Initial presentation
A 30-year-old male presented for a scleral contact lens fitting as part of the management of his long standing ‘dry eye’ problems. Scleral contact lenses had been suggested as a potential management option at his last eye examination by a colleague at another practice.
At present his only management was the use of Systane Gel Drops as needed, which in practice might be as frequent as every 10 minutes.
Key points from his history included the following;
Clinical assessment
Initial clinical findings are summarised in table 1.
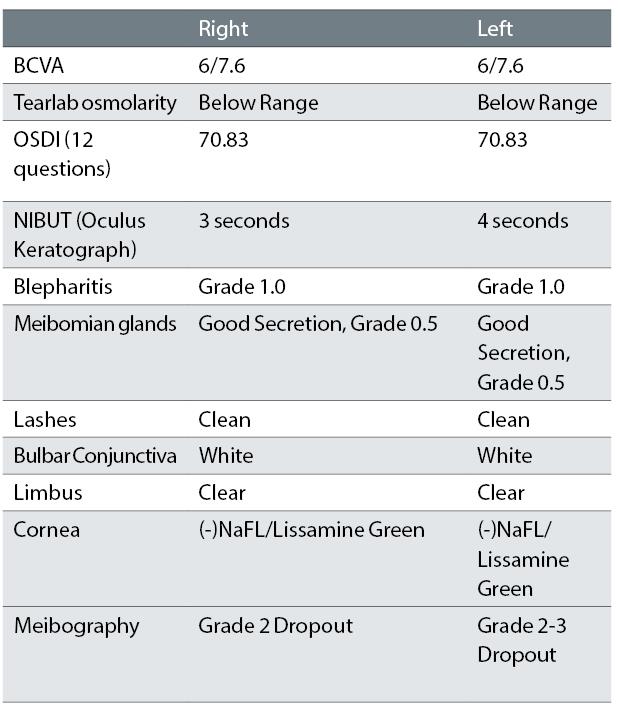
Table 1: Clinical findings at first presentation to author
Register now to continue reading
Thank you for visiting Optician Online. Register now to access up to 10 news and opinion articles a month.
Register
Already have an account? Sign in here

