Acanthamoeba keratitis (AK) is increasingly hitting the headlines of newspapers and magazines that report devastating stories of individuals who encounter this painful and life-changing corneal disease. These are so important to raise awareness and publicise the tragic possibility of losing eyesight and the experience of traumatic treatment. It is of utmost importance that practitioners understand what Acanthamoeba is and what its impact is on human health and eye function. Herein, a broad overview is presented discussing what Acanthamoeba is, how it causes Acanthamoeba keratitis and what the symptoms are, and what can be done to diagnose and treat Acanthamoeba keratitis and prevent it completely.
What is Acanthamoeba?
Acanthamoeba is a common eukaryotic single cell microorganism of a ubiquitous nature. It is normally free-living and a bacteriovore. It has become critically important in ophthalmology as it causes Acanthamoeba keratitis, now classified as a global emerging ocular health problem that can cause blindness and life-changing experiences in those who suffer from it. Information about incidence is limited as there is no requirement to report cases as yet. It is mainly, but not exclusively, associated with contact lens use. Being a eukaryotic cell, its structure and biochemical pathways are similar to human cells, and this is important to keep in mind as we understand how Acanthamoeba keratitis is treated.
Life cycle and typical habitat
Figure 1: Acanthamoeba can live in virtually any environment due to its simple life cycle
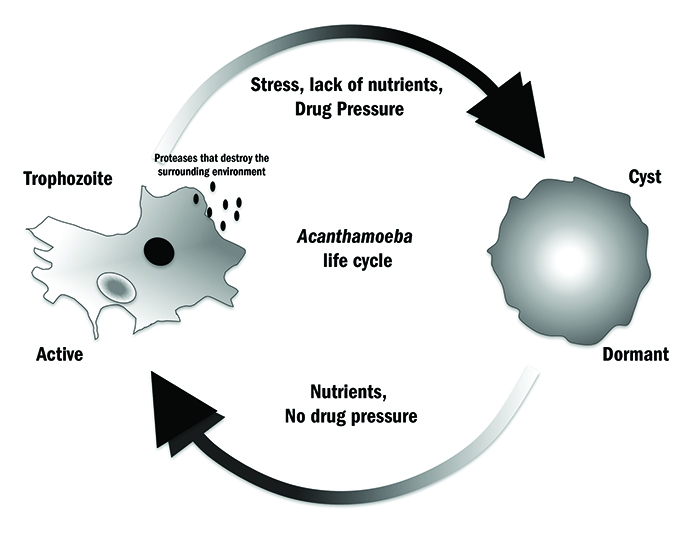
The reason behind Acanthamoeba being such a highly successful microorganism is that it can live in virtually every environment, and this is due mainly to its simple life cycle (figure 1). During this cycle it exists in one of two main forms: an active trophozoite and an inactive cyst. The trophozoite is the growing and replicating form; it develops when Acanthamoeba is in a nutrient-rich environment.1 This usually consists of bacteria and other free nutrients. Its relationship with bacteria is quite complex, as not only can the organism feed on them, but certain bacteria can also hide within the Acanthamoeba, thus making it a vector for bacterial disease. This has been reported for Legionella pneumophila, Staphylococcus aureus, Escherichia coli and Pseudomonas aeruginosa. P aeruginosa is commonly associated with eye infections.
When trophozoites are void of nutrition or in hostile environments (high and low temperatures, lack of moisture, etc), the trophozoite does not die but simply shuts down; it stops growing and replicating, transforming itself into the inactive cyst. This process is called ‘encystment’. Acanthamoeba will remain a cyst until the environment becomes more favourable for growth and the trophozoite will re-emerge in a process called ‘excystment’. Cysts can remain dormant for many years and still remain viable, and studies have shown that trophozoites can re-emerge and continue to replicate.2 The Acanthamoeba life cycle has allowed adaptation to natural habitats, but is also closely linked to the organism’s success as an opportunistic pathogen and its impact on ocular disease.1
Acanthamoeba keratitis: an incidental infection
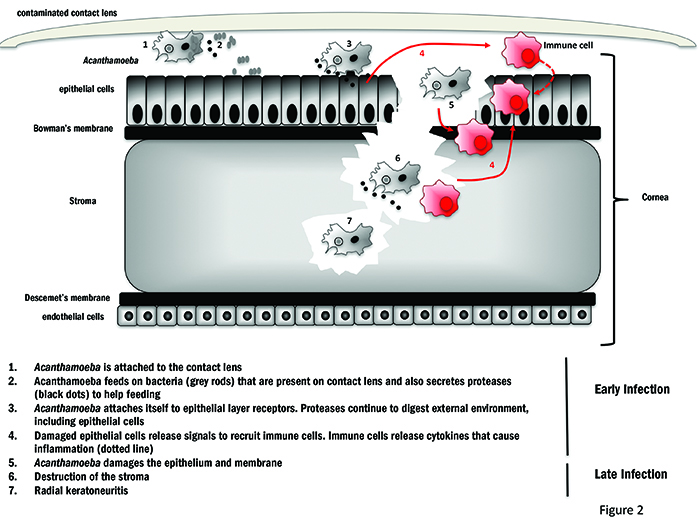
When analysing how Acanthamoeba causes keratitis, we have to remember that Acanthamoeba has not evolved to cause disease. It is normally a free-living bacteriovore.3 Its presence on the cornea is purely incidental and this is why it is labelled as an opportunistic pathogen. Scientists and clinicians often refer to it as a parasite, as it feeds on its host so causing harm. The corneal surface provides an ideal environment for the trophozoite to grow and, as it grows, progressive destruction of the epithelium, Bowman’s membrane and stroma can occur which will attract immune cells causing inflammation4 (figure 2). Acanthamoeba can infect the cornea both as a trophozoite or a cyst. If the trophozoite is the predominant form during invasion, this indicates it was already in a nutritious environment prior to infection with bacteria readily available. The trophozoites will continue to grow and multiply, feeding on any co-contaminant bacteria and the corneal cells. Cysts can also initiate infection, and when they are in the favourable environment of the cornea, they excyst to trophozoites. Acanthamoeba keratitis (AK) is usually associated with the contamination of contact lenses and cases with tap water or dust. Contact lens use is a risk factor, together with being under 25 years old and male5, but there are reports of infection in non-contact lens users,6 usually through a scratch on the corneal surface. This helps us to understand that the reason why contact lens users are at a higher risk and is likely tied to the fact that contact lenses can create micro-lesions on the cornea, which offers an ‘open door’ to Acanthamoeba.7 This is even more plausible when one considers that many individuals have antibodies to Acanthamoeba even though they have never suffered previous symptomatic infection. The presence of antibodies in individuals with no disease symptoms is not surprising as Acanthamoeba is present throughout our environment.
Figure 2: Mechanism of Acanthamoeba infection of the cornea of a contact lens wearer
Incidence rate and role of the contact lens
Information about incidence is limited as there is no requirement to report cases as yet. Data from older studies on incidence depend on geographical location, and it is highly variable, ranging from 0.33 to 1.49 per 10,000.8 Now, that diagnosis is improving, it would be beneficial to publish an up to date report on the incidence of AK. Since the infection is fortunately quite rare, patients have found support in a growing patient community that communicate and share information through social media groups. For example, the Acanthamoeba keratitis Facebook page9 currently has 795 members worldwide and new members are joining every day. In their posts, they share experiences with their diagnosis, treatment options, and medical centres. Above all, many write about their relief to have found such a community and the knowledge that they are not alone.
There has been much speculation and that the type of lens used may be responsible for promoting Acanthamoeba attachment. In 2003, Beattie et al, reported on the enhanced Acanthamoeba attachment to extended-wear silicone hydrogel lenses compared to conventional hydrogel lenses.10 Since then, studies of both hydrogel lenses11 and rigid gas permeable lenses12 have found no significant preference of Acanthamoeba to one or the other, but rather that Acanthamoeba attachment is actually associated with the moisture content of the lens.11,13,14 In the future, we will be able to identify patients who may be more susceptible to AK, as we learn more about the human host’s molecular determinants that influence disease.15 This knowledge will be gained through a better understanding of the immune mechanisms that trigger inflammation16, 17 and lead to personalised medical management, including genome sequencing.18
Ocular Impact
AK sufferers describe the infection as life-changing, emotionally consuming, extremely painful and of living in fear of losing eyesight and of the recurrence of infection. Early symptoms are similar to those of bacterial, fungal and viral keratitis. Typically in the clinic, when patients present with keratitis, they are normally treated with a broad spectrum of anti-infective agents designed to treat the more common causes of keratitis, such as bacteria.1 This is because AK is difficult to diagnose, particularly in the early stages.19
Early infection, symptoms, clinical features
Early in infection there is quite a discrepancy between patients’ symptoms and this adds to the already challenging diagnosis of AK. Some patients report intense pain that may be disproportionate to the clinical features they present, while others report the sensation of a foreign body in the eye for a number of weeks. In the early stages, a patient may experience photophobia, redness and tearing.20,21 All these symptoms, including extreme pain, may come as no surprise to eye care professionals as the eye is an immunoprivileged site and any foreign body that triggers an immune response is likely to lead to an adverse inflammation.22 The cornea is also one of the most sensitive organs in the human body with a high level of pain receptors. It is extremely important to highlight that any symptoms at this early stage can vary and this may lead to misdiagnosis of AK with devastating effects at later stages. In the early infection stages, it is generally recommended to look for punctate epithelial erosion (PEE), as this presents in approximately 55% of AK patients.23 PEE is the non-specific description of microscopic defects in the corneal epithelium that stain with fluorescein.
Late infection, clinical features
At the later stages of infection, Acanthamoeba irreparably damages the cornea and causes decreased vision due to inflammation. Some patients may experience microcystic oedema (32%) and limbitis (24%); only about 10% of patients present with a dendritic ulcer and only 4% will have a ring infiltrate.24 The ring-like infiltrate is considered the hallmark of Acanthamoeba keratitis and only develops after the amoebae have penetrated the full depth of the cornea and invaded the stroma. It is hoped that AK is diagnosed prior to this. At this point, the patient’s sight is severely under threat and treatment can be completely ineffective as the Acanthamoeba is no longer on the corneal surface. A corneal graft is usually a last resort, but there are many cases where Acanthamoeba in the stroma reactivate and cause infection in the graft.19
Diagnosis
Diagnosis in the ophthalmology clinic is made on the basis of the clinical picture, including symptoms and features as previously described, along with confocal microscopy to detect Acanthamoeba trophozoites and cysts. The challenge with this technique is that Acanthamoeba cells look like the human macrophages16, 25 that are recruited to the cornea during the inflammatory process, so it may be difficult to distinguish between cells. In addition, the cost of confocal microscopy is very high and is not always available. Furthermore, a corneal scrape is usually taken so that organisms can be isolated from a corneal culture or detected in histopathology. The current gold standard test is the polymerase chain reaction assay, in which amplification of an Acanthamoeba gene on extracted DNA from the corneal scrape confirms the presence of Acanthamoeba.26
Treatment
Early features of AK can overlap with that of bacterial, fungal and viral keratitis. Initial therapy is often broad spectrum antibiotics until the causative agent is identified, but this delay in treatment could have negative impact on the prognosis of Acanthamoeba keratitis. On one hand, antibiotics will only kill bacteria, allowing the Acanthamoeba to continue its pathogenesis, and on the other hand, some antibiotic drops may contain excipients that could make Acanthamoeba less active or encyst, thus turning into a subclinical infection, that may then flare up at a later date.1 This may have impact on the ‘real’ incidence figures on Acanthamoeba keratitis.
For cases of proven or very likely AK, specific treatment consists of frequent topical anti-infective drops, such as 0.02% polyhexamethylene biguanide (PHMB) or chlorhexidene in combination with propamidine or hexamidine. Triazole compound agents, such as ketoconazole27 and voriconazole,28 have been used with varying degrees of success and these are less likely to cause more damage to the human cells as triazole compounds target ergosterol, a sterol that is not present in human cell membranes.
Despite these treatments, poor clinical outcome is common even after corneal grafts and this could be due to the delay in diagnosis, misdiagnosis itself or differences in the Acanthamoeba strains that have been found to cause AK.
Acanthamoeba strains and other free-living amoebae that can cause keratitis
Acanthamoeba is a term describing a genus comprising a number of species including at least 20 genotypes that in turn include different strains.29 The species typically associated with Acanthamoeba keratitis is A castellanii of the T4 genotype, but others are also known to cause disease, such as A polyphaga, A culbertsoni, A hatchetti, A rhysodes, A lugdunensis, A quina and A griffini. It is possible that different Acanthamoeba species respond differently to treatments and, for this reason, some clinical outcomes may be better than others. Future directions include genotyping the Acanthamoeba from each infection to ensure that each treatment is tailor-made for the specific cause of infection.26 In addition, it is important to remember that keratitis can also be caused by other free-living amoebae aside from Acanthamoeba, although this is rare. There have been a few cases caused by Hartmanella and Vahlkampfia,30 and recently a case of newly discovered Allovahlkampfia 31 was isolated from an Egyptian patient. From this, it is clear that it is important to fully characterise the specific amoeba causing infection and research efforts are focusing on the development of new treatments and drug sensitivity assays for each of these.1,32-35
Prevention
It is difficult to avoid Acanthamoeba as it surrounds us in the environment, but since most AK cases (but not all) are associated with contaminated contact lenses, it is crucial to highlight that this infection can be prevented. The best practice is to engage in conversation with the contact lens user to ensure they are aware of the risks of Acanthamoeba infection, however small that may be. Contact lens care is of utmost importance to ensure that there is no contact with water, soil or significant sources of dust.
Tap water is not sterile. Free-living amoebae, including Acanthamoeba were isolated from 89% of homes in the UK and their presence varied with tap water temperature and location. Hot water taps in bathrooms harbour the most Acanthamoeba (76%) while cold water kitchen taps carry the least number (16%).36 There is a significant push to raise awareness among both practitioners and contact lens wearers, with the most prominent campaign led by AK sufferer Irenie Ekkeshis. This campaign called the No Water Initiative won the Vision Pioneer Award for Campaign of the Year 2016 and aims to raise awareness of the risks associated with using non-sterile water to clean contact lenses. She designed No Water stickers (figure 3) that are now supported by the BCLA, who have produced these for use on contact lens packaging.37 ‘No water’ includes no contact lens wear in the shower, in the swimming pool, lakes, rivers or even the sea as Acanthamoeba has been isolated from all these environments and prevention becomes challenging when contact lenses are used for convenience in sporting activities, such as sailing and surfing. Fortunately, the dangers are now being highlighted in sports medicine books and journals.38
Figure 3: Stickers from the ‘no water’ initiative

Another control measure to help in the prevention of infection is to change contact lens cases frequently. The moisture in cases can lead to a build-up of biofilm, a thin layer of microorganisms gathered in a matrix, often of a mucus-like consistency. This biofilm contains environmental-originated bacteria, fungi and amoeba (including Acanthamoeba) that have the potential to cause infection and inflammation in the eye. Biofilms are extremely common in the household setting; just think of what we might commonly and mistakenly call ‘mildew’, the grey or pinkish slime in a poorly ventilated bathroom, and it is easy to see how quickly and efficiently Acanthamoeba can spread.
Soil and dust can also contain Acanthamoeba. Acanthamoeba cysts can be carried in the wind,39 thus providing another route for contact lens contamination. Therefore, the lens care routine is crucial in managing the prevention of disease. Washing hands thoroughly with soap and drying with a clean towel reduces the chances of cross-contamination. Using the appropriate care system for lens type is essential so that the lens can be cleaned properly and a rubbing motion when cleaning will free the lens from any dirt, oil, makeup and protein residues that Acanthamoeba can stick too. Storing contact lens care system appropriately, such as recapping the bottle, is important as these solutions do not kill either Acanthamoeba trophozoites or cysts 40. If the solution becomes accidentally contaminated, Acanthamoeba can encyst to then reactivate once on the cornea.
Advice to practitioners
Contact lens user education about Acanthamoeba keratitis is imperative to prevent this disease, as is clinician hygiene measures in practice. The biggest obstacle for any practitioner is patient compliance. There will always be contact lens wearers who will go against advice and swim, shower and allow tap water to contaminate the lens. Therefore, practitioners need to be aware that Acanthamoeba may be the cause of a painful keratitis, and be able to intervene at earlier stages through immediate referrals to local ophthalmology clinics. When a patient presents with an eye irritation, the moisture content of the lenses should be ascertained. Questions should focus on the contact lens cleaning regime and a history of activities that the patient has engaged in. Have they been involved in water sport activities, have they visited countries or islands where there is a high prevalence of Acanthamoeba in the water (for example, the Canary Islands or Jamaica) or do they recall eye exposure to high winds or rubbing their eyes after gardening? Examination should include a detailed viewing of the cornea for any micro lesions. Finally, if Acanthamoeba keratitis cannot be ruled out, immediate referral to a consultant ophthalmologist must be made so that full clinical and laboratory diagnosis can be carried out and the treatment regime started.
Fiona L Henriquez is Professor of Parasitology and infection and microbiology group leader at the Institute of Biomedical and Environmental Health Research, University of the West of Scotland.
References
1 Roberts CW, Henriquez FL. Drug target identification, validation, characterisation and exploitation for treatment of Acanthamoeba (species) infections. Exp Parasitol. 2010 Sep;126(1):91-6.
2 Campbell SJ, Ingram PR, Roberts CW, Henriquez FL. Induced encystment improves resistance to preservation and storage of Acanthamoeba castellanii. Parasitology. 2008 Oct;135(12):1401-5.
3 Balczun C, Scheid PL. Free-Living Amoebae as Hosts for and Vectors of Intracellular Microorganisms with Public Health Significance. Viruses. 2017 Apr1;9(4).
4 Lorenzo-Morales J, Martín-Navarro CM, López-Arencibia A, Arnalich-Montiel F, Piñero JE, Valladares B. Acanthamoeba keratitis: an emerging disease gathering importance worldwide? Trends Parasitol. 2013 Apr;29(4):181-7.
5 Brown AC, Ross J, Jones DB, Collier SA, Ayers TL, Hoekstra RM, Backensen B, Roy SL, Beach MJ, Yoder JS; Acanthamoeba Keratitis Investigation Team. Risk Factors for Acanthamoeba Keratitis – A Multistate Case-Control Study, 2008-2011. Eye Contact Lens. 2017 Jan 17.
6 Jain R, Garg P, Motukupally SR, Geary MB. Clinico-microbiological review of non-contact-lens-associated acanthamoeba keratitis. Semin Ophthalmol. 2015 Jul;30(4):281-8.
7 Marciano-Cabral F, Cabral G. Acanthamoeba spp. as agents of disease in humans. Clin Microbiol Rev. 2003 Apr;16(2):273-307.
8 Seal D. Treatment of Acanthamoeba keratitis. Expert Rev Anti Infect Ther.2003 Aug;1(2):205-8.
9 https://www.facebook.com/groups/2407518611/
10 Beattie TK, Tomlinson A, Seal DV. Surface treatment or material characteristic: the reason for the high level of Acanthamoeba attachment to silicone hydrogel contact lenses. Eye Contact Lens. 2003 Jan;29(1 Suppl):S40-3
11 Reverey JF, Fromme R, Leippe M, Selhuber-Unkel C. In vitro adhesion of Acanthamoeba castellanii to soft contact lenses depends on water content and disinfection procedure. Cont Lens Anterior Eye. 2014 Aug;37(4):262-6.
12 Cope JR, Collier SA, Schein OD, Brown AC, Verani JR, Gallen R, Beach MJ, Yoder JS. Acanthamoeba Keratitis among Rigid Gas Permeable Contact Lens Wearers in the United States, 2005 through 2011. Ophthalmology. 2016 Jul;123(7):1435-41.
13 Lee GH, Lee JE, Park MK, Yu HS. Adhesion of Acanthamoeba on Silicone Hydrogel Contact Lenses. Cornea. 2016 May;35(5):663-8.
14 Lee GH, Yu HS, Lee JE. Effects of multipurpose solutions on the adhesion of Acanthamoeba to rigid gas permeable contact lenses. Ophthalmic Physiol Opt. 2016 Mar;36(2):93-9.
15 Niederkorn JY, Alizadeh H, Leher H, McCulley JP. The pathogenesis of Acanthamoeba keratitis. Microbes Infect. 1999 May;1(6):437-43.
16 Mattana A, Sanna M, Cano A, Delogu G, Erre G, Roberts CW, Henriquez FL, Fiori PL, Cappuccinelli P. Acanthamoeba castellanii Genotype T4 Stimulates the Production of Interleukin-10 as Well as Proinflammatory Cytokines in THP-1 Cells, Human Peripheral Blood Mononuclear Cells, and Human Monocyte-Derived Macrophages. Infect Immun. 2016 Sep 19;84(10):2953-62.
17 Cano A, Mattana A, Woods S, Henriquez FL, Alexander J, Roberts CW. Acanthamoeba activates macrophages predominantly through TLR4 and MyD88-dependent mechanisms to induce IL-12 and IL-6. Infect Immun. 2017 Mar 27.
18 Ginsburg GS, Willard HF. Genomic and personalized medicine: foundations and applications. Transl Res. 2009 Dec;154(6):277-87.
19 Lorenzo-Morales J, Khan NA, Walochnik J. An update on Acanthamoeba keratitis: diagnosis, pathogenesis and treatment. Parasite. 2015; 22:10.
20 Gupta R, Gorski M, Henderson T, Lazzaro D, Haseeb MA. Clinical Course of Unilateral Acanthamoeba Keratitis in a Cosmetic Contact Lens Wearer. Ann Clin Lab Sci. 2015 Spring;45(3):366-70.
21 Cristina S, Cristina V, Mihaela P. Acanthamoeba keratitis challenges a case report. Rom J Ophthalmol. 2016 Jan-Mar;60(1):40-2.
22 Ksander BR, Sano Y, Streilein JW. Role of donor-specific cytotoxic T cells in rejection of corneal allografts in normal and high-risk eyes. Transpl Immunol. 1996 Mar;4(1):49-52.
23 http://www.reviewofcontactlenses.com/content/d/irregular_cornea/c/37560/
24 Ibrahim YW, Boase DL, Cree IA. How Could Contact Lens Wearers Be at Risk of Acanthamoeba Infection? A Review. Journal of Optometry. 2009;2(2):60-66.
25 Escoll P, Rolando M, Gomez-Valero L, Buchrieser C. From amoeba to macrophages: exploring the molecular mechanisms of Legionella pneumophila infection in both hosts. Curr Top Microbiol Immunol. 2013;376:1-34.
26 Alexander CL, Coyne M, Jones B, Anijeet D. Acanthamoeba keratitis: improving the Scottish diagnostic service for the rapid molecular detection of Acanthamoeba species. J Med Microbiol. 2015 Jul;64(7):682-7.
27 Wynter-Allison Z, Lorenzo Morales J, Calder D, Radlein K, Ortega-Rivas A, Lindo JF. Acanthamoeba infection as a cause of severe keratitis in a soft contact lens wearer in Jamaica. Am J Trop Med Hyg. 2005 Jul;73(1):92-4.
28 Bang S, Edell E, Eghrari AO, Gottsch JD. Treatment with voriconazole in 3 eyes with resistant Acanthamoeba keratitis. Am J Ophthalmol. 2010 Jan;149(1):66-9.
29 Corsaro D, Walochnik J, Köhsler M, Rott MB. Acanthamoeba misidentification and multiple labels: redefining genotypes T16, T19, and T20 and proposal for Acanthamoeba micheli sp. nov. (genotype T19). Parasitol Res. 2015 Jul;114(7):2481-90.
30 Pinna, A, Porcu, T, Boscia, F, Cano, A, Erre, G and Mattana, A. (2016), Free living amoebae (FLA) keratitis. Acta Ophthalmol, 94
31 Tolba ME, Huseein EA, Farrag HM, Mohamed Hel D, Kobayashi S, Suzuki J, Ali TA, Sugano S. Allovahlkampfia spelaea Causing Keratitis in Humans. PLoS Negl Trop Dis. 2016 Jul 14;10(7):e0004841.
