 Health policy makers have been alerted to new findings that cancer drug Avastin (bevacizumab) has similar side-effects to the more expensive and commonly used eye treatment Lucentis (ranibizumab).
Health policy makers have been alerted to new findings that cancer drug Avastin (bevacizumab) has similar side-effects to the more expensive and commonly used eye treatment Lucentis (ranibizumab).
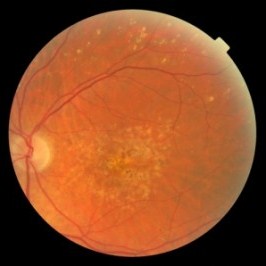
A study published this week by The Cochrane Library journal found Avastin does not appear to increase deaths or serious side-effects compared with Lucentis in people with neovascular age-related macular degeneration (AMD).
The review noted that Avastin had been developed to treat cancer while Lucentis was marketed specifically for AMD.
Authors concluded that health policies that favoured the more costly Lucentis instead of Avastin for macular degeneration for reasons of safety were not supported by current randomised controlled trial evidence.
Register now to continue reading
Thank you for visiting Optician Online. Register now to access up to 10 news and opinion articles a month.
Register
Already have an account? Sign in here
