In our everyday practice we regularly meet patients who complain of headaches. Careful assessment is always important and, very occasionally, the decisions we make can have far reaching consequences for the patient’s health. Fortunately, most of the patients that experience the most severe forms of headaches usually present immediately to hospital and receive prompt treatment.
Case 1 – Internal carotid aneurysm
A 47-year-old male patient (SB), first started suffering ‘terrible’ headaches at the end of March 2011. He saw his general practitioner who diagnosed ‘migraine’. A week later the patient also started experiencing numbness on the left side and what he described as ‘strange visual sensations’ along with the headache. He was referred to a consultant neurologist who undertook an MRI scan. The scan revealed an aneurysm which had leaked slightly (Figure 1).
[GalleryComponent="84"]
The diagnosis was made of a ‘ruptured aneurysm of the left internal carotid artery’. This rupture caused a subarachnoid haemorrhage and this in turn caused the headaches. Scans confirmed the following:
- An aneurysm of the left carotid artery close to the chiasma and the cavernous sinus; a saccular aneurysm of the left carotid artery is close to the ‘s’ bend of the artery
- The maximum intensity projection (MIP) CT scan, (Figure 1a and close up 1b) showed the left internal carotid artery aneurysm as a ‘top hat’
- A selective left internal carotid artery lateral oblique angiogram (Figure 1c) showed the aneurysm in vivid detail.
- The decision was made to manage the aneurysm with a surgical coil procedure the next day using a technique called aneurysm embolisation. The reasons for the early intervention included the rupture of the aneurysm, the severity of the patient’s headaches and the apparent elevation of the left optic nerve and chiasma. Figure 2 (a to d) shows the procedure of coiling and I have added the time-line on the images. The patient had three coils inserted in the form of a ball (Figure 2c) which filled the whole aneurysm, leaving an empty sac at the end of the process (Figure 2d).
[GalleryComponent="85"]
Case 2 – Subarachnoid haemorrhage
In July 2007, a 70-year-old female patient reported ‘thumping’ headaches, double vision, losses of balance and hallucinations on waking one morning. Fortunately, her husband rang for an ambulance and she was transferred to a hospital specialising in the management of intracranial vascular disease. She was diagnosed with a subarachnoid haemorrhage originating from what I understand was a basilar artery aneurysm. The urgency of the situation required an immediate CT scan to ascertain the cause of the headaches and symptoms, so unfortunately no images of the initial assessment are available for this article. The patient was given a copy of the post-coiling CT scan (Figure 3) which she consented to my publishing here. The images show an artifact detected after coiling of the basilar tip aneurysm (Figures 3a and b). The scans showed no evidence of hydrocephalus and minimum widening of the lateral ventricles (Figures 3c and d). There was no evidence of a re-bleed and, fortunately, no discernible ischaemia.
[GalleryComponent="86"]
Over the next four weeks the headaches, hallucinations and imbalance improved. After around six weeks the 6th nerve palsy (the cause of the double vision) began to resolve. The patient vividly remembered noticing acuteness of her hearing during this episode, and reported being able to hear noises four floors away in the hospital. A year later, further scans were undertaken (Figure 3e). Magnetic resonance angiograms (MRAs) showed the blood vessels clearly enough to allow a careful search for leakage or damage. What was noted at this assessment was thought by one radiologist to be a recurrent tiny aneurysm at the tip of the basilar artery while another radiologist thought it was more likely to be a residual aneurysm. Further scans over the year confirmed that this was a residue left post-coiling and no further leaks were evident, nor did the patient suffer any more symptoms. Figure 3e shows the 2mm residual aneurysm of the basilar tip with no leakage and the coiling which I suggest would be above this residual aneurysm, and is not seen on the MRA except on the axial scan where one can see what appears to be a rounded anomaly. I have been able to acquire an image of a basilar tip aneurysm from a radiologist colleague which I have included for illustrative purposes (Figure 4).
[CaptionComponent="940"]Discussion
Subarachnoid haemorrhage is usually due to a ruptured cerebral aneurysm and describes bleeding into the space between the two outer meninges, the arachnoid mater and the pia mater (the subarachnoid space). Subarachnoid haemorrhages account for 4 per cent of all strokes and are medical emergencies. Around 50 per cent are potentially fatal. Those that survive often have residual neurological deficit or cognitive impairment. The classic symptoms of subarachnoid haemorrhage include ‘thunderclap’ headache, neck stiffness, seizure, confusion, vomiting and coma. Cerebral aneurysms may be classified as follows:
- Saccular – bulges from the side of the artery with a distinct neck at the base (as in Figure 2)
- Fusiform – bulges in all directions with no neck
- Giant – greater than 2.5cm in size, either saccular or fusiform.
I find it easy to remember the commonest sites for cerebral aneurysms by reciting the following nonsensical mnemonic; ‘A PC made in Basildon Picasso’ (Table 1 and Figure 4). 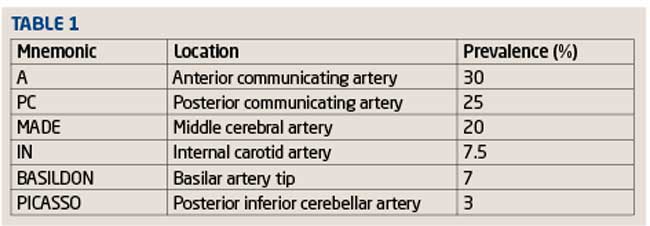
Aneurysm embolisation
The usual treatment of a ruptured intracranial aneurysm involves open surgery to clip the aneurysm inside the skull. Endovascular techniques, avoiding the need for open surgery, approach the aneurysm from within the affected vessel. A thin tube containing a platinum coil is inserted into the femoral artery in the groin and advanced through the aorta to the carotid and vertebral arteries. The coil is detached from the catheter and densely packed inside the aneurysm (Figure 2). This obliterates the aneurysm by causing a blood clot to form inside the aneurysm.[GalleryComponent="87"]
Magnetic resonance angiography (MRA)
During this series I have mentioned MRA a few times. MRA is a technique based on magnetic resonance interferometry (MRI) and used to image blood vessels to look for stenosis, occlusion or aneurysms. The technique acquires images of blood flow. Intravenous contrast agents (such as Godolinium DTPA) shorten the longitudinal relaxation time (T1) of blood to 200ms, less than the T1 of all other tissues except fat. Short TR sequences produce bright images of the blood (Figure 5a). There are a number of techniques for acquisition:
- Flow-dependent angiography (FDA) – this is based on blood flow within the vessels and used to distinguish vessels from other static tissues. Phase-contrast MRA utilises phase differences to distinguish blood from static vessels while ‘time of flight’ MRA exploits the fact that blood turbulence results in fewer excitation phases than static tissue (Figure 5c)
- Flow-independent angiography (FIA) – this does not rely on blood flow but instead on the differences of T1, T2 (transverse relaxation time) and the clinical shift of the different tissues acquired in 3D CT or MRI scans. The technique is good at imaging regions of slow flow, often indicative of vascular disease (Figure 5b). FIA does not require contrast agents
- Maximum intensity projection (MIP) – this describes a technique whereby the computer stimulates rays through a stack of slices and selects the ones with the highest value or intensity for display (Figures 1 and 2).
Conclusion
Rare as some of the cases I have outlined in this series may be, they show how a primary care optometrist is an integral part of community healthcare and emphasise how much systemic disease has an ocular impact and therefore may present to us. Look out for a forthcoming interactive CET exercise aimed at summarising the knowledge gained from this series and allowing you to discuss your own experience. Read more
Systemic disease: Heteronymous hemianopias Systemic disease: Acromegaly and its diagnosis
Systemic disease: Vascular disorders with neurological consequence
Systemic disease: Vascular disorders with neurological consequence
Systemic disease in practice: Pontine haemorrhage and diplopia
Kirit Patel has an independent practice in Radlett, Herts
