Stroke (or cerebrovascular accident — CVA) represents a common neurological emergency that can often result in disability or even death. Around 100,000 strokes occur every year in the UK (one every five minutes) and, at the moment, there are over 1.2 million stroke survivors in the country. In England alone, the total cost of stroke is over £1.7 million (State of the nation – Stroke statistics, January 2017).
The vast majority of strokes occur when an area of the brain is deprived of blood either through vessel occlusion or blockage. This is described as ischaemic stroke and accounts for 85% of cases. There are also cases of haemorrhagic stroke (15%), when the principal cause is a vessel rupture and subsequent bleeding into a brain area.
As primary health care providers, optometrists can play an important role in prevention, diagnosis and also in the care of patients that survived a stroke. This article will look at stroke before it happens, during its occurrence, and after it has happened.
Before stroke
The risk factors for stroke include:1
- Age: the risk doubles every 10 years after the age of 55.
- Gender: higher risk in men prior to the age of 65 years; the risk is higher in women above the age of 65 years.
- Race: Afro-Caribbeans have a higher risk than White European.
- Family history: greater risk when a first-degree relative has suffered a stroke.
- Previous history of stroke: the risk is double in those who have already suffered a stroke.
- Smoking.
- Sedentary life style.
- Systemic pathology such as high blood pressure (BP — the most important risk factor), carotid artery disease, cardiac arrhythmias, diabetes, hyperlipidaemia.
- History of transient ischaemic attacks (TIA) or amaurosis fugax (see below).
- Patients diagnosed with age-related macular degeneration (AMD) are twice as likely to develop a stroke than the normal population.2
Let us now look at some of these risk factors in more detail.
Transient Ischaemic attacks
It is often the case that before a stroke occurs, a patient would have had symptoms of the so-called transient ischaemic attack (TIA) or ‘mini stroke.’ During a TIA, the vascular disturbances (vasospasm and blood flow interruption) are temporary and do not cause structural brain damage. Nevertheless, the pathophysiology of ischaemic stroke and TIA is identical and, therefore, both require rapid and accurate diagnosis. Moreover, 15 to 30% of strokes are preceded by TIA often in the same 24-hour period. Therefore, a quick positive diagnosis of TIA is highly important. This depends, however, on access to specialist support services, such as MRI, that are sometimes difficult to access within a 24-hour period (the time limit required for TIA diagnosis). Consequently, a positive diagnosis of TIA is often made based on a patient’s history and the clinician’s experience.3
Symptoms of TIA can include:
- Hemiparesis
- Aphasia
- Transient visual loss (TVL)
- Hemianopia
- Diplopia
- Vertigo
- Limb weakness
- Hearing loss
These symptoms occur suddenly and then gradually disappear over minutes and usually in less than one hour. TIA attacks should be differentiated from migraine aura, seizures, syncope and even from anxiety attacks.3 By quickly recognising TIA with visual symptoms and promptly referring the patient, the optometrist can play a vital role in stroke prevention. However, TVL is only one symptom of TIA and can also be seen associated with other conditions. Monocular TVL can be associated with giant cell arteritis (GCA), ocular migraine, or closed angle glaucoma, while binocular TVL can also be associated with epilepsy and complex migraine states.4 The optometrist should ask the appropriate questions to determine the possible cause behind the TVL, namely asking details about:
- Pattern
- Duration
- Associated symptoms
- Other medical history
Nevertheless, regardless of the cause, patients with TVL need to be referred urgently for appropriate investigation and treatment.
Carotid artery disease
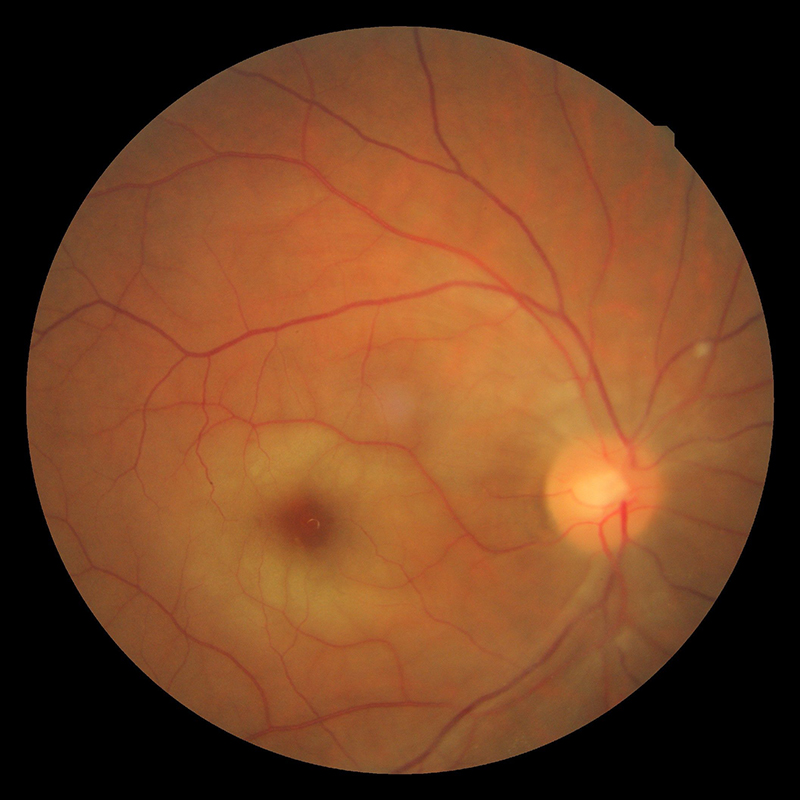
Figure 1: Central retinal artery occlusion
Carotid artery disease occurs when there is over 50% occlusion of the carotid arteries due to atherosclerosis. It can manifest also through various ophthalmic symptoms due to retinal emboli, insufficient blood flow to the retina and choroid, or chronic hypoperfusion of the globe (ocular ischaemic syndrome).5
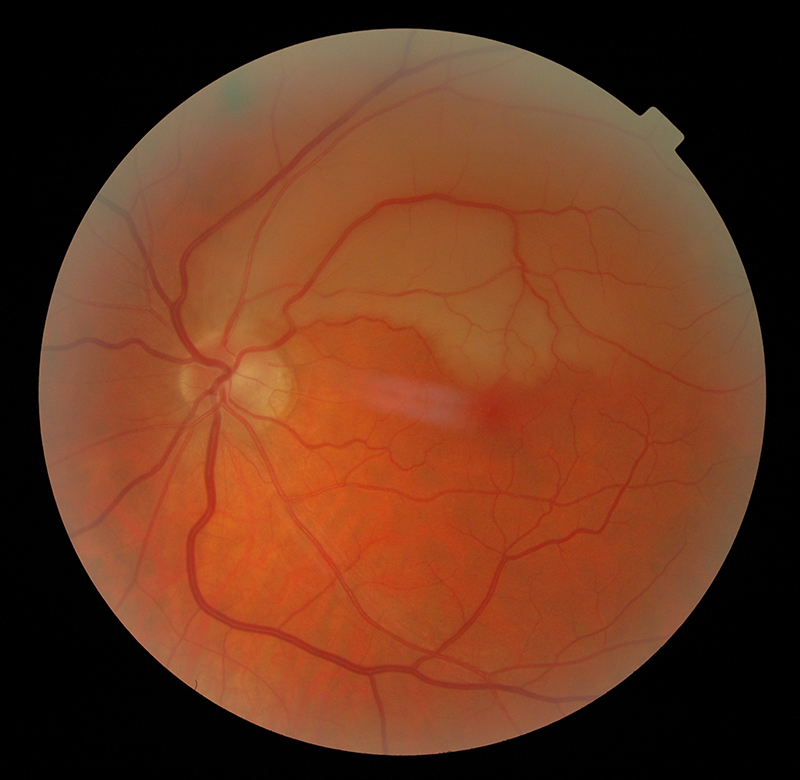 Figure 2: Superior branch retinal artery occlusion
Figure 2: Superior branch retinal artery occlusion
The visual symptoms associated with carotid artery disease vary from TVL caused by a retinal embolus, to central and branch retinal artery occlusions (figures 1 and 2), anterior ischaemic optic neuropathy (figure 5) and ocular ischaemic syndrome (OIS). The ocular changes and symptoms associated with carotid artery disease are summarised in table 1.5 An early accurate diagnosis will help preventing ischaemic stroke, which sometimes occurs after only a few days from the onset of these symptoms.
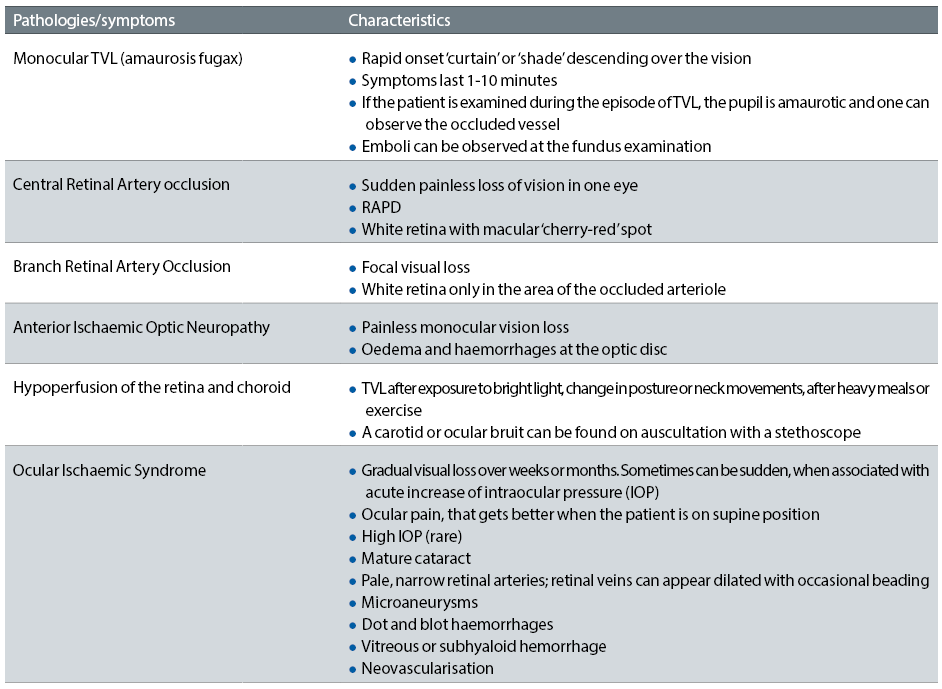
Table 1: Ocular changes with carotid artery disease
Systemic arterial hypertension
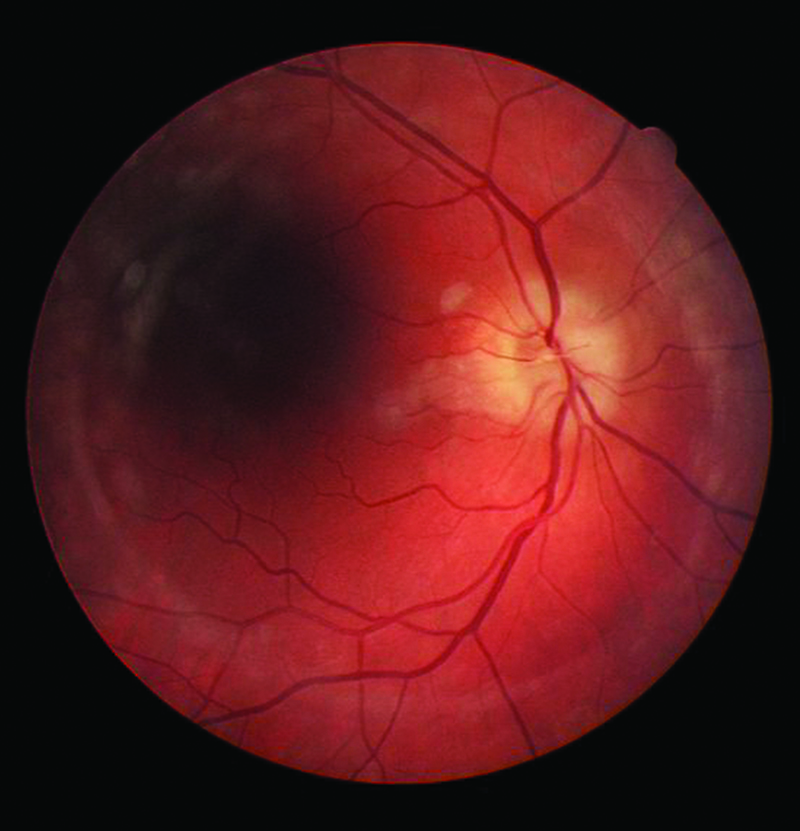
Figure 3: Anterior ischaemic optic neuropathy (non-arteritic)
Systemic arterial hypertension represents the main cause of stroke and a major risk factor for heart disease, such as heart attack and heart failure. It can also induce severe kidney disease and ultimately, kidney failure. Approximately one-third of people with systemic hypertension are still undiagnosed and the disease is responsible for over 62,000 preventable deaths through stroke and heart disease each year (data from Blood Pressure UK). High blood pressure (BP) is defined as values of systolic BP at or above 140mmHg and of diastolic BP at or above 90mmHg (figure 4).

Figure 4: Blood pressure measurement by electronic or sphygmomanometer is the best way to detect systemic hypertension
At the ocular level, the first signs of high BP are represented by retinal arteriolar narrowing (figures 5 and 6).
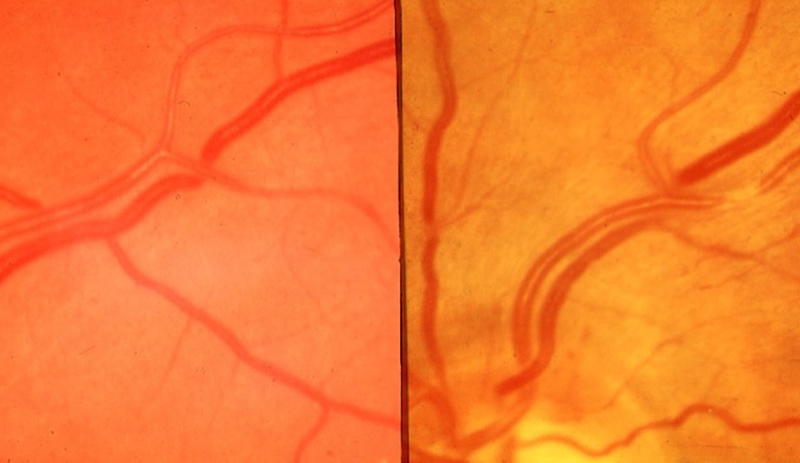
Figure 5: Early hypertensive changes including nicking, generalised and focal narrowing
However, one of the unresolved issues in the association between narrowed retinal arterioles and hypertension is whether arteriolar narrowing is antecedent to the development of hypertension by altering vascular haemodynamics, which results in a re-setting of BP, or whether changes in retinal vessel calibre reflects physiological adaptations to increases in BP. Focal retinal arteriolar narrowing and arteriovenous nicking are structural vascular signs that reflect high BP. In addition, wider retinal veins are commonly associated with an increased risk for stroke.6,7
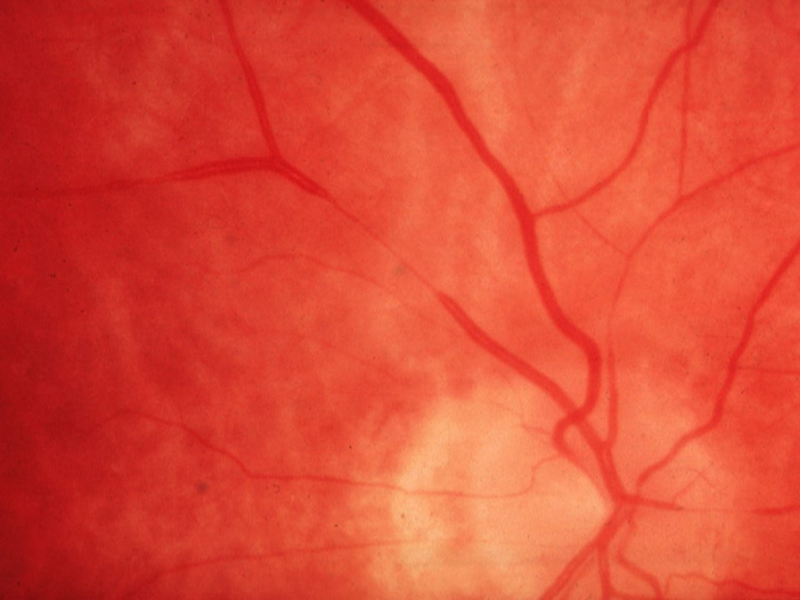
Figure 6: Focal narrowing
Hypertensive retinopathy, depending on the grade, is characterised by the presence of arteriovenous nicking, focal and generalised retinal arteriolar narrowing (figure 5), cotton-wool spots, micro-aneurysms, flame and blot-shaped retinal haemorrhages, exudative maculopathy and papilloedema (figure 7).
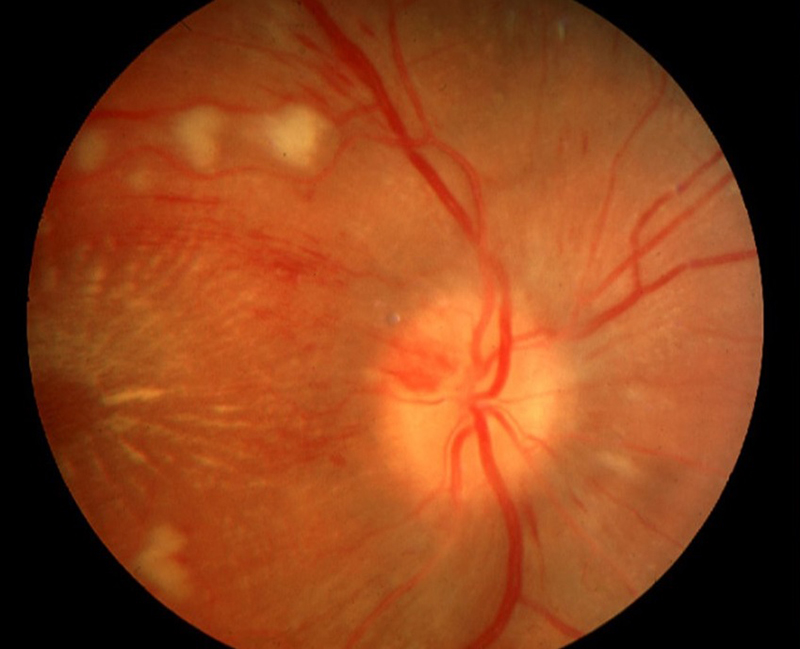
Figure 7: Malignant hypertensive retinopathy (grade 3)
The fundoscopic appearance and systemic association of various grades (simplified to three) are listed in table 2 (adapted from eight).
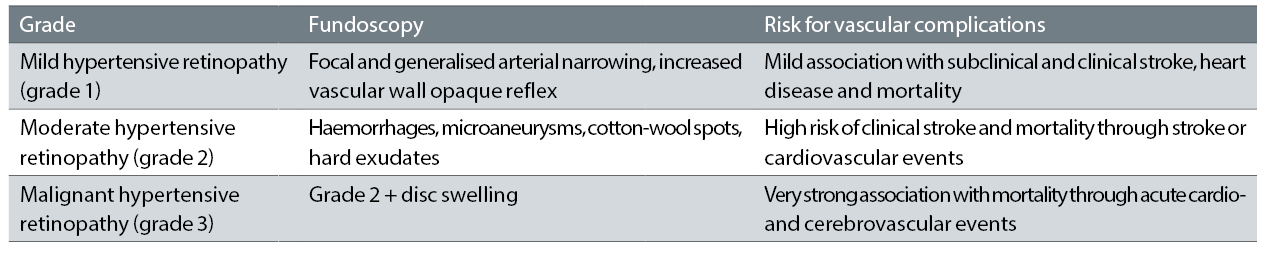
Table 2: Hypertensive retinopathy grades
In addition to detecting and monitoring the above fundoscopic changes, optometrists can also play a role in the BP monitoring. It is often the case that our patients have a well-controlled systemic hypertension through their treatment. However, in other situations the patients are either non-compliant with their treatment regime or the medication is inefficient. Therefore, on the spot check of BP by the optometrist can often detect abnormal values in individuals with fundoscopic changes that suggest systemic hypertension. Nevertheless, despite the fact that measuring systemic BP does not require specialist training, only a small proportion of optometrists use BP monitors in their practice.9 There is, therefore, a high need to increase awareness among optometrists and possibly offer them specialist training for measurement and interpretation of BP values.
Age-related macular degeneration (AMD)
AMD represents a chronic, degenerative ocular condition associated with loss of central vision (figure 8). Several reports found that patients suffering from AMD are at higher risk than age-matched healthy individuals for developing CHD and stroke. The Atherosclerosis Risk in Communities (ARIC) Study found that persons with early AMD signs had double the risk of incident stroke over 10 years.10 Moreover, they also are more likely to have an increased mortality compared to the age-matched healthy population. Moreover, AMD and stroke share common risk factors (hypertension, carotid artery disease, smoking) and also genetic background (APOE genes).11 Therefore, early screening of AMD patients for such pathologies could represent an important step of prevention and disease-modifying interventions.
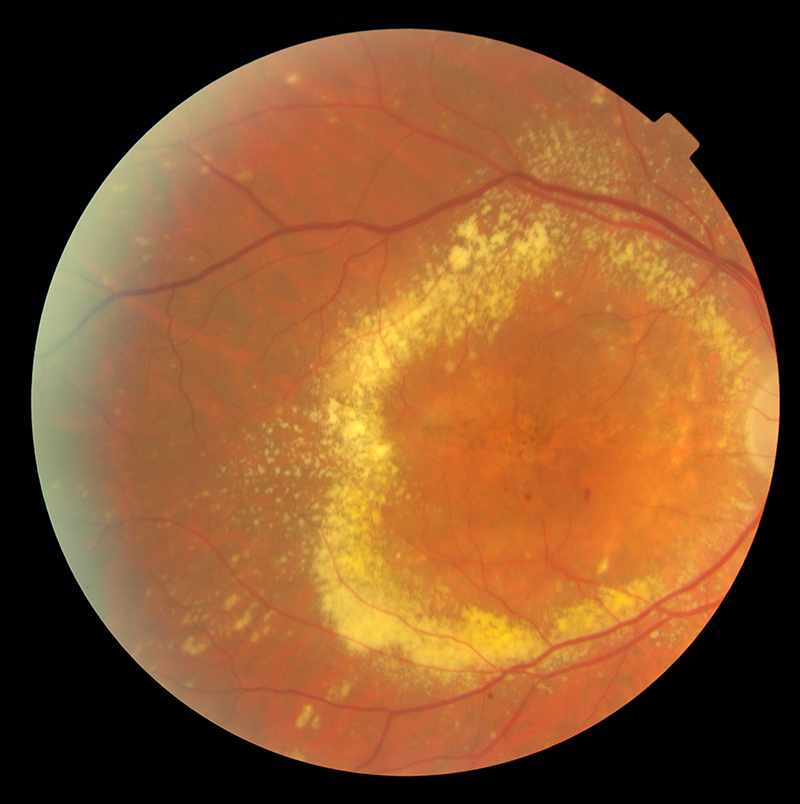
Figure 8: Wet age-related macular degeneration
It has been suggested that some anti-VEGF treatments could increase the risk for stroke; and this possibility could have important therapeutic implications.12 It has been shown that there is higher risk of stroke among persons with a history of stroke or arrhythmias treated with higher ranibizumab doses.13 Therefore, the clinicians should be aware of the above and monitor closely the patients with AMD not only for further loss of visual function but also for risk of stroke.
Diabetic retinopathy
Diabetes mellitus (DM) represents a growing health problem that currently costs the UK £23.7 billion (State of the Nation Statistics 2016). Diabetes doubles the risk of cardiovascular events and stroke when comparing to individuals without DM. Diabetic retinopathy (DR) occurs in 40 % of people with type 1 diabetes and in 20% of people with type 2 diabetes (figure 9). On average 1,280 new cases of blindness caused by diabetic retinopathy are reported each year in England alone, while a further 4,200 people per year are thought to be at risk of DR-related vision loss.14
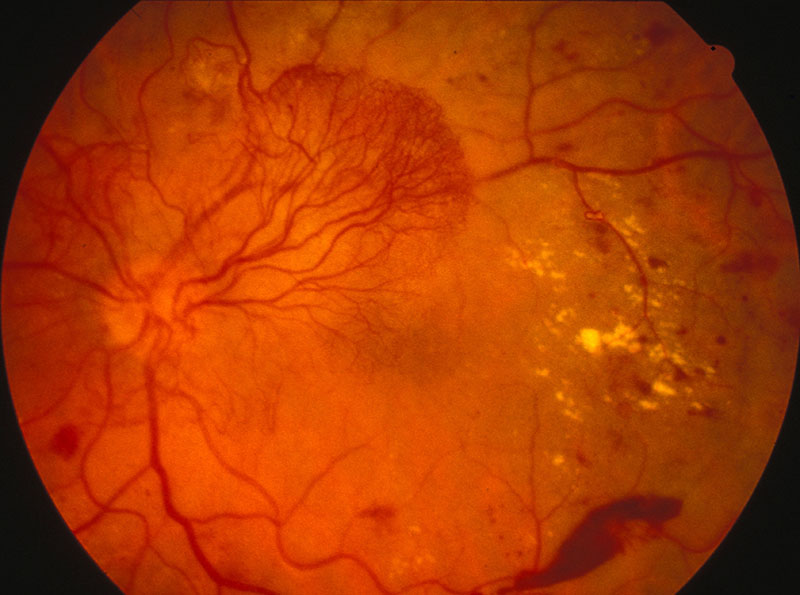
Figure 9: Proliferative diabetic retinopathy
DR increases with the overall duration of DM and poor glycemic control and is associated with increased risk of stroke in both type 1 and 2 diabetes. It has been found that the risk of incident ischemic stroke was two to three times higher in individuals with non-proliferative diabetic retinopathy (NPDR) when comparing individuals without diabetic retinopathy.15 In addition, the risk of the stroke is six times higher in patients with proliferative DR (PDR), and the risk of stroke mortality doubles in these patients when compared to patients without PDR.16
Optometrists play an important role in DR screening. By understanding the link between DM, DR and stroke, optometrists can play an important role in prevention and appropriate referral in cases at high risk.
During stroke
It is possible that a stroke occurs in one of our patients while waiting for or during an eye examination. During a stroke, the patient can exhibit various symptoms, such as weakness or paralysis on one side of the body (face, arm, leg), slurred speech, confusion, loss of balance, tingling, burning or numbness of the skin, headache and vision loss. eFAST helps the optometrist to quickly assess the need for immediate help:
- Eyes: Conjugate ocular deviation towards the affected hemisphere.
- Face: Ask the patient to smile and assess the asymmetry of the face.
- Arms: Ask the patient to raise both arms and observeweakness.
- Speech: Slurring when asked to repeat a simple phrase.
- Time: Call 999 immediately if any of the above occur.
By acting eFAST the optometrists can offer help immediately and contribute to saving the life of the patient. In addition, optometrists should receive formal first aid training, a skill that all health care providers should have.
After stroke
Around 65% of all stroke survivors will display some sort of visual disability. This, in association with other post-stroke sequelae, can have disastrous effects on patients’ independence and mental health. Nevertheless, there is currently no standardised visual screening tool which can accurately assess all potential post-stroke visual impairments.17
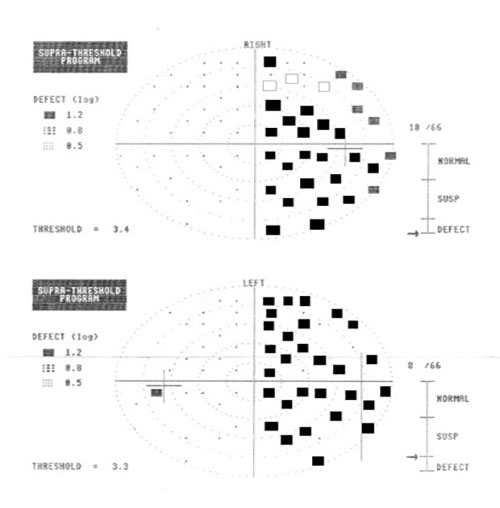
Figure 10: Right homonymous hemianopia field loss due to stroke
After stroke, basic visual processes, such as motility, accommodation, convergence, fusion, stereopsis and vision perception can be profoundly disturbed. The most common visual-related problems encountered after stroke are detailed in table 3.18 These patients should benefit from a careful history, to include previous TIA/TVL or strokes. In addition, the optometrist should note any behaviours, such as head tilt or closing one eye, that point towards a possible ocular problem.19 Visual acuity, complete field analyses (central, peripheral, mono- and binocular), cover tests, pupillary reactions, ocular motility, reading tests, testing for nystagmus, complete fundus evaluation should be part of the examination process in this patients.
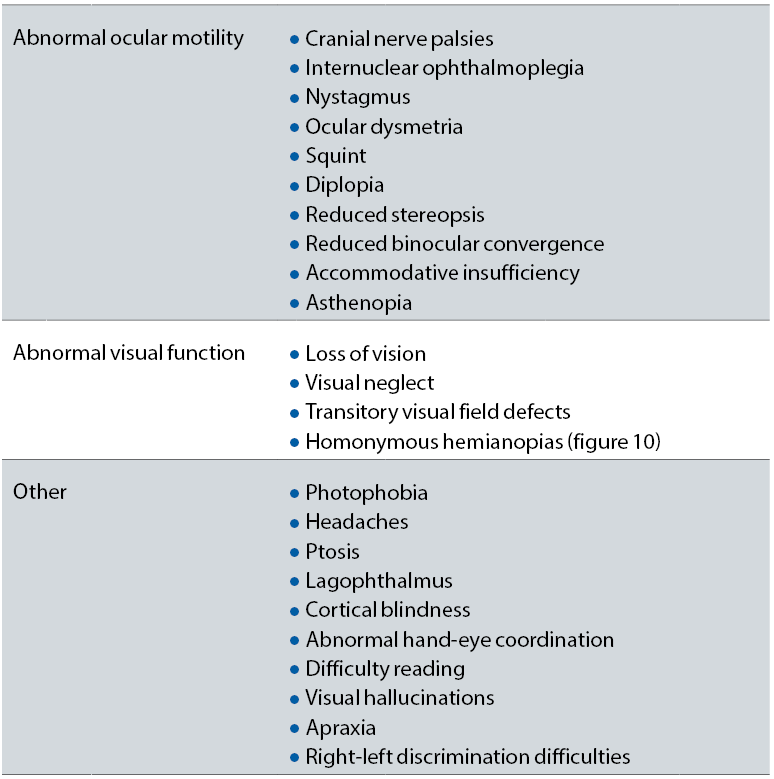
Table 3: Visual features of patients who have suffered from a stroke
From table 3, one very important symptom that is represented is the so-called ‘visual or hemispatial neglect’, a visual attention dysfunction in which the patient is not aware of half of their visual surroundings. This problem can occur with or without a visual field defect. However, even if there is no associated homonymous hemianopia, the patient cannot recognise one side of their field of vision. It is diagnosed by observing the patient behaviour and by using some specialised tests, such as visual and sensory confrontation tests and the line bisection test.20 These patients are more disabled and take longer to recover than the others and, therefore, need special multidisciplinary approach for their management.
One of the most important challenges when examining patients that have suffered a stroke is represented by the inability to communicate efficiently with them. Communication disorders such as aphasia (impaired speech and language understanding), dysarthria (difficulties in talking caused by muscle weaknesses) and speech apraxia (inability of patients to express themselves correctly and consistently) are common after stroke. Added to the motor and other type of impairments, the above changes can result in a major psychological trauma for the patients suffering from this disease. The patients may be irritable, anxious, and frustrated. Therefore, the eye care provider should look at ways to improve comprehension and communication that would finally lead to better management of these individuals. Some useful tips include:
- Always address the patient directly.
- Speak clearly and slowly using few words, which the patient can understand.
- Listen patiently and encourage the patient to speak.
- Do not patronise the patient.
- Use large print.
- Always praise the patient.
Appropriate correction of any refractive error, use of prisms and monocular patching for binocular vision problems and visual training procedures to compensate for disability should be considered in order to visually rehabilitate these patients. Consultation with other health professionals involved in the patient’s management should always be considered a priority.
Dr Doina Gherghel is a lecturer in ophthalmology at the School of Life and Health Sciences, Aston University.
References
- Graybeal LE, Whitaker NA. (2006) Visual problems result from severe stenosis. Review of Optometry. 143;11:1-7.
- Wong TY, Klein R, Sun C, et al. (2006). Age-related macular degeneration and risk for stroke. Ann Intern Med. 145:98-106.
- Nadarajan V, Perry RJ, Johnson J, Werring DJ (2014). Transient ischemic attacks: mimics and chameleons. Pract Neurol 14: 23-31
- Pula JH, Kwan K, Yuen CA, Kattah JC (2016). Update on the evaluation of transient visual loss. Clin Ophthalmol 10: 297-303
- Arthur A, Alexander A, Bal S, Aaron S (2014). Ophthalmic masquarades of the atherosclerotic carotids. Indian J Ophthalmol 62: 472-476
- Lindley RI, Wang JJ, Wong MC, Mitchell P, Liew G, Hand P, Wardlaw J, De Silva DA, Baker M, Rochtchina E, Chen C, Hankey GJ, Chang HM, Fung VS, Gomes L, Wong TY (2009). Retinal microvasculature in acute lacunar stroke: A cross-sectional study. The Lancet. Neurology. ;8:628-634
- McGeechan K, Liew G, Macaskill P, Irwig L, Klein R, Klein BE, Wang JJ, Mitchell P, Vingerling JR, de Jong PT, Witteman JC, Breteler MM, Shaw J, Zimmet P, Wong TY (2009). Prediction of incident stroke events based on retinal vessel caliber: A systematic review and individual-participant meta-analysis. American journal of epidemiology. 170:1323-1332
- Wong TY, Mitchell P (2004). Hypertensive retinopathy. New England J Med 351: 2310-2317
- Hurcomb PG, Wolffsohn JS (2005). The management of systemic hypertension in optometric practice. Ophthalmic Physiol Opt 25: 523-533
- Wong TY, Klein R, Sun C, Mitchell P, Couper DJ, Lai H, Hubbard D, Sharrett AR; Atherosclerosis Risk in Communities Study (2006). Age-related macular degeneration and risk for stroke. Ann Intern Med. ; 145: 98–106
- Wong TY (2010). Age-related macular degeneration. Why should stroke physicians care? Stroke; 41: 575-576
- Wong TY (2009). Age-related macular degeneration and cardiovascular disease in the era of anti-vascular endothelial growth factor therapies. Am J Ophthalmol.; 148: 327–329
- Schmidt-Erfurth U (2010). Clinical safety of ranibizumab in age-related macular degeneration. Expert Opin Drug Saf.; 9: 149–165
- Public Health England (2016). NHS Diabetic Eye Screening Programme: Information for health professionals
- Cooper LS, Wong TY, Klein R, et al (2006). Retinal microvascular abnormalities and MRI-defined subclinical cerebral infarction: The Atherosclerosis Risk in Communities Study. Stroke. 37:82-6.
- Klein R, Klein BE, Moss SE, Cruickshanks KJ (1999). Association of ocular disease and mortality in a diabetic population. Arch Ophthalmol.117:1487-95
- Hanna KL, Hepworth LR, Rowe F (2016). Screening methods for post-stroke visual impairment: a systematic review. Disabil Rehabil 26: 1-13
- Jones SA, Shinton RA. (2006). Improving outcome in stroke patients with visual problems Age and Aging. 35: 560-565
- Cohen AH, Soden R. An optometric approach to the rehabilitation of the stroke patient. Journal of the American Optometric Association 52: 795-800
- Kalra L, Perez I, Gupta S, Wittink M. (1997) . The influence of visual neglect on stroke rehabilitation Stroke. 28: 1386-1391
