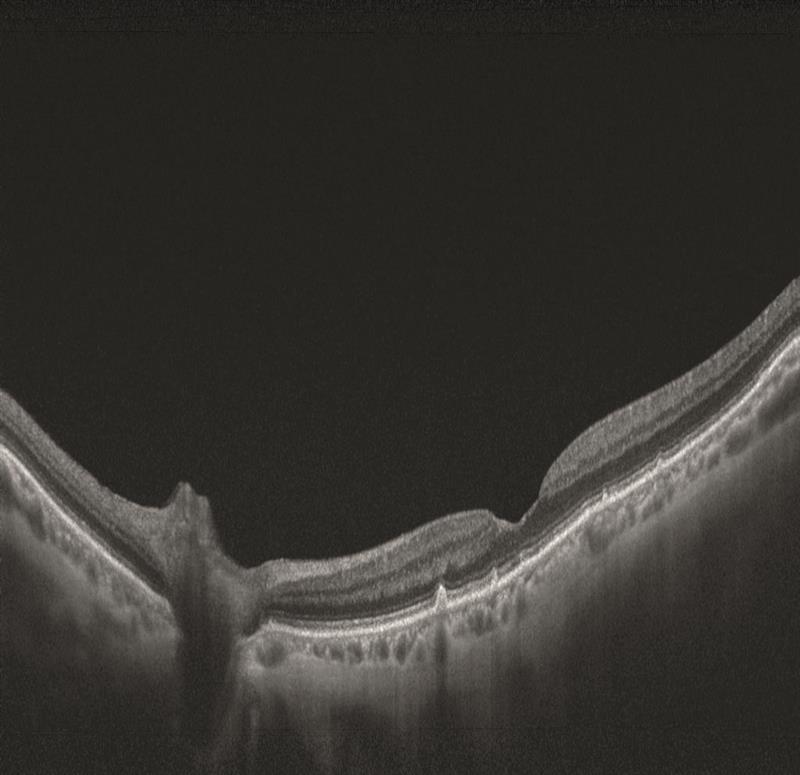
Figure 15
For these reasons, swept-source is allowing excellent visualisation of the vitreous (figure 13) and of the choroid (figure 13 is of an 18-year-old and figure 14 of an 83-year-old – note the much thinner choroid of the latter).
