In an ideal world the visual axes of our two eyes would always coincide precisely and effortlessly at the object of regard at whatever distance or direction of gaze it happens to be. The astute practitioner will have realised, however, that our world falls a little short of this ideal.
Compensated and uncompensated heterophoria
The majority of us exhibit heterophoria. The visual axes do achieve union, but at the cost of some effort by the fusional reserves. These are elements of convergence and divergence which are in place to maintain binocular single vision and, provided that they achieve this task without undue strain, the heterophoria can be regarded as compensated. A compensated phoria can be defined as one that causes no inconvenience to the patient and requires no intervention by the clinician. It is therefore important that uncompensated phorias be detected and managed reliably in primary care practice.
Tests for decompensation
A number of methods have been employed to test for compensation. Most are now historical, but among the survivors are those listed below. None is entirely reliable in isolation, so if time allows use of more than one test should allow more certain management.
Near point of convergence
Observation of the patient converging on to a target (RAF rule – figure 1, budgie stick target or the point of a writing implement) can give valuable insight, as those with defective convergence tend to struggle not only at the near point but over a greater range of fixation distances, so the ease and smoothness of the movements is reduced and fatigue sets in quickly on repetition. ‘Jump’ convergence, where the patient jumps from distant to near targets, is even more demanding. Some patients who struggle with either convergence test will close one eye or adjust their head to increase the fixation distance, so careful observation may pay dividends.
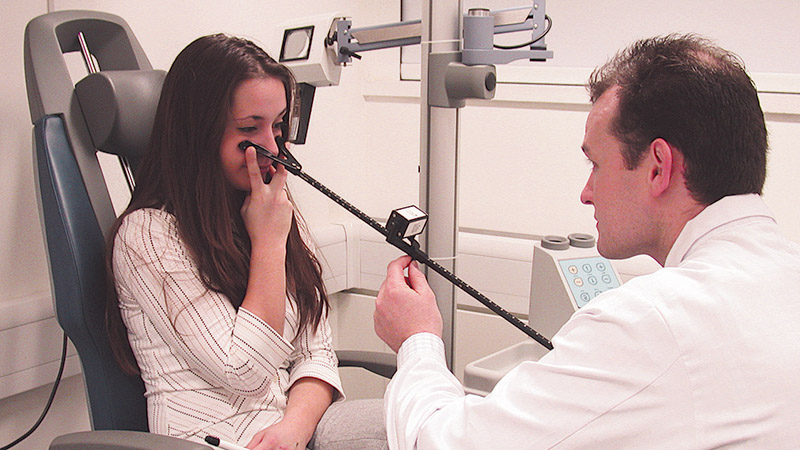
Figure 1 Near point of convergence measured by an RAF rule
Recovery movement on cover test
The speed and quality of the recovery movement made by the non-fixing eye when the cover is removed can be assessed by the observer (figure 2). This is quite popular as it is quick and needs very little equipment. A jerky or sluggish movement can be abetted by the addition of small degrees of prism until it improves. The recovery movement is much easier to observe if there is a large phoria to recover from, but not all uncompensated phorias are large, so reliance on this method alone is not recommended.

Figure 2: The nature of the recovery movement on cover test for a phoria indicates levels of compensation
Measurement of fusional reserves
This can be done in several ways. Usually only the reserve opposing the phoria is measured and if the former is two or three times the magnitude of the latter (depending whose criterion you adopt) all is thought to be well. Alternatively, the reserves in both directions can be measured (the ‘middle third’ method) but this takes longer as 30-60 minutes recovery time needs to be allowed between the two. Various graphical methods, some quite complex, have been used and tend to be popular on the other side of the Atlantic, where there may be fewer time constraints. Apart from the time involved, the readings taken will vary with the instrumentation used, the target, the speed at which the test is applied, and the subjective response of the patient. All of these can be allowed for, but the major limitation of these techniques is the time they take to perform and analyse.
Dynamic retinoscopy
This is a technique more talked about than actually performed in primary practice these days. In experienced hands, it can be very useful but experience has to be gained and the skill element is fairly high. Occasional employment of this technique is of limited value.
Fixation disparity tests
What is required in everyday practice is a method which can detect an uncompensated phoria quickly. For that reason, the methods most frequently employed by primary care optometrists are near point of convergence, the cover test recovery movement and the Mallett unit or similar fixation disparity tests.
Theoretical basis of fixation disparity tests
Each point on the retina of one eye corresponds to an area on the retina of the other eye, rather than a single point. These areas are named Panum’s fusional areas after their discoverer and are believed to be cortical in origin. They are oval, with the longer axis horizontal and subtending around five minutes of arc in the central area (possibly even less for the very central foveal elements), becoming progressively larger in more peripheral areas where they may subtend several degrees of arc due to decreasing cone density.
The spatial counterpart, Panum’s fusional space, is contained between two curved surfaces, either side of the horopter. Objects within Panum’s fusional space are seen singly, though depth perception is possible due to the slight angular disparity between the retinal elements which make up Panum’s areas. Objects lying outside Panum’s fusional space appear in physiological diplopia.
When the fusional reserves are struggling to overcome the phoria, Panum’s areas may be used to maintain single vision by allowing a slight misalignment of the visual axes to occur without diplopia (figure 3). Typically, this will be around the five minutes of arc associated with the central Panum’s areas regardless of the actual size of the uncompensated phoria, as the larger more peripheral Panum’s areas cannot be utilised for this unless the more central ones are suppressed. The extent of deviation from aligned fixation without diplopia is termed the fixation disparity.
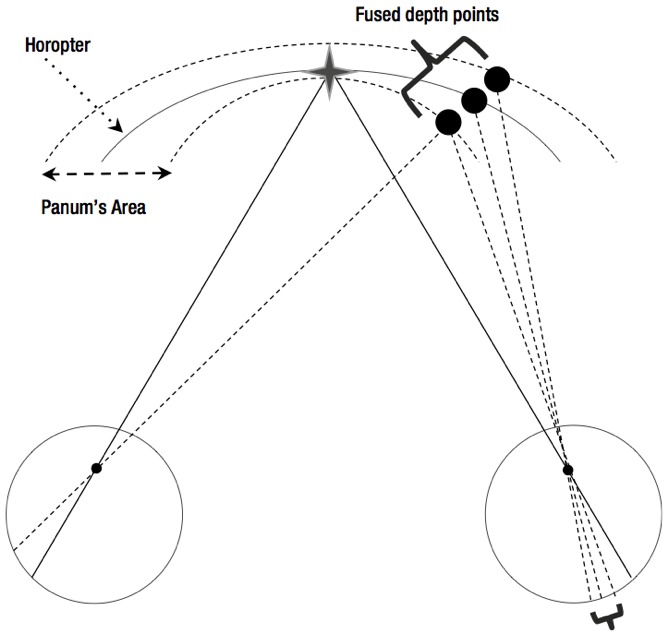
Figure 3: Objects lying outside Panum’s fusional space appear in physiological diplopia. Panum’s areas may be used to maintain single vision by allowing a slight misalignment of the visual axes to occur without diplopia
Though measurement of the fixation disparity (or ‘retinal slip’) itself is possible in a lab setting, this is not done in the so-called fixation disparity tests commonly employed clinically. The ubiquitous Mallet unit (figure 4) and its relatives (figure 5) all measure how much assistance the fusional reserves need to eliminate the fixation disparity under binocular conditions. This assistance may be in the form of a spherical lens (the ‘relieving sphere’) or a prism (‘relieving prism’ or ‘associated phoria’) which reduces the phoria to a size which can be managed by the fusional reserve.
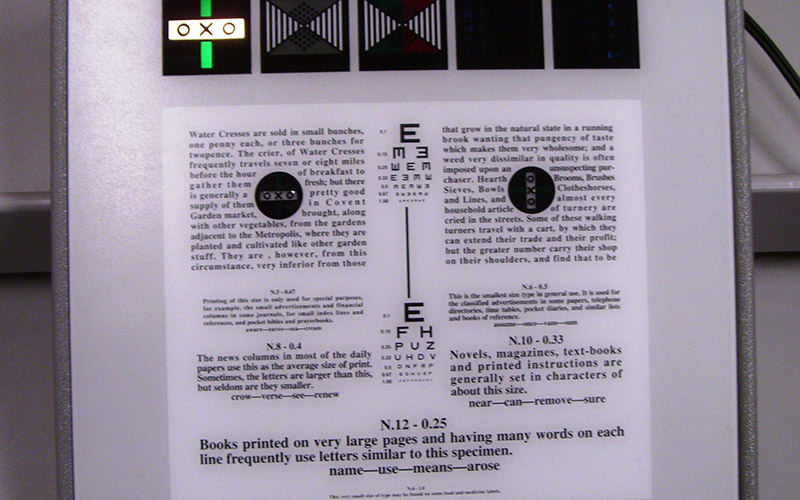
Figure 4: The Mallett unit
Nature of fixation disparity tests
The requirements for such a test include a binocular lock or locks which can be seen by both eyes, and markers that indicate the position of the individual eyes, so are seen uniocularly. The latter need to be visible enough for the patient to report their position accurately, but unobtrusive enough to give rise to minimal interference with fusion. The size and position of the binocular locks will influence the results obtained, and Mallett1 describes how the design was effectively tuned until good correlation with symptoms was obtained (with adult symptomatic phoria patients).

Figure 5: The EyeGenius system
The principles of all fixation disparity techniques are similar. The patient views the target binocularly under conditions as close to those associated with the symptoms being investigated as possible. The monocular markers, which in the case of the Mallet unit are coloured polarised strips, are aligned either with prism or spherical lenses and the minimum power needed to align the strips is recorded.
Clinical application
What could possibly go wrong?
From my own observations as an optometrist and as a College assessor and examiner, quite a lot. For a start, there are lots of dysfunctional Mallet units sitting forlornly in consulting rooms and that cupboard where things go to be forgotten. Rechargeable ones that will not recharge, because they need new batteries, or bulbs that have blown and never been replaced are commonplace. Then there are the polarised visors which allow the marker strips to be seen monocularly. At least, that is, if the direction of polarisation of the visor matches that of the unit. Some units have their nonius markers polarised at 90° and 180°, some at 45° and 135°, and this also applies to the visors. These are eventually replaced over the course of the unit’s working life, often with the wrong one because the person ordering the replacement may not be aware that there is a right one.
Unless absolutely certain, it is always best for the practitioner to test out themselves before using a unit (and not the patient during the test as it will dissociate them) to ensure that each eye can see only one marker. If this is not the case, the incidence of uncompensated phorias among your patient base may seem rather low.
Which patients should you check fixation disparity on?
Many maintain that only the symptomatic patient is worth testing, but there is a case for collecting background data on asymptomatic patients too if time permits. Fixation disparity units do give false positives on some patients, especially with distance fixation, and it is worth knowing this before the patient presents with symptoms. Also, many a patient may be symptomatic but not report this as they feel the symptom to be a normal everyday occurrence, such as fatigue or tiredness.
At what point in the routine should it appear?
Since it is a test which has been designed to be performed with the eyes fully associated it should ideally precede any test that dissociates the eyes, though in many routines observed it is tacked on after the cover test or other dissociation tests, not to mention retinoscopy and an entire monocular refraction. Possibly fundoscopy. Did I mention that false positives occur?
Points on technique
- Binocular vision should be stabilised, by reading a line of letters or words before the assessment of fixation disparity. This is particularly important if the patient has been dissociated recently, and as discussed, they probably have.
- It should be remembered that the polarised visor used significantly reduces light levels and ideally illumination should be adjusted accordingly. This is easier to arrange when testing at near, but rarely done.
- The horizontal slip is usually done first from habit, and Mallett described this in his original papers. But sometimes a vertical imbalance can destabilise the horizontal ocular motor balance and correcting it first may reduce the horizontal prism relief needed. So it is best to keep an open mind.
- Before applying the visor, the patient should be asked ‘Are the two red (or green) lines exactly in line with each other or are they out of line?’ It is surprising how often a patient will report displacement of the lines even without the visor. This is worth knowing as you may be making prescribing decisions based on this patient’s observations.
- Having established that the patient is not likely to lead you astray, repeat the previous question with the visor in place. Leading questions should be avoided, especially when dealing with children. There is evidence that the way the instructions are phrased can influence the results even in adults.2 If the patient reports that the lines are out of alignment ask the patient ‘Which one is out of line with the X ?’ You should know before you start the test which line is seen by which eye. If you cannot remember if from last time, it is simply a matter of looking at the target through the visor yourself. Under no circumstance cover up one of the patient’s eyes and say ‘which line can you see now?’ This is called dissociation. Then ask ‘Is it to the left or to the right?’ or ‘Is it towards me or away from me?’ With children (and indeed some adults), the latter question may be more sensible, as they do tend to mix up their lefts and rights on occasion. The minimum prism or modification to the spherical element of the Rx which aligns the polarised lines should be noted (figure 6).
- When assessing fixation disparity for near, we do not need to be restricted to one target position, as the unit is very portable. Many patients read or look at paperwork and display screens in a variety of positions. Lots of them have ‘A’ and ‘V’ patterns and small degrees of incomitancy are not rare. It does not take long to check if the patient is still compensated when the eyes are elevated or depressed, or in any other relevant position that a careful history might suggest.
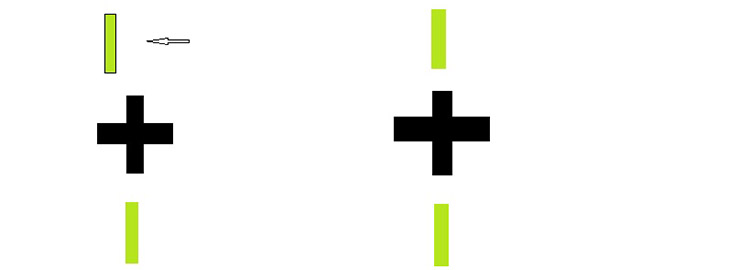
Figure 6: Slip of a monocular nonius line is indicative of decompensation, left, while aligned suggests compensation, right, where a phoria exists. The central cross is the binocular lock
Interpretation
Until you have done a cover test, you will not necessarily know what the fixation disparity findings mean. No slip found on a patient with a phoria would indicate that the phoria is compensated, at least for the moment, but the same finding on a strabismic subject would indicate harmonious anomalous retinal correspondence. Small degrees of slip usually point towards an uncompensated phoria, especially if there are symptoms, but a larger slip may be associated with microtropia. Sometimes the slip seems to vary at random, and the markers may oscillate across the neutral point. This is indicative of binocular instability. The condition is associated with uncompensated heterophoria and the fusional reserves tend to be low. In general, if you keep adding small amounts of prism or sphere the slip will stabilise, and the patient will be happier.
When to prescribe
False positives (did I mention false positives?) and negatives do happen with the Mallett unit. False positives, as discussed earlier, are sometimes associated with imprecise methodology which creates dissociation, or by over-enthusiastic subjective responses, because the patient is eager to please and the instructions given not completely neutral. False negatives may occur because the test is not being carried out under the same conditions that give rise to the patient’s symptoms. The magnitude of the phoria may vary with fixation distance and direction of gaze. The opposing fusional reserve will be diminished by fatigue, so the time of day that the patient is seen may also be a factor.
In general, it is not worth prescribing to a patient who is asymptomatic, but we need to be aware what could qualify as a ‘symptom’ in this context. It could include:
- The classic asthenopic symptoms (headache, ‘eyestrain’ , tiredness). These are more likely in adults and older children who are also more likely to persist with difficult tasks.
- Poor concentration or attention span, especially in the younger patient, who may not persist in a task which is not easy to perform. School performance may be affected.
- Intermittent diplopia is a clear giveaway.
- Closure of one eye to prevent any diplopia is another.
- Intermittent blurring.
- Spasm of the near reflex.
- Reduced stereopsis and low-grade suppression.
- Slow acquisition of reading skills. Any child suspected of dyslexia should be thoroughly examined for binocular anomalies before any other assumptions are made.
What to prescribe
We have two choices:
- Improving the fusional reserves by exercise.
- Reducing the offending phoria by sphere manipulation or prisms.
Assuming that the orthoptic exercises are either rejected by the patient or not carried through (which sadly is common), we need to prescribe something to relieve the symptoms. Having measured our relieving sphere or relieving prism (‘associated phoria’), what are we going to do with it? In the case of a relieving sphere, the general consensus is to prescribe it if it does not interfere with vision unduly, and the same is true of relieving prism for vertical elements. Prescribe what the Mallett unit tells you to. Even cyclodeviations (figure 7) are fairly straightforward. If there is a decent sized cylinder in one or both eyes, fiddle with the axis until the bars straighten out, and if that means going back to the axis they came in with before you changed it, do not be surprised.

Figure 7: Indication of a cyclodeviation
If only horizontal prism was so simple. For many, including the author, it mostly is. Prescribing the full relieving prism seems the obvious thing to do because it is likely that the symptoms will be relieved. This is on the grounds that the unit was designed and tuned that way by Mallett. However, there are other schools of thought. One advocates routinely prescribing around half of the measured relieving prism and, to be fair, there are some of the larger exophores who seem to get a little greedy for base in, at least initially until fusion stabilises. Jennings3 has described a time-honoured method of routinely prescribing 2∆ base in each eye for symptomatic exophoria which, while of dubious scientific provenance, is successful enough to have become a tradition of sorts. The disparity of these prescribing methods, and their persistence, suggests that we do not need to get too precious about prescribing prism. It mostly works, and is probably going to be better than not prescribing it in a symptomatic patient.
Fear of over-prescribing prism, and the patient soaking up progressively larger prismatic corrections is widespread but, in the authors experience, illusory. In normal asymptomatic patients, one can induce an associated phoria with an adverse prism and this will halve within around 3.5 minutes provided the opposing fusional reserve is robust.4,5 However, this does not usually happen in symptomatic patients and this lack of prism adaptation can itself be a powerful (and quite quick) indicator of decompensation due to inadequate fusional reserves. It is also rather useful in that, for example, if more base in prism is required to compensate the phoria at near than at distance, a slight over-correction in the distance will almost certainly be adapted to by the patient. It will be their more robust divergent fusional reserve having to adapt rather than the enfeebled convergent one. Furthermore, it will only take a few minutes to find out.
If routinely prescribing a reduced relieving prism did not work at all, it would probably have died of natural causes long ago, but there is the danger that we could be converting an uncompensated phoria into, um, another uncompensated phoria. In this case, assuming the patient returns, we will have to increase the prism so that eventually we reach the level that will relieve the symptoms properly. To the timid prescriber, this could look rather like the patient soaking up the prism, whereas in fact it is just the opposite.
Finally, should we prescribe the prism unilaterally or split it equally between the eyes? Mallett advocated prescribing according to what was found by the test and in general that seems to work well for the small degrees of prism we usually need. However, there comes a point where loading all of the prism into one spectacle lens starts to give rise to cosmetic issues so for larger prismatic corrections splitting is widely done and rarely if ever seems to cause a problem.
Andrew Franklin is an optometrist and college assessor and examiner with a special interest in binocular vision.
References
1 Mallett RFJ (1964) The investigation of heterophoria at near and a new fixation disparity technique. Optician December 1964:4-5.
2 Karania R, Evans BJW. The Mallett Fixation Disparity Test: influence of test instructions and relationship with symptoms Ophthal. Physiol. Opt. 2006 26: 507-522.
3 Jennings A (2001) Anomalies of Convergence in Evans BJW, Doshi S. Binocular Vision and Orthoptics :37 Butterworth Heinmann, Oxford.
4 North R, Henson DB (1981) Adaption to prism induced heterophoria in subjects with abnormal binocular vision or asthenopia. Am. J. Otom. and Physiol. Optics. 58(a):746-52.
5 North R, Sethi B, Owen K. (1990)Prism Adaptation and viewing distance. Ophthalmic and Physiological Optics 10
