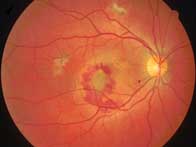
 The National Institute for Health and Clinical Excellence (NICE) has been forced to rethink its controversial decision to restrict the use of Lucentis and Macugen on the NHS to treat patients with wet age-related macular degeneration (AMD) in England and Wales.
The National Institute for Health and Clinical Excellence (NICE) has been forced to rethink its controversial decision to restrict the use of Lucentis and Macugen on the NHS to treat patients with wet age-related macular degeneration (AMD) in England and Wales.
A meeting scheduled to take place on Monday was cancelled by NICE and instead the body said in a statement: 'A rescheduled committee date is planned for the autumn to discuss the revised economic model as well as responses to the consultation, and it is anticipated that a second appraisal consultation document will be released following the meeting.
Register now to continue reading
Thank you for visiting Optician Online. Register now to access up to 10 news and opinion articles a month.
Register
Already have an account? Sign in here
