Numerous studies over the past 15 years have indicated the significant frequency and intensity of dryness and discomfort-related symptoms patients experience during contact lens wear.
Across the various polymer materials, modalities and wearing schedules, this research has indicated that about 50 per cent of contact lens wearers are clinically symptomatic. Further, more recent studies have also indicated that these symptoms increase over the course of a day's wear of contact lenses. Dryness symptoms can lead to reduced wearing times, dissatisfaction with contact lenses, and ultimately to failure of lens wear.
Weekly disposable lenses were first introduced in 1987 by Johnson & Johnson Vision Care (Acuvue, etafilcon A), followed soon thereafter by the world's first daily disposable lens in June 1993 (1 Day Acuvue, etafilcon A).
Daily disposables eliminate the need for a daily lens care regimen, offering a significant convenience advantage to the patient. Further, there is little potential for solution-related sensitivities associated with this modality. While the daily disposable modality is associated with improved health and comfort-related outcomes, to date there has been relatively little indication that the daily disposable modality has had a significant impact on over-arching, comfort-related issues associated with traditional contact lens materials and modalities. While daily disposables are novel in terms of their lack of corresponding deposition-related problems, these lenses are typically manufactured from the same or similar polymers from which their frequent replacement counterparts are derived.
In this regard, improvements in polymers associated with the daily disposable modality may too lead to significant improvements in patient comfort, which in turn will allow wearers to appreciate the full spectrum of comfort and health benefits associated with this unique modality. For example, while we don't completely understand the role of the silicone hydrogel lenses in terms of their impact on contact lens-related dry eye, there have been reports suggesting that these new polymers can be associated with improved patient comfort. This too could be especially true of novel polymers developed specifically for the daily disposable modality.
Recently, Johnson & Johnson Vision Care and CIBA Vision released two daily disposable lenses, both based on existing polymer materials, but that have incorporated ingredients via new technologies. The Focus Dailies with AquaComfort lens is derived from its nefilcon A material (FDA Group II), but now includes more polyvinyl alcohol (PVA) in the polymer.
Johnson & Johnson Vision Care's new lens, 1 Day Acuvue Moist, is made from its etafilcon A material (FDA Group IV), but now includes a moisture retaining agent, polyvinyl pyrrolidone (PVP) throughout the polymer matrix. Through a proprietary 'Lacreon' technology, PVP is locked into the etafilcon A matrix. This technology enhances moisture retention and significantly reduces the coefficient of friction of the lens surface. The PVP is not released from the polymer over a day's wear, and thus is not blinked away.
The primary purpose of this report is to compare subjective outcomes associated with these two lenses.
MATERIALS and METHODS
Eligibility and baseline visit
This study was approved by an ethics committee, in accordance with good clinical practice. This was a bilateral, randomised, patient-masked, cross-over clinical trial with a target completion of 40 patients. All participants were required to wear both lens types (as pairs) for a one-week period. Eligible participants were current soft contact lens wearers of at least 18 years of age with good visual acuity (better than 6/9 in each eye with the study lenses).
Refractive requirements included a contact lens prescription between -1.00 and -6.00DS and less than or equal to 1.00DC. Each had to be willing to review and sign an Informed Consent form, and be willing and able to follow the study protocol. Exclusion criteria included medication usage, or systemic or ocular conditions that would contra-indicate soft contact lens wear.
Patients attended for their initial visit without wearing contact lenses. At this visit, ocular and contact lens history information was obtained, which was followed by a manifest refraction and high and low contrast logMAR visual acuity, autokeratometry, slit-lamp biomicroscopy, and lens fitting. Lenses were randomly assigned to patients, and allowed to settle on the eyes for at least 10 minutes prior to the fitting and visual evaluations (Table 1 lists the comparative lens parameters).
Standard clinical lens fitting assessments, including centration, coverage, and movement were performed. Patients were finally asked to provide initial subjective scores relating to comfort, vision, and overall impression.
Each patient was then discharged with instructions to return after seven days of daily lens wear (at least six hours each day) and disposal; instructions were given that no lens care products were to be used during the study, but rather, to simply replace the lens if the need presented.
Follow up and cross-over visits
All subsequent visits took place after at least two hours of lens wear on the day of the examination. Subjects were initially asked to complete subjective outcome assessments, including a variety of relevant topics on comfort on insertion, comfort before removal, overall comfort, dryness before removal, overall dryness, vision, vision at night, fluctuating (variable) vision, lens handling, wearing time, and overall impression of their lens wearing experience. All variables were assessed on a five-point scale; a typical example of this is: excellent, very good, good, fair and poor.
Results are presented as 'top 2 box' percentage; excellent and very good responses by patients. Monocular logMAR visual acuity was recorded, followed by an assessment of lens fit using a slit-lamp biomicroscope. Lenses were then removed and discarded, which was followed by a complete slit-lamp biomicroscopy examination.
A pair of the appropriately powered alternate lens type was inserted, and after a brief (~10 minute) settling time, a dispensing visit was conducted in an identical fashion as the first visit. Again, the subject was asked to return to the clinic after seven days of wear for the second follow up visit. This visit was conducted in an identical fashion as the first follow up visit, with the exception that subjects were also asked their overall preference for the lens types, and a slit-lamp biomicroscopic examination was performed prior to study exit.
For the slit-lamp biomicroscopy and lens surface examinations, observations were graded to the nearest 0.1 by the investigator using the Efron Grading Scales when applicable.
Data analysis
Descriptive statistics including means, standard deviations, frequencies, and percentages were used to summarise all data. Hypothesis testing was performed using paired t-tests (for comparison of normally distributed outcomes that were continuous in nature), Wilcoxon's signed rank tests (for comparison of non-normally distributed outcomes that were continuous in nature), and chi-square analyses (for categorical outcome comparisons).
Most outcomes were compared for data from the subjects' right eyes to avoid the advanced statistical analyses that are associated with using data from both eyes, which is correlated rather than independent. For all statistical comparisons, a p-value of <0.05 was considered statistically significant.
RESULTS
Sample descriptors
Forty-four patients were initially recruited to participate in the clinical trial, with a mean (± standard deviation) age of 27.2±7.6 years (63.6 per cent of the sample was female). The mean (± standard deviation) best sphere (right eye) was -3.20±1.40D. Forty-one patients completed the clinical trial, and of the three that discontinued, two had inadequate lens fits, and one discontinued due to an unrelated medical problem. Of the two patients who could not be fitted in 1 Day Acuvue Moist, this was due to excessive lens movement. It should be noted, the flatter 9.0mm base curve was the only lens available for this study, and the 8.5 base curve is the manufacturer's first fit recommended choice.
Subjective outcomes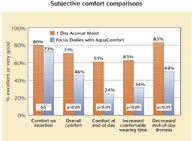 The average wearing time for 1 Day Acuvue Moist was 6.7±0.7 days per week, which was no different from the 6.5±0.8 days per week associated with Focus Dailies with AquaComfort (p = 0.21).
The average wearing time for 1 Day Acuvue Moist was 6.7±0.7 days per week, which was no different from the 6.5±0.8 days per week associated with Focus Dailies with AquaComfort (p = 0.21).
Likewise, there was no difference in daily wearing times associated with each lens, with the average wearing time in 1 Day Acuvue Moist at 12.2±2.3 hours per day and the average in Focus Dailies with AquaComfort at 11.9±2.2 hours per day (p = 0.28). As shown in Figure 1, however, a significant number of patients reported significantly improved comfortable wearing times while wearing 1 Day Acuvue Moist compared to the Focus Dailies with AquaComfort .
As expected, there was also no difference in logMAR visual acuity results when comparing follow-up results from each of the follow-up visits, as on average for both lens types, patients had slightly better than 6/6 visual acuity. At the initial dispense, there were no differences in self-reported comfort (91.1±8.2 for 1 Day Acuvue Moist versus 89.6±8.8 for Focus Dailies with AquaComfort, p = 0.45), self-reported vision (90.7 for 1 Day Acuvue Moist versus 88.6±9.1 for Focus Dailies with AquaComfort , p = 0.11), and overall impressions (89.3±7.7 for 1 Day Acuvue Moist versus 88.1±8.2 for Focus Dailies with AquaComfort, p = 0.48). Thus, there is no evidence to differentiate the lenses relative to comfort on insertion, unlike the large differences found relative to overall and end-of-day comfort described below.
There were numerous differences in subjective comfort outcomes when comparing the results from the follow-up visits for the lenses, which are listed in Figure 1.
These differences primarily relate to improved comfort and reduced dryness with 1 Day Acuvue Moist compared to Focus Dailies with AquaComfort. Across all comfort and dryness outcomes, including both end-of-day and overall ratings, 1 Day Acuvue Moist outperformed Focus Dailies with AquaComfort. The most significant difference (p = 0.01) between the lenses was comfort prior to lens removal (namely, end-of-day), which is a critical finding in this study given the annoyance patients experience with discomfort at the end of a day's wear of lenses. As shown in Figure 1, subjects had significantly improved overall comfort and end-of-day comfort, in addition to their eyes feeling more 'moist' and 'smooth' and 'fresher' when in 1 Day Acuvue Moist compared to Focus Dailies with AquaComfort. Subjects had a significant reduction in the need to use re-wetting drops and the need to remove lenses at the end of day due to discomfort.
To summarise, in the majority of comfort variables questioned, 1 Day Acuvue Moist significantly outperforms Focus Dailies with AquaComfort. 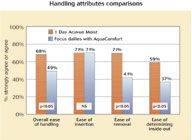 Figure 2, shows the comparative handling responses, with superior performance demonstrated for 1 Day Acuvue Moist, in particular for removal.
Figure 2, shows the comparative handling responses, with superior performance demonstrated for 1 Day Acuvue Moist, in particular for removal.
Other findings
Overall, there were no differences in biomicroscopic measures of ocular physiology that related to wearing of the study contact lenses over a one week period. There was no statistical difference in the percentage of 'acceptable' fits when comparing the lenses.
DISCUSSION
Contact lens-related discomfort dryness is one of the most important factors impacting our patients and practices today. Ultimately, these symptoms during lens wear can lead to a significant reduction in wearing time, patient frustration and dissatisfaction, and potential drop out. Thus, the focus of both contact lens manufacturers, in addition to care solution manufacturers, has recently shifted toward improving upon these outcomes during contact lens wear.
One of the major focuses in this regard is the development of novel materials that lead to improvements in comfort. Certainly, one of the more exceptional options in this regard would be a novel daily disposable lens material designed to improve upon comfort, while at the same time, providing the full spectrum of health benefits associated with the daily disposable modality.
Both Johnson and Johnson Vision Care and CIBA Vision recently introduced daily disposable lenses targeted at this concept of unique polymer development, and to our knowledge, this is the first reported head-to-head comparison of these two novel lenses. Both lens polymers are unique in terms of the attempt to associate a 'moisturising' ingredient (PVP and PVA, respectively), although the lenses are significantly different in terms of the incorporation of the ingredients within the polymer matrix and, ultimately, permanence of these ingredients throughout a day's wear.
While this concept could appear to be minor at the outset, the evidence from this study regarding improved comfort and reduced dryness associated with 1 Day Acuvue Moist clearly suggests that the permanence of PVP within the etafilcon matrix has substantial subjective comfort benefits to the patient. This was true across all comfort and dryness related assessments tested in this study. In addition, patients reported better feeling eyes, and a reduced dependence on using re-wetting drops and removing their lenses due to end-of-day discomfort when wearing 1 Day Acuvue Moist. The lens preference scores confirmed all of these subjective findings with comfort-related head-to-head preference comparisons all favouring 1 Day Acuvue Moist.
As previously discussed, recent studies9,10 have shown that there is a significant diurnal variation in symptoms of comfort and dryness in lens wearers, with a massive increase in these symptoms at the end of a day's wear. In this regard, it is critical that we as practitioners find ways to improve upon this very common end-of-day dryness experienced by our patients. As shown in this study, subjective scores relating to lens comfort (specifically, comfort before removal, overall comfort, dryness before removal and overall dryness) clearly favoured the
1 Day Acuvue Moist lens. Again, the most significant difference in comfort comparisons between these lenses was comfort prior to lens removal at the end of the day. These findings should be considered when choosing the optimal daily disposable lens to prescribe.
In summary, evidence from this study suggests that the 1 Day Acuvue Moist lens has a positive impact on our patients' comfort-related experiences. This unique lens will allow for the continuation of lens wear for those patients struggling with discomfort, in addition to significant potential growth of our practices in terms of previous discomfort-related contact lens drop-outs.
References
1 McMonnies CW, Ho A. Marginal dry eye diagnosis: history versus biomicroscopy. In: Holly FJ, ed. The Preocular Tear Film in Health, Disease, and Contact Lens Wear. Lubbuck, TX: Dry Eye Institute; 1986:32-40.
2 Brennan NA, Efron N. Symptomatology of HEMA contact lens wear. Optom Vis Sci, 1989;66:834-838.
3 Doughty MJ, Fonn D, Richter D, Simpson T, Caffery B, Gordon K. A patient questionnaire approach to estimating the prevalence of dry eye symptoms in patients presenting to optometric practices across Canada. Optom Vis Sci, 1997;74:624-631.
4 Vajdic C, Holden BA, Sweeney DF, Cornish RM. The frequency of ocular symptoms during spectacle and daily soft and rigid contact lens wear. Optom Vis Sci, 1999;76:705-711.
5 Begley CG, Caffery B, Nichols KK, Chalmers R. Responses of contact lens wearers to a dry eye survey. Optom Vis Sci, 2000;77:40-46.
6 Begley CG, Chalmers RL, Mitchell GL et al. Characterization of ocular surface symptoms from optometric practices in North America. Cornea, 2001;20:610-618.
7 Guillon M, Cooper P, Maissa C, Girard-Claudon K. Dry eye symptomatology of contact lens wearers and nonwearers. Adv Exp Med Biol, 2002;506:945-949.
8 Guillon M, Maissa C. Dry eye symptomatology of soft contact lens wearers and nonwearers. Optom Vis Sci, 2005;82:829-834.
9 Nichols JJ, Ziegler C, Mitchell GL, Nichols KK. Self-reported dry eye disease across refractive modalities. Invest Ophthalmol Vis Sci, 2005;46:1911-1914.
10 Chalmers RL, Begley CG. Dryness symptoms among an unselected clinical population with and without contact lens wear. Cont Lens Anterior Eye, 2006.
11 Schlanger JL. A study of contact lens failures. J Am Optom Assoc, 1993;64:220-224.
12 Pritchard N, Fonn D, Brazeau D. Discontinuation of contact lens wear: a survey. ICLC, 1999;26:157-162.
13 Nilsson SEG and Soderqvist M. Clinical performance of a daily disposable contact lens: a three-month prospective trial. J BCLA, 1995; 18:3 81-86.
14 Solomon et al. A three-year prospective study of the clinical performance of daily disposable contact lenses compared with frequent replacement and conventional daily wear contact lenses. CLAO J, 1996: 22:250-7.
15 Hamano et al. A study of complications induced by conventional and disposable contact lenses. CLAO J, 1994 20:103-8.
16 Chalmers RL, Dillehay S, Long B et al. Impact of previous extended and daily wear schedules on signs and symptoms with high Dk lotrafilcon A lenses. Optom Vis Sci, 2005;82:549-554.
17 Malet F, Pagot R, Peyre C et al. Subjective experience with high-oxygen and low-oxygen permeable soft contact lenses in France. Eye Contact Lens, 2003;29:55-59.
18 Efron N. Grading scales for contact lens complications. Ophthalmic Physiol Opt, 1998;18:182-186.
Jane Veys is clinical affairs director, and John Meyler is senior director - professional affairs, both are from Johnson & Johnson Vision Care Europe, Middle East & Africa
