With the expansion of independent prescribing in the UK, the gap between what optometrists are able to achieve either side of the Atlantic is closing. For this reason, it is useful to keep an eye on the latest developments in anterior eye assessment and treatment on show at the AAO conference, as many of these are likely to appear in the UK soon after. This article mentions some new products that caught my attention at this year’s conference.
Tonometry
The use of a flat applanation by contact tonometers results in as much as 50% error, according to Dr Sean McCafferty, CEO of CATS tonometers. The problem is that it is impossible to identify which patients are those likely to show error when IOP is measured in the usual way, where the flattening of the cornea results in corneoscleral buckling, which skews results (figure 1). The CATS tonometer prism uses a curved applanation surface which, according to McCafferty, reduces mechanical error on applanation and reduces error caused this way to just 3%. There are a number of recent studies verifying this claim.1 Also of interest, the CATS appears not to suffer the post-refractive surgery drop in IOP that usually occurs when the cornea is thinned by laser treatments. The prism is disposable and fits into the same mounting as a traditional Goldmann prism.
Nanodropper
Dr Allisa Song is CEO of a company responsible for the first FDA-listed volume-reducing adaptor for dropper bottles. Millions of dollars (and pounds) are wasted each year due to excess medication being expressed from eye dropper bottles. Readers will be more than familiar with the trickle of fluid running down a patient’s cheek after application of drops. Standard dropper bottles produce drops that are too big for the tear reservoir, often five times too big,2 hence the overspill and the waste. When smaller drops are introduced, and no drug is wasted, the efficacy of the drug is just as good but none is lost and the treatments last longer, something essential whether the drug is paid for by the patient or by a health service.
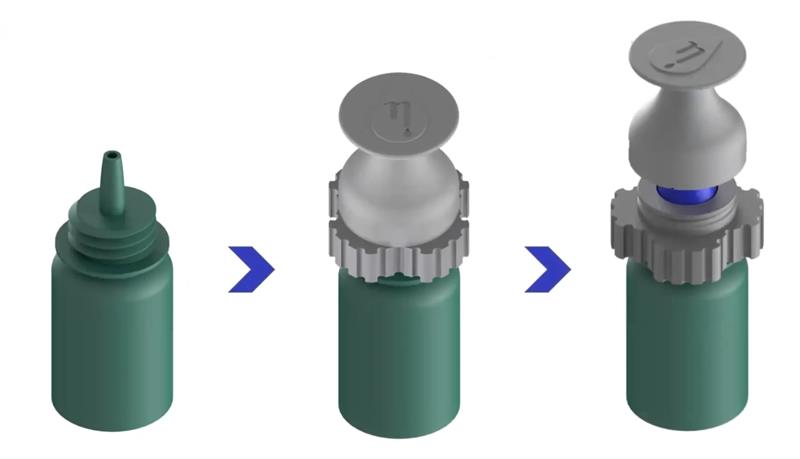
The Nanodropper is a sterile adaptor that can be fitted to dropper bottles (figure 2, left) and reduces the drops produced from 40µL to just 10.2µL, an amount that can be fully absorbed by the eye without waste. This means that a bottle will last some three to four times longer. The device is now familiar throughout all of the United States and is standard in most of the leading paediatric hospitals.
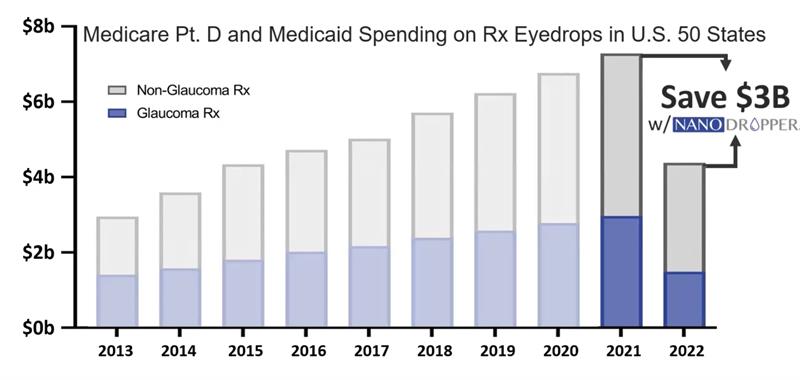
With annual costs of eye drops in the US amounting to just under $8 billion, widespread use of the Nanodropper could have cut this spending almost in half (figure 3). I love how such a simple device has such a significant impact.
Figure 3: Nanodropper cuts the cost of topical drop expenditure nearly in half
Vyluma
Vyluma is a private biopharmaceutical company ‘focused on developing novel topical treatments to address significant unmet needs for ophthalmic diseases that are prevalent worldwide.’ These treatments include the use of a miotic to improve depth of focus for presbyopes (NVK029) currently undergoing a phase II dose confirmation study. Another is NVK031, a miotic able to reduce pupil size by 1 to 2mm, the aim of which is to reduce glare and so be of help for those finding night driving problematic.
Perhaps of more relevance to UK readers, Vyluma is responsible for a low dose atropine for use in myopia management.

The drug (NVK002) is a preservative-free, single dose low concentration atropine (figure 4, right), which is administered nightly and has been found to be both safe and effective for children aged three to 17 years in the childhood atropine for myopia progression (CHAMP) study. The CHAMP study is currently testing two concentrations in the United States and Europe, and preliminary results of the phase III, four-year study, are expected in late 2022 and full results one year later.3 Based on initial results, Vyluma is submitting the drug for FDA approval in the coming months.
Optejet
Dr Beth Scott, of Eyenovia, was keen to promote a novel smart drug delivery system called the Optejet. Smart drug delivery requires a method that is not dependent on gravity, that is able to avoid contamination, that can deliver a precise dose to limit adverse effects and waste, and which allows remote tracking to monitor compliance with dosing. The Optejet (figure 5), yet to receive FDA clearance, is once such system and is proving a boon among paediatric patients.4 As dosing relies upon the press of a button, the system is also going to be useful to those older patients who might otherwise struggle with squeezing a dropper bottle, something I am sure readers have heard about from some of their older patients.
Figure 5: Controlled dosing made easy with the Optejet

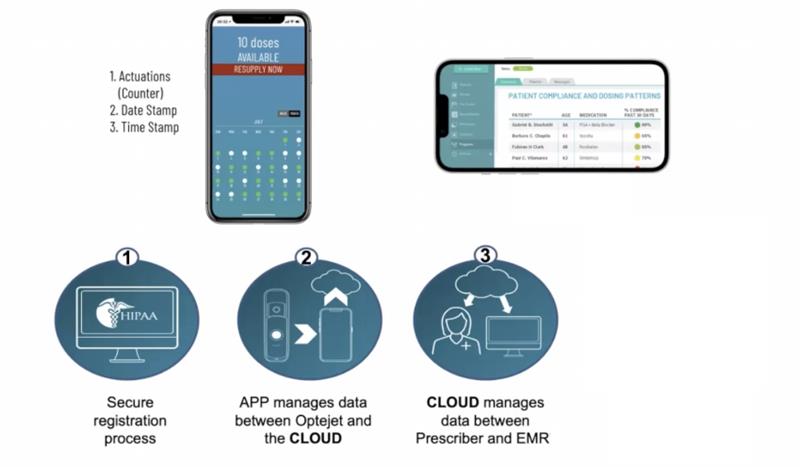
Also, as the drug is administered as a mist, it appears that drug absorption is maximised. This has benefits in reducing the time for drug response, for example achieving dilation of ≥6mm in 74% of eyes in just 15 minutes with a topical mydriatic. There are also benefits in a significant reduction in plasma levels of the drug and also local cytotoxicity, so minimising any ocular or systemic adverse effects. Compliance with treatments will soon be enhanced with the development of a secure, cloud-based data transfer system allowing the ECP to monitor a patient’s drug use remotely (figure 6).
Figure 6: Remote monitoring of drug use will soon be available for the Optejet system (EMR = electronic medical record)
CureSight Eye Tracking

The CureSight system, which was the main focus at the Academy meeting, is a method of treating amblyopia and aims to offer a more effective and easier to comply with treatment than occlusion. CureSight achieves this by the use of a home treatment that is both easy to perform and attractive to most kids as it exploits their watching a film on a digital screen (figure 8) while wearing a colour filter spectacle.
Figure 8: The CureSight; an effective alternative to eye patching
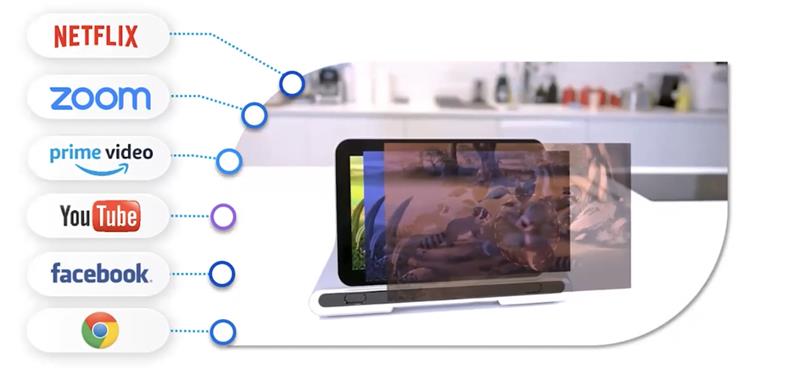
The CureSight display device allows the child to watch anything they wish to stream, whether from YouTube, Netflix or Prime, and the image is split into two channels by the visor while a real-time image adaptation is also applied to blur the centre of the screen image to the better sighted eye. The level of the applied blur is detected by the depth of amblyopia and, importantly, is adjusted over time as the amblyopia is treated. The reason for the buzz around the device this year is that it has now been granted full FDA approval as an alternative to traditional patching. Indeed, a randomised controlled study has found the CureSight to be more effective than patching over a 16-week treatment period, with a two-line acuity improvement in 80% of subjects as compared with 60% with patching. I assume it is more popular with the patients too.
Immunomodulation for Conjunctivitis
Dr Susan Resnick presented details of a new treatment for vernal keratoconjunctivitis (VKC) in children. VKC is a severe form of allergic conjunctivitis, which occurs mainly in males, usually children, and can result in sight-threatening complications, such as limbal stem cell deficiency, keratoconus and glaucoma. Traditional treatment with steroids also has the potential for adverse responses such as posterior subcapsular cataract.
Verkazia is a cyclosporine emulsion (0.1%), which acts as a calcineurin inhibitor immunosuppressant and has now been FDA approved for topical use on VKC patients. Key to the effectiveness of Verkazia is its incorporation of a unique cationic emulsion, which limits washout and enhances the spread of the drug over the cornea, maintaining drug presence for longer than would be the case with a standard drop formulation.5 Recent studies confirm that this formulation nearly doubles the effectiveness of the drug.6
Nasal Spray for Dry Eye
Dr Jeffrey Nau, CEO of Oyster Point Pharma, presented an innovative new way of treating dry eye disease. The nerve pathways involved in tear production include afferent signals from the anterior ethmoid nerve in the nose via the trigeminal nerve, which trigger efferent parasympathetic responses via the same third nerve (figure 9). These stimulate activity in the lacrimal glands, meibomian glands and the goblet cells of the ocular surface.
Tyrvaya is an FDA-approved nasal spray containing 0.03mg varenicline, the chemical found in the smoking cessation product called Champix, and is believed to activate the trigeminal parasympathetic pathway and so promote tear stability.7 Recent studies, such as the MYSTIC study, have confirmed its effectiveness in helping to minimise the impact of dry eye disease on patients.8
References
- McCafferty SJ et al. Modified Goldmann prism intraocular pressure measurement accuracy and correlation to corneal biomechanical metrics: multicentre randomised clinical trial. British Journal of Ophthalmology, 2019;103:1840-1844
- Mishima S et al. Determination of tear volume and tear flow. Investigative Ophthalmology, 1966 Jun;5(3):264-76
- https://clinicaltrials.gov/ct2/show/NCT03350620
- Wirta DL et al. Mydriasis with micro-array print touch-free tropicamide-phenylephrine fixed combination MIST: pooled randomized Phase III trials. Therapeutic Delivery, 2021 Mar;12(3):201-214
- Lallemand F et al. Successfully improving ocular drug delivery using the cationic nanoemulsion, novasorb. Journal of Drug Delivery, 2012;2012:604204
- Leonardi A et al; VEKTIS Study Group. A randomized, controlled trial of cyclosporine a cationic emulsion in pediatric vernal keratoconjunctivitis: The VEKTIS Study. Ophthalmology, 2019 May;126(5):671-681
- Labetoulle M et al. Role of corneal nerves in ocular surface homeostasis and disease. Acta Ophthalmologica, 2019 Mar;97(2):137-14
- Quiroz-Mercado H et al. A phase II randomized trial to evaluate the long-term (12-week) efficacy and safety of OC-01 (varenicline solution) nasal spray for dry eye disease: The MYSTIC study. Ocular Surface, 2022 Apr;24:15-21



