
A return to contact lens practice after the COVID-19 lock down requires an enhanced awareness of the risks and possibilities of contamination in the practice, and how to manage these. Patients can be triaged via a survey and telehealth before the appointment, and then precautions taken to assure distancing and protection as far as possible during their time in the practice. Staff need to be trained in new procedures and protected from exposure via screens and masks. Regular, rigorous cleaning of surfaces and equipment needs to be carried out between appointments and re-useable diagnostic lenses will need to be disinfected according to rigorous guidelines. However, with adequate guidance and care, contact lens wear continues to be a good option for patients.
Background
The arrival of COVID-19, the disease caused by the coronavirus SARS-CoV-2, has had world-changing implications. From its first appearance in January 2020, numbers of cases have escalated rapidly, with the total at time of writing exceeding 13 million confirmed cases and half a million deaths, both of which are still increasing at pace.1 The epi-centre has moved from Wuhan province in China to Spain and Italy, to the UK and Iran, the USA and with mounting cases now in India, Latin America and Africa. Control of the disease has varied widely with the level of lock-down, in which citizens are confined at home to contain transmission, with the accompanying fallout of business failure and loss of jobs. In optics and other primary care services, this has resulted in the closure of practices for everything but emergencies, and, as the profession returns from lockdown, a slowing of the normal rhythm as additional hygiene procedures are implemented to protect patients and staff from potential contamination. This is a snapshot of what is known at the present time about COVID-19 and how it should inform contact lens practice moving forward. This picture of best evidence-based practice will continue to evolve because unprecedented levels of research are bringing forward new knowledge every day. Currently, a publication search on ‘COVID-19’ brings up more than 26,000 refereed publications, increasing by an average of over 4,000 per month or 150 per day. Similar searches on ‘COVID-19 + eye’ and ‘COVID-19 + CL’ yield 337 and 9 publications respectively.
Coronaviruses
The novel coronavirus that results in COVID-19 contains RNA surrounded by a protein shell, called a nucleocapsid, which is in turn surrounded by a lipid bilayer or envelope. This lipid layer makes it extremely susceptible to the action of surfactants or soaps, which cause the virus to essentially fall apart, leading to recommendations for frequent hand washing with soap to control the contagion. Anchored in the envelope and protruding from it are a number of spike proteins, giving the virus the appearance of a crown, hence its name from the Latin, ‘corona’. The spikes are able to bind onto receptors on human tissue called angiotensin converting enzyme 2 (ACE2)2 and this gives them access into the host cells, in which they can replicate (figure 1). A protease on the host cell surface, TMPRSS2, primes the spike protein for interaction with ACE2. SARS-CoV-2 has a 10-20 times greater affinity for binding to ACE2 than the first SARS-CoV,3 enhancing its ability to bind to cells and cause COVID-19.
SARS-CoV-2 interactions with the ocular surface
ACE2 is strongly expressed in the linings of the nose, tongue and the alveoli in the lungs 4,5,6 making them a particularly good entry point for COVID-19. In the eye, ACE2 is mainly expressed in the retina and retinal pigment epithelium. Studies have found ACE2 to be expressed at the ocular surface albeit with a lower presence than in the nose, tongue and lungs,7,8,9,10 which suggests a possible but lesser susceptibility to infection.7,11 Other studies12,13 have concluded that the eye is not a preferred gateway for entry and the risk of entry through tears is low.14,15 Recent clinical and laboratory evidence suggests the conjunctiva is rarely involved in SARS-CoV-2 infection and is neither a preferred tissue for the virus, nor a preferred gateway for entry into the respiratory tract.16 Further work concludes that the risk of transmission through the tears is low,17 transmission via the conjunctiva is not supported18 and for patients without conjunctivitis no COVID-19 was detected in the tears and conjunctival secretions, suggesting they are not a common route for transmission.19
In addition to a number of case study reports on individuals with COVID-19 in their tears or on the ocular surface,20,21,22 cohort studies published to date have found a low presence of COVID-19 of about three percent in confirmed, symptomatic patients, with a range between zero and seven percent (table 1).
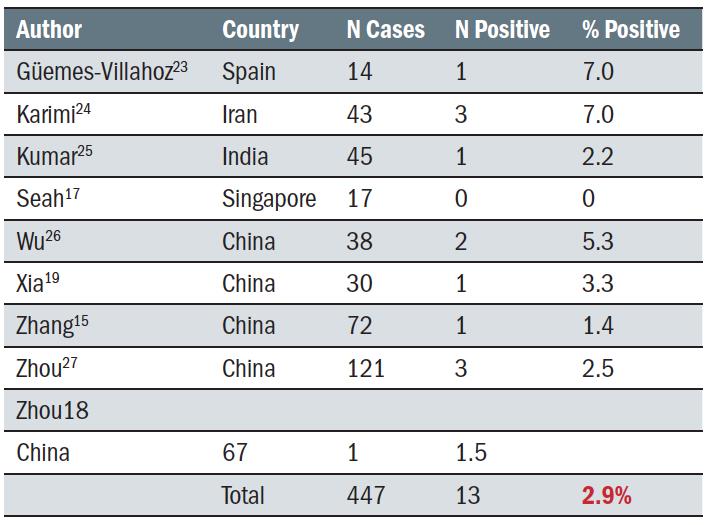 Table 1: Number of COVID-19 positive cases found with the virus on their ocular surface or in their tears
Table 1: Number of COVID-19 positive cases found with the virus on their ocular surface or in their tears
There are several reports of patients with COVID-19 presenting with viral conjunctivitis. Some reports suggest that it is the presenting sign of the virus, occurring between three hours and four days prior to the COVID-19 diagnosis.20,28,29,30 Scalinci et al reported five asymptomatic cases with viral conjunctivitis which they attributed to COVID-19, although as viral conjunctivitis can result from many possible sources, causality is questionable.31 Table 2 summarises cohort studies reporting incidence of conjunctivitis in COVID-19 patients. Within this summary, two studies stand out; Docherty has a very large number of subjects (more than all the other studies put together) and a very low incidence of conjunctivitis, and Wu has a much smaller subject total and a significantly higher proportion of conjunctivitis. Including Docherty, the incidence of conjunctivitis was a very low 0.56%, whereas if the Docherty study is excluded and the others considered in total, the average increases to 2.5%, which is similar to the proportion of people reported in table 1 who had the virus detected on the ocular surface.
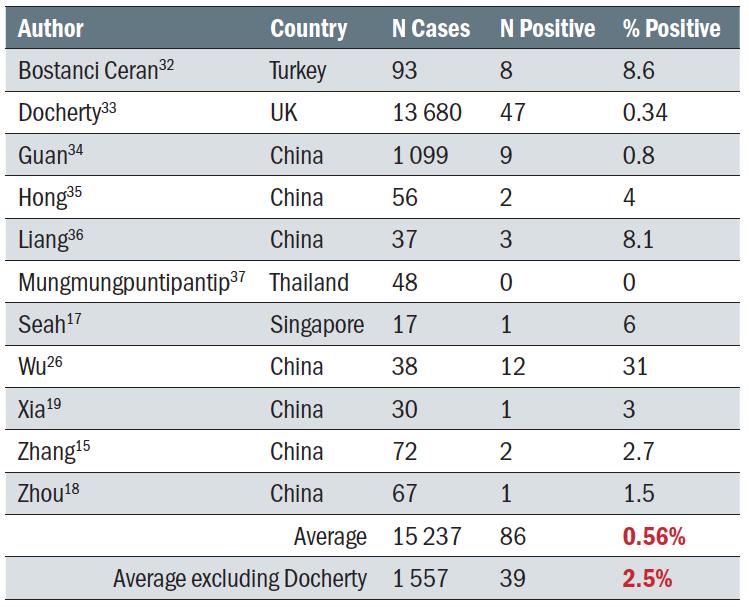 Table 2: The incidence of conjunctivitis in COVID-19 positive subjects
Table 2: The incidence of conjunctivitis in COVID-19 positive subjects
A systematic review of 11 studies38 concluded that at the moment, owing to limited and conflicting evidence it could not be established whether SARS-CoV-2 only colonises ocular structures or is able to invade them. A second review paper concluded that while precautions by eye care practitioners and patients remain necessary, the virus appears thus far unlikely to bind to the ocular surface to initiate infection, as SARS-CoV-2 has been found rarely in tears, and then predominantly only in confirmed, symptomatic patients.39
Changes to Eye Care Practice Post COVID-19
COVID-19 undoubtedly impacts normal practice, and eye care practitioners need to consider what changes to normal practice are necessary, especially in the fitting and management of contact lens patients. This has been widely considered, and advice is available from many organizations in both the profession and the industry, for instance the American Optometric Association and the British Contact Lens Association, the latter of which has published guidance in 13 languages. CORE has an online resource, COVIDEyeFacts.org, with guidelines for patients in 29 languages as well as videos and other materials that can be used in the practice. Additionally, peer-reviewed advice is available from several publications.40,41,42,43 Drawing from these, advice falls into four main areas; patient management ahead of and during the visit, use of personal protective equipment (PPE), disinfection of contact lens equipment and diagnostic sets and, finally, considerations when undertaking contact lens fits.
Patient Management
One of the first considerations for patients coming into a practice is to determine if they could possibly be COVID-19 positive. Patients can be screened by means of a simple survey, preferably carried out in advance of their visit via the practice website, a phone app or by a telephone call from staff. The questionnaire should ask if patients have had any potential symptoms such as a fever, dry cough, sore throat, headache or loss of taste or smell. The survey should further explore if the patients have been in close contact with any confirmed or suspected COVID-19 cases, have recently been to any country where the virus is endemic or have experienced any conjunctivitis or ‘pink-eye’. Any patient answering positively to one or more of these should preferably not come in to the practice at that time. The rules which apply to patients also apply to practice staff, who should not come in to work if they are unwell or have been potentially exposed to the virus, avoiding the risk of transfer to other staff members and patients.
Patients who do need to come in to the practice should be triaged via telehealth44,45,46,42,47 or remote consultation via phone, video call or phone app, and could (for instance) send an image of their eye(s) to the practice for the eye care practitioner to check. If they are suspected COVID-19 positive but must be seen because of an emergency, they should only be given an appointment if the practice and staff are suitably equipped to protect them from contamination. Otherwise they should be referred to somewhere where full protection is in use, such as a local hospital ophthalmology department or a practice with full protection measures. Patients should be asked to arrive punctually and shown straight to the consulting room to avoid them gathering in the waiting room.
It should be remembered, not all COVID-19 positive patients are symptomatic, and a review of 21 papers found a dramatic range of between five and 80% of asymptomatic patients tested positive for COVID-19.48 This means that any symptom-based screening will miss some people and some asymptomatic cases will become symptomatic over the next week; a group of people called ‘pre-symptomatics.’ Bear in mind that the chances of contamination from the tears or ocular surface of these people are very small, and the concern is rather for the more general forms of transmission via coughing, sneezing and contact with contaminated surfaces.
Personal Protective Equipment (PPE)
In considering protective measures for staff and patients, some of the environmental controls are similar to the ones we are now seeing in shops and supermarkets.40,41,42,43 Waiting areas should be re-arranged to increase physical distancing, and transparent separation screens can be placed at reception and dispensing areas between staff and patients. Patients should be asked to arrive wearing a mask and just in time for the appointment and be taken immediately to the examination room to avoid them gathering in the waiting area. Hand sanitisers should be available at the entrance to the practice and at other suitable locations in the consulting room and the dispensing area. High traffic areas should be cleaned at the beginning and end of the day, and regularly during the day using appropriate methods. The Centers for Disease Control and Prevention (CDC) recommends three disinfectants specifically effective against COVID-19;
- 1 to 3% hydrogen peroxide
- household bleach diluted in a ratio of five tablespoons to a gallon (two dessert spoons per litre) of water
- ≥ 60% ethanol or 70% isopropanol solutions.
Cleaners and disinfectants are recommended to be left on the surface for several minutes before being wiped off in order to be effective.
Sharing objects such as clipboards and pens by staff should be avoided, and materials or processes normally shared by patients, for example, magazines and brochures and the provision of complimentary coffee or tea in the waiting room, should be removed. Scrupulous and regular hand washing with soap and water should be observed by all staff and protective ‘breath shields’ put in place for any face-to-face procedures such as slit lamp examinations (figure 2).
 Figure 2: A Perspex breath shield fitted to the slit lamp provides another layer of protection where close face-to-face proximity is necessary
Figure 2: A Perspex breath shield fitted to the slit lamp provides another layer of protection where close face-to-face proximity is necessary
These, along with the wearing of masks by both staff and patients, reduce the chance of airborne droplet and aerosol contamination. Examination room surfaces and equipment should be disinfected between patients, including both clinical equipment such as slit lamps and breath shields and non-clinical items such as desk tops, keyboards, door handles and chairs. As these procedures and the explanation of good hygiene procedures to patients will add time, consultation schedules should be adjusted to allow sufficient time and as a result fewer patient encounters can be expected in a day.
The need for PPE is different when seeing symptomatic patients and non-symptomatic ones. The former would require full PPE, for which most practices will not be equipped. However, most practices will be dealing with non-symptomatic patients, where some basic safety precautions should be applied.
Minimizing conversation when at close face-to-face distances such as at the slit lamp or during ophthalmoscopy will reduce the chances of airborne contamination, so here ask patients not to speak unless prompted, and keep your own requests to them to a minimum. Goggles or face shields will be needed when seeing symptomatic patients, but are generally not recommended for asymptomatic ones. The CDC and World Health Organization (WHO) recommend the use of face masks in any situation where physical distancing is not possible which includes supermarkets, public transport and of course primary care practice, where both patients and staff should wear them. With asymptomatic patients these can be surgical or fabric masks, but if the patient is symptomatic higher specification N95 masks should be used for their enhanced ability to block the virus. Masks need to be worn properly, fully covering both nose and mouth and fitting flush to the face around the edge in order to be effective (figure 3). When used properly in conjunction with a breath shield on the slit lamp, masks block virtually all contamination from a cough with best protection from an N95 mask.49 An improperly worn mask which does not cover the nose and/or mouth provides substantially poorer levels of protection.
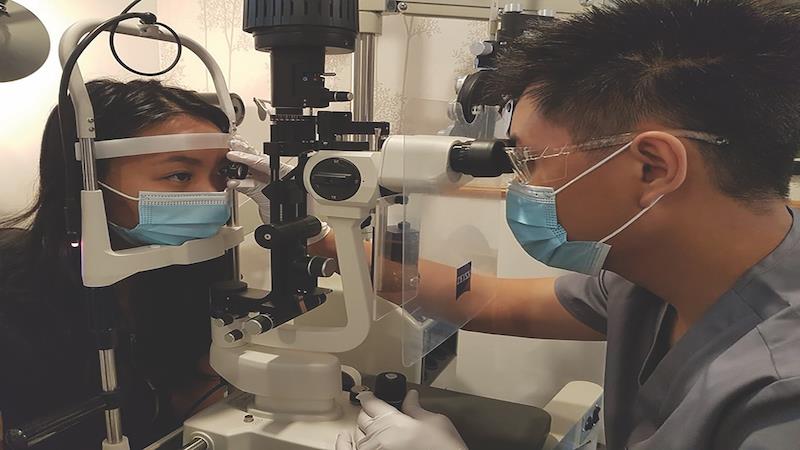 Figure 3: Face masks should be worn properly, covering both nose and mouth and fitting close to the face. Goggles may be used for additional protection
Figure 3: Face masks should be worn properly, covering both nose and mouth and fitting close to the face. Goggles may be used for additional protection
Gloves are a controversial topic and they undoubtedly increase the degree of difficulty in applying or removing contact lenses. For symptomatic patients, tight-fitting, powder-free gloves are recommended. They should be made from nitrile material to avoid potential for any allergy to latex for either practitioner or patient. Gloves would need to be discarded after each patient as they can become contaminated during use. For asymptomatic patients, scrupulous washing of the practitioners’ hands both before and after manipulating the patients’ eyes and lenses is typically considered sufficient protection by most regulatory authorities.
Disinfection of Diagnostic Sets
Disposable contact lenses, used once, offer the best option for fitting patients. However, some diagnostic lenses such as gas permeable, scleral, hybrid or custom soft lenses are not available in a disposable format, and disinfection recommendations for these have been proposed50 by both professional organizations such as the American Academy of Optometry (AAO) and American Optometric Association (AOA) and manufacturing organizations such as the Contact Lens Manufacturers Association (CLMA) and GP Lens Institute (GPLI). These recommendations are as follows;
- Gas permeable lenses; should be disinfected in non-neutralised 3% hydrogen peroxide for three hours or longer, rinsed with multipurpose solution (MPS) and then stored dry.
- Soft and hybrid lenses; should also be disinfected in non-neutralised 3% hydrogen peroxide for three hours or longer, after which they should be transferred to fresh peroxide with neutraliser for 6 hours or longer and then stored in an appropriate MPS.
Contact Lens Fits
Some eye care practitioners have concerns about fitting contact lenses at the moment, and this should be addressed for both existing and new patients. The CDC working definition of ‘exposure’ to the novel coronavirus is 15 minutes or more in close proximity to someone who is COVID-19 positive,51 which is a more extreme situation than most contact lens consultations where some level of distancing can be maintained through most of the consultation and where the majority of patients will not be tested as positive for the presence of SARS-CoV-2.
For existing patients, the time in which the practitioner and patient are in contact with each other at close quarters is short, and if combined with the use of masks, breath shields and scrupulous hand washing before and after touching the lenses or eyes, the risk of transmission in either direction is minimal.
For new contact lens patients, exposure time is necessarily longer, but steps can be taken to mitigate the risk of viral exposure. For instruction on lens application and removal, in addition to both instructor and patient wearing masks, the patient can be asked to watch a training video in advance of the appointment and a clear Perspex shield can be used between the two during instruction or a face shield worn by the instructor (figure 4). If the instruction time takes longer than 15 minutes and a physical barrier is not possible, an additional appointment can be booked.
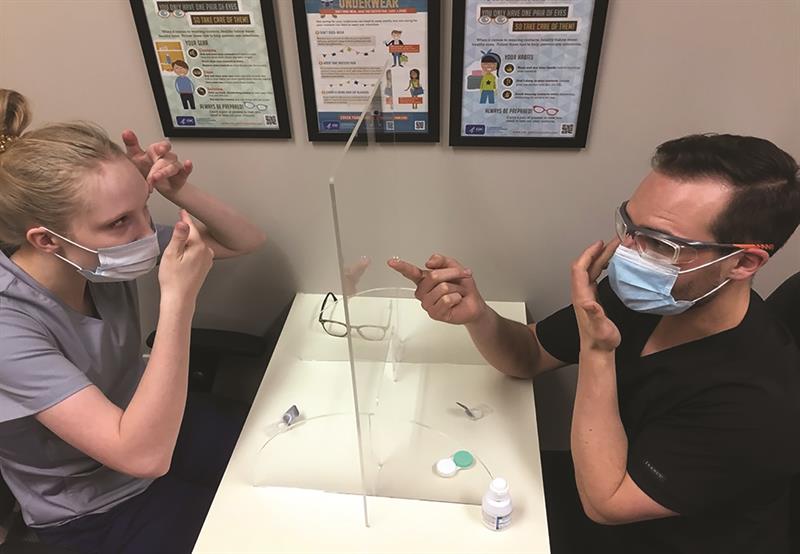 Figure 4: A Perspex shield between instructor and patient enables them to see each other clearly during instruction. In this case, the instructor is also wearing well-fitting safety goggles.
Figure 4: A Perspex shield between instructor and patient enables them to see each other clearly during instruction. In this case, the instructor is also wearing well-fitting safety goggles.
COVID-19 and Contact Lens Wear
A number of considerations come to light when reviewing the scientific evidence on COVID-19 and contact lenses.52 There is no scientific evidence of an increased risk of developing COVID-19 for contact lens wearers compared with spectacle wearers or people with no visual correction.52 There is no evidence of increased risk for any type of contact lens material because of enhanced interaction with the COVID-19 virus, nor for any wearing modality. There is no direct information about the efficacy of contact lens solutions against SARS-CoV-2, and the evidence about their efficacy against viruses in general is equivocal.53,54 The CDC makes a general recommendation for the use of hydrogen peroxide disinfection with bactericidal and viricidal activity established after 1 minute exposure to a concentration of 0.5%, well below the concentrations and exposure times normally used in contact lens disinfection.55,56 Rubbing and rinsing lenses before disinfection helps to remove viruses from lenses,54 and the surfactants present in MPS and peroxide solutions should theoretically help to kill them by acting against their lipid-based envelope, although this has not yet been shown experimentally for any coronavirus.
Practitioners should reinforce normal good hygiene practices for contact lens wear – wearers should wash their hands thoroughly with soap and water and dry them with a paper towel before any handling procedures. Lenses should be replaced on schedule, the case, if used, must be cleaned and replaced regularly and care products should be used as recommended, taking particular care to replace solution in the case at each use and not to top it up, as this increases the risk of infection.57,58,59
Whether a contact lens wearer, spectacle wearer or someone who needs no vision correction, people should avoid touching their eyes, nose or mouth with unwashed hands, as advised by the WHO60 and the CDC.61 There is no scientific evidence to support anecdotal concerns that contact lens wearers might touch their faces and eyes more often than spectacle wearers, thereby putting them at a higher risk of contracting COVID-19.52 The CDC states that regular spectacles are not considered to be adequate eye protection against COVID-19.62 Arguably contact lens wearers are less likely to have the issues of steaming and fogging spectacle wearers can have when wearing a mask, and contact lenses might therefore be a better option for this situation. Spectacle wearers tend to handle their glasses regularly during the day, and as the virus can exist on plastics and metal surfaces for 2 to 3 days or longer,62,63 their spectacles can become a vector for contamination, and should be cleaned regularly with soap and water. If they are ill at all, patients should cease contact lens wear until they are healthy again – this applies not just for COVID-19, but for any illness, particularly upper respiratory tract infections such as colds or flu, as this puts them at greater risk of microbial or infiltrative keratitis.64
Practical Eyecare Advice for Patients
As they are now working from home and have reduced opportunities for sport and socialising, patients are wearing their contact lenses less.65 Patients who are using reusable lenses should be advised that the longest recommended storage period for lenses stored in neutralised peroxide is seven days, and in MPS 30 days, after which they should re-disinfect the lenses before use. It would be worth discussing with these patients switching to daily disposable lenses for the duration, as a now more economical, convenient solution with a lower risk of adverse events.
Spectacle wearers who wear masks often have issues with their glasses fogging up as their breath is channelled up over the lenses. To manage this, they can tuck the top of their mask under the spectacles to seal it off at the nose, and supplement this with the use of some surgical tape to hold it to their face or use a mask with a nose-bridge seal. To render the spectacle lenses less prone to fogging they can be rubbed with an ani-fog cloth, or washed with soapy water and then wiped dry.66 They could also consider the option of contact lenses for the times when they wear a mask. The amount of home-improvement activity has increased during the pandemic as people have more available time at home, and this increases the risk of eye injuries.67 It is also worth reminding patients either during consultations or via a newsletter or your website, of the importance of using safety spectacles.
Conclusion
The COVID-19 pandemic has had wide-reaching consequences for which we need to change the way that we return to contact lens practice. Practitioners must review local regulatory requirements as these may vary, but inevitably some degree of practice modification will be necessary. Patients can continue wearing their contact lenses, although higher than normal levels of hygiene should be observed. They must wash their hands and spectacles regularly with soap and water to reduce the chances of viral exposure and be aware that regular spectacles do not provide guaranteed protection from COVID-19. Unwashed hands should be kept away from their eyes, nose and mouth and should they fall ill for any reason, they should discontinue contact lens wear until they are healthy again.
Lyndon Jones, Professor, School of Optometry & Vision Science. Director, Centre for Ocular Research & Education (CORE), University of Waterloo, Ontario, Canada
Karen Walsh, Professional Education Team Leader, Centre for Ocular Research & Education (CORE), University of Waterloo, Ontario, Canada
Acknowledgements
This publication follows a webinar sponsored by Alcon. The views expressed are those of Lyndon Jones and Karen Walsh and do not necessarily represent Alcon’s position.
Disclosures
In his capacity as a researcher and professor at CORE and the University of Waterloo, Lyndon Jones has received funding or honoraria from the following companies: Alcon, Allergan, Contamac, CooperVision, GL Chemtec, Johnson & Johnson Vision, Lubris, Menicon, Nature’s Way, Novartis, Ophtecs, Oté, PS Therapy, Santen, Shire, SightGlass, Visioneering Technologies. He acts as a consultant and/or serves on an advisory board for Alcon, CooperVision, Johnson & Johnson Vision, Novartis and Ophtecs.
Karen Walsh has received honoraria from Alcon, CooperVision and Johnson & Johnson Vision.
References
- World Health Organization. WHO Coronavirus Disease (COVID-19) Dashboard. World Health Organization.
- Zhou P, Yang X Lou, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020. doi:10.1038/s41586-020-2012-7
- Daniel Wrapp, Nianshuang Wang, Kizzmekia S. Corbett, et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation | Science. Science (80- ). 2020.
- Hamming I, Timens W, Bulthuis MLC, Lely AT, Navis GJ, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004. doi:10.1002/path.1570
- Xu H, Zhong L, Deng J, et al. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci. 2020. doi:10.1038/s41368-020-0074-x
- Butowt R, Bilinska K. SARS-CoV-2: Olfaction, Brain Infection, and the Urgent Need for Clinical Samples Allowing Earlier Virus Detection. ACS Chem Neurosci. 2020. doi:10.1021/acschemneuro.0c00172
- Zhou L, Xu Z, Castiglione GM, Soiberman US, Eberhart CG, Duh EJ. ACE2 and TMPRSS2 are expressed on the human ocular surface, suggesting susceptibility to SARS-CoV-2 infection. Ocul Surf. 2020. doi:10.1016/j.jtos.2020.06.007
- Zhang BN, Wang Q, Liu T, et al. [Expression analysis of 2019-nCoV related ACE2 and TMPRSS2 in eye tissues]. Zhonghua Yan Ke Za Zhi. 2020. doi:10.3760/cma.j.cn112142-20200310-00170
- Sungnak W, Huang N, Bécavin C, et al. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med. 2020. doi:10.1038/s41591-020-0868-6
- Grajewski RS, Rokohl AC, Becker M, et al. Letter to the Editor: A missing link between SARS-CoV-2 and the eye?: ACE2 expression on the ocular surface. J Med Virol. 2020;1(2).
- Hui KPY, Cheung MC, Perera RAPM, et al. Tropism, replication competence, and innate immune responses of the coronavirus SARS-CoV-2 in human respiratory tract and conjunctiva: an analysis in ex-vivo and in-vitro cultures. Lancet Respir Med. 2020. doi:10.1016/S2213-2600(20)30193-4
- Lange C, Wolf J, Auw-Haedrich C, et al. Expression of the COVID-19 receptor ACE2 in the human conjunctiva. J Med Virol. 2020. doi:10.1002/jmv.25981
- Xiang M, Zhang W, Wen H, Mo L, Zhao Y, Zhan Y. Comparative transcriptome analysis of human conjunctiva between normal and conjunctivochalasis persons by RNA sequencing. Exp Eye Res. 2019. doi:10.1016/j.exer.2019.04.005
- Sun C Bin, Wang YY, Liu GH, Liu Z. Role of the Eye in Transmitting Human Coronavirus: What We Know and What We Do Not Know. Front Public Heal. 2020. doi:10.3389/fpubh.2020.00155
- Zhang X, Chen X, Chen L, et al. The evidence of SARS-CoV-2 infection on ocular surface. Ocul Surf. 2020. doi:10.1016/j.jtos.2020.03.010
- Liu Z, Sun C bin. Conjunctiva is not a preferred gateway of entry for SARS-CoV-2 to infect respiratory tract. J Med Virol. 2020. doi:10.1002/jmv.25859
- Seah IYJ, Anderson DE, Kang AEZ, et al. Assessing Viral Shedding and Infectivity of Tears in Coronavirus Disease 2019 (COVID-19) Patients. Ophthalmology. 2020. doi:10.1016/j.ophtha.2020.03.026
- Zhou Y, Zeng Y, Tong Y, Chen C. Ophthalmologic evidence against the interpersonal transmission of 2019 novel coronavirus through conjunctiva. medRxiv. 2020. doi:10.1101/2020.02.11.20021956
- Xia J, Tong J, Liu M, Shen Y, Guo D. Evaluation of coronavirus in tears and conjunctival secretions of patients with SARS-CoV-2 infection. J Med Virol. 2020. doi:10.1002/jmv.25725
- Cheema M, Aghazadeh H, Nazarali S, et al. Keratoconjunctivitis as the initial medical presentation of the novel coronavirus disease 2019 (COVID-19). Can J Ophthalmol. 2020. doi:10.1016/j.jcjo.2020.03.003
- Chen L, Liu M, Zhang Z, et al. Ocular manifestations of a hospitalised patient with confirmed 2019 novel coronavirus disease. Br J Ophthalmol. 2020. doi:10.1136/bjophthalmol-2020-316304
- Colavita F, Lapa D, Carletti F, et al. SARS-CoV-2 Isolation From Ocular Secretions of a Patient With COVID-19 in Italy With Prolonged Viral RNA Detection. Ann Intern Med. 2020. doi:10.7326/M20-1176
- Güemes-Villahoz N, Burgos-Blasco B, Arribi-Vilela A, et al. SARS-CoV-2 RNA detection in tears and conjunctival secretions of COVID-19 patients with conjunctivitis. J Infect. 2020. doi:10.1016/j.jinf.2020.05.070
- Karimi S, Arabi A, Shahraki T, Safi S. Detection of severe acute respiratory syndrome Coronavirus-2 in the tears of patients with Coronavirus disease 2019. Eye. 2020;34(7):1-4.
- Kumar K, Prakash AA, Gangasagara SB, et al. Presence of viral RNA of SARS-CoV-2 in conjunctival swab specimens of COVID-19 patients. Indian J Ophthalmol. 2020. doi:10.4103/ijo.IJO_1287_20
- Wu P, Duan F, Luo C, et al. Characteristics of Ocular Findings of Patients with Coronavirus Disease 2019 (COVID-19) in Hubei Province, China. JAMA Ophthalmol. 2020. doi:10.1001/jamaophthalmol.2020.1291
- Zhou Y, Duan C, Zeng Y, et al. Ocular Findings and Proportion with Conjunctival SARS-COV-2 in COVID-19 Patients. Ophthalmology. 2020. doi:10.1016/j.ophtha.2020.04.028
- Daruich A, Martin D, Bremond-Gignac D. Ocular manifestation as first sign of Coronavirus Disease 2019 (COVID-19): Interest of telemedicine during the pandemic context. J Fr Ophtalmol. 2020. doi:10.1016/j.jfo.2020.04.002
- Khavandi S, Tabibzadeh E, Naderan M, Shoar S. Corona virus disease-19 (COVID-19) presenting as conjunctivitis: atypically high-risk during a pandemic. Contact Lens Anterior Eye. 2020. doi:10.1016/j.clae.2020.04.010
- Ying NY, Idris NS, Muhamad R, Ahmad I. Coronavirus Disease 2019 Presenting as Conjunctivitis. Korean J Fam Med. 2020. doi:10.4082/kjfm.20.0090
- Scalinci SZ, Trovato Battagliola E. Conjunctivitis can be the only presenting sign and symptom of COVID-19. IDCases. 2020. doi:10.1016/j.idcr.2020.e00774
- Bostanci Ceran B, Ozates S. Ocular manifestations of coronavirus disease 2019. Graefe’s Arch Clin Exp Ophthalmol. 2020. doi:10.1007/s00417-020-04777-7
- Docherty AB, Harrison EM, Green CA, et al. Features of 16,749 hospitalised UK patients with COVID-19 using the ISARIC WHO Clinical Characterisation Protocol. medRxiv. 2020. doi:10.1101/2020.04.23.20076042
- Guan W, Ni Z, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020. doi:10.1056/NEJMoa2002032
- Hong N, Yu W, Xia J, Shen Y, Yap M, Han W. Evaluation of ocular symptoms and tropism of SARS-CoV-2 in patients confirmed with COVID-19. Acta Ophthalmol. 2020. doi:10.1111/aos.14445
- Liang L, Wu P. There may be virus in conjunctival secretion of patients with COVID-19. Acta Ophthalmol. 2020. doi:10.1111/aos.14413
- Mungmungpuntipantip R, Wiwanitkit V. Ocular manifestation, eye protection, and COVID-19. Graefe’s Arch Clin Exp Ophthalmol. 2020. doi:10.1007/s00417-020-04662-3
- Aiello F, Gallo Afflitto G, Mancino R, et al. Coronavirus disease 2019 (SARS-CoV-2) and colonization of ocular tissues and secretions: a systematic review. Eye. 2020. doi:10.1038/s41433-020-0926-9
- Willcox MDP, Walsh K, Nichols JJ, Morgan PB, Jones LW. The ocular surface, coronaviruses and COVID-19. Clin Exp Optom. 2020. doi:10.1111/cxo.13088
- Amesty MA, Alió del Barrio JL, Alió JL. COVID-19 Disease and Ophthalmology: An Update. Ophthalmol Ther. 2020. doi:10.1007/s40123-020-00260-y
- Lai THT, Tang EWH, Chau SKY, Fung KSC, Li KKW. Stepping up infection control measures in ophthalmology during the novel coronavirus outbreak: an experience from Hong Kong. Graefe’s Arch Clin Exp Ophthalmol. 2020. doi:10.1007/s00417-020-04641-8
- Safadi K, Kruger JM, Chowers I, et al. Ophthalmology practice during the COVID-19 pandemic. BMJ Open Ophthalmol. 2020. doi:10.1136/bmjophth-2020-000487
- Zeri F, Naroo SA. Contact lens practice in the time of COVID-19. Contact Lens Anterior Eye. 2020. doi:10.1016/j.clae.2020.03.007
- Bowe T, Hunter DG, Mantagos IS, et al. Virtual Visits in Ophthalmology: Timely Advice for Implementation During the COVID-19 Public Health Crisis. Telemed e-Health. 2020. doi:10.1089/tmj.2020.0121
- Kalra G, Williams AM, Commiskey PW, et al. Incorporating Video Visits into Ophthalmology Practice: A Retrospective Analysis and Patient Survey to Assess Initial Experiences and Patient Acceptability at an Academic Eye Center. Ophthalmol Ther. 2020. doi:10.1007/s40123-020-00269-3
- Nagra M, Vianya-Estopa M, Wolffsohn JS. Could telehealth help eye care practitioners adapt contact lens services during the COVID-19 pandemic? Contact Lens Anterior Eye. 2020. doi:10.1016/j.clae.2020.04.002
- Saleem SM, Pasquale LR, Sidoti PA, Tsai JC. Virtual Ophthalmology: Telemedicine in a COVID-19 Era. Am J Ophthalmol. 2020. doi:10.1016/j.ajo.2020.04.029
- Henneghan C, Brassey J, Jefferson T. COVID-19: What Proportion Are Asymptomatic? Oxford, UK; 2020.
- Felfeli T, Batawi HI, Aldrees SS, Hatch W, Mandelcorn ED. Infection Control Measures During Simulated Slit-Lamp Examination. American Academy of Ophthalmology. https://www.aao.org/clinical-video/infection-control-measures-during-simulated-slit-l. Published 2020.
- Barnett M. In-office contact lens disinfection. Contact Lens Spectr. 2020;35(January):17.
- CDC. Public health guidance for community-related exposure. cdc.gov. https://www.cdc.gov/coronavirus/2019-ncov/php/public-health-recommendations.html.
- Jones L, Walsh K, Willcox M, Morgan P, Nichols J. The COVID-19 pandemic: Important considerations for contact lens practitioners. Contact Lens Anterior Eye. 2020. doi:10.1016/j.clae.2020.03.012
- Kowalski RP, Sundar-Raj C V, Romanowski EG, Gordon YJ. The disinfection of contact lenses contaminated with adenovirus. Am J Ophthalmol. 2001;132(5):777-779. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=11704040.
- Heaselgrave W, Lonnen J, Kilvington S, Santodomingo-Rubido J, Mori O. The disinfection efficacy of MeniCare soft multipurpose solution against Acanthamoeba and viruses using stand-alone biocidal and regimen testing. Eye Contact Lens. 2010;36(2):90-95. doi:10.1097/ICL.0b013e3181d13c2d
- Centers for Disease Control and Prevention. Guideline for Disinfection and Sterilization in Healthcare Facilities, 2008; Miscellaneous Inactivating Agents. CDC website. 2013. doi:1
- Kampf G, Todt D, Pfaender S, Steinmann E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J Hosp Infect. 2020. doi:10.1016/j.jhin.2020.01.022
- Chang DC, Grant GB, O’Donnell K, et al. Multistate outbreak of Fusarium keratitis associated with use of a contact lens solution. JAMA. 2006;296(8):953-963. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16926355.
- Joslin CE, Tu EY, Shoff ME, et al. The association of contact lens solution use and Acanthamoeba keratitis. Am J Ophthalmol. 2007;144(2):169-180. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17588524.
- Saw SM, Ooi PL, Tan DT, et al. Risk factors for contact lens-related fusarium keratitis: a case-control study in Singapore. Arch Ophthalmol. 2007;125(5):611-617. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17502498.
- World Health Organization. Coronavirus disease (COVID-19) advice for the public. Coronavirus disease 2019.
- Cdc.gov/coronavirus. What you should know about COVID-19 to protect yourself and others. Cdc. 2020.
- National Center for Immunization and Respiratory Diseases, Division of Viral Diseases. Interim Infection Prevention and Control Recommendations for Patients with Suspected or Confirmed Coronavirus Disease 2019 (COVID-19) in Healthcare Settings. CDC. 2020.
- Van Doremalen N, Bushmaker T, Morris DH, et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020. doi:10.1056/NEJMc2004973
- Sankaridurg PR, Willcox MD, Sharma S, et al. Haemophilus influenzae adherent to contact lenses associated with production of acute ocular inflammation. J Clin Microbiol. 1996;34(10):2426-2431.
- Morgan PB. Contact lens wear during the COVID-19 pandemic. Contact Lens Anterior Eye. 2020. doi:10.1016/j.clae.2020.04.005
- Malik SS, Malik SS. A simple method to prevent spectacle lenses misting up on wearing a face mask. Ann R Coll Surg Engl. 2011. doi:10.1308/rcsann.2011.93.2.168b
- Hamroush A, Qureshi M, Shah S. Increased risk of ocular injury seen during lockdown due to COVID-19. Contact Lens Anterior Eye. 2020. doi:10.1016/j.clae.2020.04.007
