Optical coherence tomography (OCT) was first introduced commercially in 1996, measuring reflected light to produce detailed cross-sectional and 3D images of the retina. However, it was not until 2002 that the technology was widely accepted in clinical ophthalmology and optometry and began helping eye care professionals visualise retinal changes in a way they had never seen before.
The first OCT technology widely accepted in clinical practice was time-domain OCT (TD-OCT), and it transformed the practice of medical and surgical retina. However, TD-OCT used a moving reference mirror for measuring the time it takes for light to be reflected and this relatively slow, mechanical process limited both the amount of data that could be captured as well as image quality. TD-OCT data was acquired at approximately 400 A-scans per second with an axial resolution of 8-10µm in tissue. This meant that image acquisition times were slow, and no eye tracking facility was offered, so images were greatly impacted by motion artefacts. Additionally, image resolution and the density of volume scans were low, limiting the practicality of using TD-OCT for the diagnosis and management of patients.
Spectral domain
Spectral domain OCT (SD-OCT) was introduced commercially in 2006. The technology eliminated the moving parts that limited TD-OCT technology so acutely, and instead detected the light echoes simultaneously by measuring the interference spectrum, using an interferometer with a high-speed spectrometer.
SD-OCT acquires data at speeds from 26,000 to 85,000 A-scans per second with an axial resolution of 5-7µm in tissue, depending on the device used. SD-OCT enables much faster image acquisition times, high-density volume scans and higher resolution images with better visualisation of individual retinal layers compared to TD-OCT (figure 1). For these reasons, SD-OCT has become the gold standard non-invasive imaging technique for visualising structural changes in the retina.
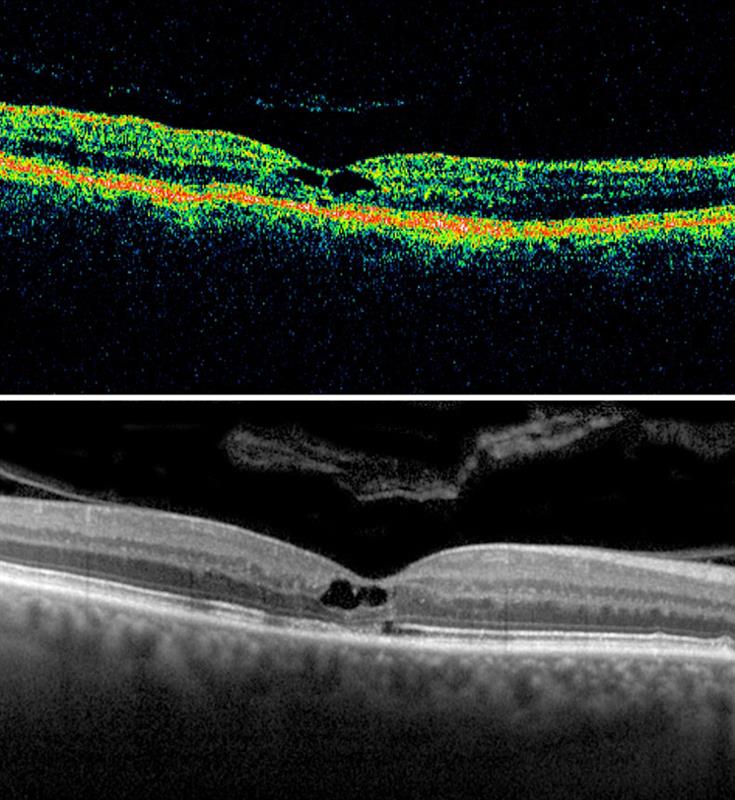
Figure 1: TD-OCT and SD-OCT of the same patient with macular telangiectasia
Though A-scan rates in SD-OCT are much faster in comparison to TD-OCT, fast scan speeds cannot completely eliminate motion artefacts. This limitation was quickly addressed in 2006 with the introduction of patented dual beam active eye tracking technology to mitigate motion artefacts. Dual beam active eye tracking simultaneously images the eye with two beams of light. One beam uses more than 1,000 data points on the retina to track eye movement, while the other beam uses the mapped image as a reference and is directed to the desired location irrespective of blinks or saccadic eye movements. The result is minimised motion artefact with point-to-point correlation between fundus and OCT scans, greater image detail and clarity, and more confident assessment of small change to within one micron at every follow-up visit for improved patient care (figure 2). Since the introduction of dual beam active eye tracking, several other eye tracking technologies have been introduced, many of which rely on post-processing of the image to remove motion artefact.
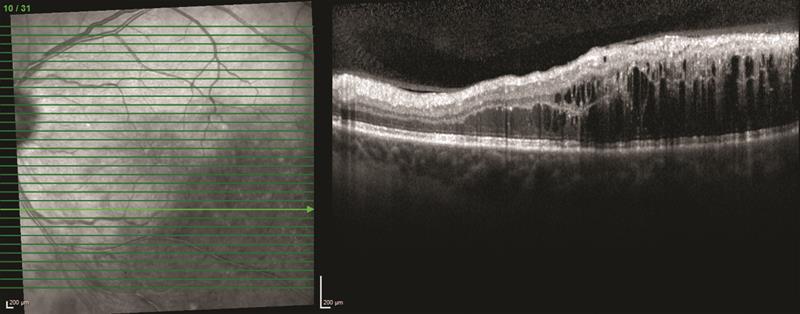
Figure 2: Blood vessel alignment across all visits demonstrates precise rescan placement using dual beam active eye tracking
The development of SD-OCT technology is certainly not exhausted. A-scan rates and axial resolutions have improved year on year across the board resulting in a catalogue of OCT devices that are faster and providing more detailed images than ever before.
Typically, SD-OCT instruments use near-infrared wavelengths of approximately 850nm that penetrate well into the retina and provide better contrast of retinal structures and better axial resolution compared to longer wavelengths of 1,000-1,300nm.1 However, the signal is reduced with increasing depth into the tissue and details of the choroid become less visible.
Swept-source
To achieve deeper penetration into the choroid, enhanced depth imaging (EDI) was introduced in 2012 as a novel SD-OCT imaging technique that enables high-resolution imaging of external retinal layers, the choroid and lamina cribrosa. Swept-source OCT has emerged in recent years and there is much interest around its application in visualising the choroidal structures. Swept-source OCT uses a laser that sweeps across a narrow band of wavelengths with each scan. It does not use a spectrometer but instead a complementary metal oxide semiconductor camera is employed, along with two fast parallel photodiode detectors. The result is a fast scanning speed of 100,000 A-scans per second and longer wavelength of around 1,050nm to allow for a deeper range of imaging the retina and improved image acquisition time.
Comparison
Several studies have compared visualisation of the inner retina and choroid using SD-OCT and swept-source OCT. It has been shown that swept-source OCT imaging does indeed provide sharper visualisation of the choroidal structures. However, it has also been shown that swept-source OCT technology is restricted in its ability to provide simultaneous visualisation of the vitreous structures and the choroid (figure 3).2
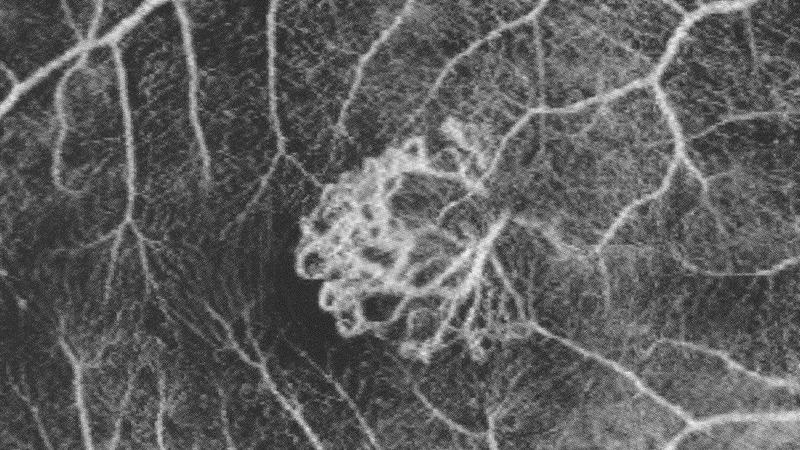
Figure 3: A full depth (FDI) SD-OCT image and swept-source OCT image of the same eye, which clearly show the full thickness of the retina and choroid. However, SD-OCT imaging provides superior visualisation of the premacular bursa with vitreomacular traction.2
SD-OCT permits a higher axial resolution (5µm compared to 8µm in swept-source OCT) and inner retinal contrast in comparison to swept-source OCT2, so there is a trade-off between resolution in the inner retina and visualisation of the choroid. While it has been suggested that the choroid thins in cases of neovascular age-related macular degeneration and thickens in central serous retinopathy, there are many diseases in which the role of the choroid is not apparent or unknown. Therefore, trading off resolution in the inner retina for deeper choroidal penetration may not be clinically advantageous at this time.
Research has shown that the signal penetration depths of swept-source OCT and SD-OCT using the EDI technique are very similar, and allow examination of the choroidal layer in its entire extent.3 EDI can be used alongside regular SD-OCT imaging to provide a full depth image (FDI) of the vitreoretinal-choroidal structures, providing greater visualisation of the vitreous and a similar visualisation of the choroid to swept-source OCT (see figure 3).2 Utilising SD-OCT technology in this way provides a good compromise between vitreous and choroidal visualisation.
The latest commercially available innovation in SD-OCT technology goes further and negates the need to use the EDI or FDI techniques for simultaneous visualisation of the vitreous and choroid. It does this by using a combination of dual beam active eye tracking with an A-scan rate of 85,000 for fast image acquisition and improved image contrast and quality all the way from vitreous through to choroid in one shot (figure 4).
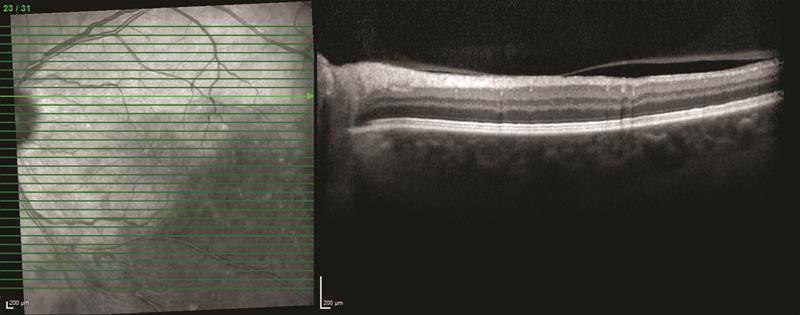
Figure 4: SD-OCT image taken using Spectralis OCT2
Research into the clinical application of swept-source OCT technology continues and interestingly, it has proven particularly suited to imaging the anterior segment and enables high-resolution imaging from the epithelium to the posterior surface of the crystalline lens, allowing clinicians to see what they measure, and measure what they see, for the first time.
Another important consideration when adopting a new imaging technology, such as swept-source OCT, is that it may render historical patient data obsolete, removing the clinician’s ability to monitor disease progression over time using images and data from their existing device. It is important to balance the novelty of adopting a new imaging technology, with clinical relevance of the technology and the preservation of historical data. Technologies that are upgradeable are a good investment and may enable the clinic to keep up with the latest advances in OCT imaging while retaining valuable patient data (figure 5).
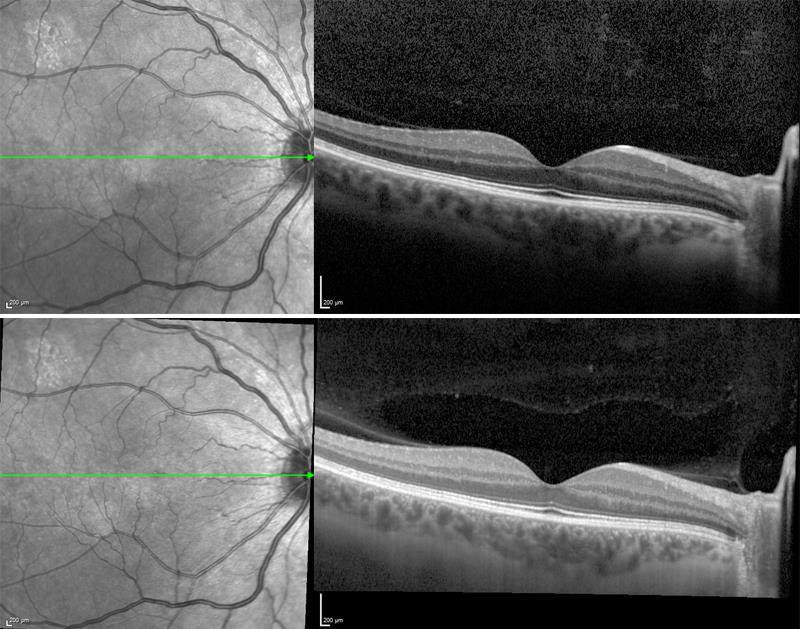
Figure 5: Below: an image of the macular taken using Spectralis OCT. Bottom: a follow up image of the same eye taken in the same anatomic location using Spectralis OCT2 and dual beam active eye tracking
OCT imaging has transformed the diagnosis and treatment of eye disease both for retina specialists and high street optometrists. Recent advances in SD-OCT technology to increase scan speeds and range, provides more detail in the vitreous and choroid without sacrificing detail in the inner retina, or continuity of patient data. The clinical application of swept-source OCT continues to be researched, with significant clinical value being placed on its application in imaging the anterior segment. SD-OCT imaging remains the gold standard in clinical practice due to the excellent image contrast and resolution in the neurosensory retina.
Christopher Mody is director of clinical services at Heidelberg Engineering.
References
1 Barteselli, G et al. Combined depth imaging technique on spectral-domain optical coherence tomography. Am J Ophthalmol. 2013, 155(4):727-32.
2 Barteselli, G. et al. Real-time full-depth visualisation of posterior ocular structures: comparison between full-depth imaging spectral domain optical coherence tomography and swept-source optical coherence tomography. Retina, 2016, 36(6):1153-61.
3 Waldstein, SM et al. Comparison of penetration depth in choroidal imaging using swept source vs spectral domain optical coherence tomography. Eye (Lond). 2015, Mar;29(3):409-15.
