It is an emerging feature of healthcare that there is increased focus on identification of elements associated with patient care such as the use of medical equipment/devices and drugs. When such systems are ‘smart’, a mechanism is provided to safeguard against, for example, administering a drug to the wrong person, or administering the wrong dosage or using the wrong surgical implant. This is achieved by checking the identification of the patient and the identification of the items associated with the patient intervention. Also, in situations of recall of medical devices or drugs, the use of procurement and stock management systems which employ efficient and accurate identification will improve patient safety. This approach of implementing systems with an awareness of a ‘get it right’ philosophy has arisen out of an often painful experience of processes which can fail in patient care.
Systems, for example, for the delivery of prescription medication within hospital acute condition departments have been identified as ones which would significantly benefit from such an approach, and currently in the NHS around 10% of all adverse patient incident reports involve medication errors.1 This approach of appropriate identification and recording of ‘elements’ of patient care is, however, one which is likely to see progressive application throughout the patient journey. It may be necessary, however, to introduce obligatory systems of safety, since initiatives to implement ‘best practice’ tend to become ‘lost in transition’.
GS1 and GTIN
Such initiatives, to achieve implementation of safer identification of devices/drugs on a global scale, require international standards to be developed, accepted and maintained. A key initiative is that of the GS1 identification protocol which provides a framework within which various levels of identification have been defined. The GS1 protocol is, in fact, a global standard for element identification across all industry sectors, and where the implementations in healthcare mirror implementations within other commercial sectors. Within the GS1 system of standards, the Global Trade Item Number (GTIN) facilitates a global supply chain solution which makes possible the unique identification of any trade item that may be priced, ordered or invoiced at any point in the supply chain (figure 1).

Figure 1: GS1 identification protocol ensures accurate identification throughout the global supply chain
The framework of the Global Trade Item Number (GTIN) management standard allows commerce to make consistent decisions relating to the unique identification of goods, for example to identify if new GTIN numbers are required in the event of modification of contents of a specific product. At a trading level, in the context of logistics of transportation of goods across international borders, the use of the GTIN system improves the efficiency of movement of such goods and ultimately improves the safety of trade. A specific GS1 system has been specifically identified for healthcare.2
UDI
For medical devices, the UDI (unique device identification) is defined as the combination of DI (device identification) and PI (process identification). The DI is a mandatory, fixed element of the UDI which identifies the labeller of the device and the specific version of model of a device. The PI contains specific additional information relating to the production of the item and can include the lot or batch number within which a device was manufactured, the serial number of a specific device, the expiration date of a specific device and the date upon which a specific device was manufactured. In the United States, the DI component is allocated by specific licenced agencies such as GS1, HIBCC and ICCBBA.
GMDN Classification System: Medical Devices
With the implementation of the Medical Device Directive within the European Community, it was identified that an appropriate nomenclature system was required for Medical Devices within a framework that would emerge as ISO 15225.3 With financial support from the European Commission, the GMDN classification system was established to create this global resource framework.4 The GMDN classification system was based on six previously existing classification systems for medical devices, and which included system developed by the FDA (CNMD), the European Diagnostic Manufacturers Association (EDMA) and the Japanese system (JFMDA). Initially this set of GMDN classification consisted of around 13,500 unique entries. The first version of the GMDN nomenclature was issued as a CEN Report CR 14230 in 2001. The GMDN system is structured and maintained/administered by the GMDN Agency where the set of GMDN definitions are currently used by over 70 national regulatory agencies.

Table 1: Key fields of GMDN entry
The way in which the GMDN codes are structured allows the creation of a library of generic terms with typically less than 25,000 entries (see table 1). These generic terms can in turn be linked to the much larger set of manufacturers’ products each with its own UDI definitions (see table 2) – currently estimated to be in excess of 5,000,000. The link between the two is expected to be recorded in a relational database.
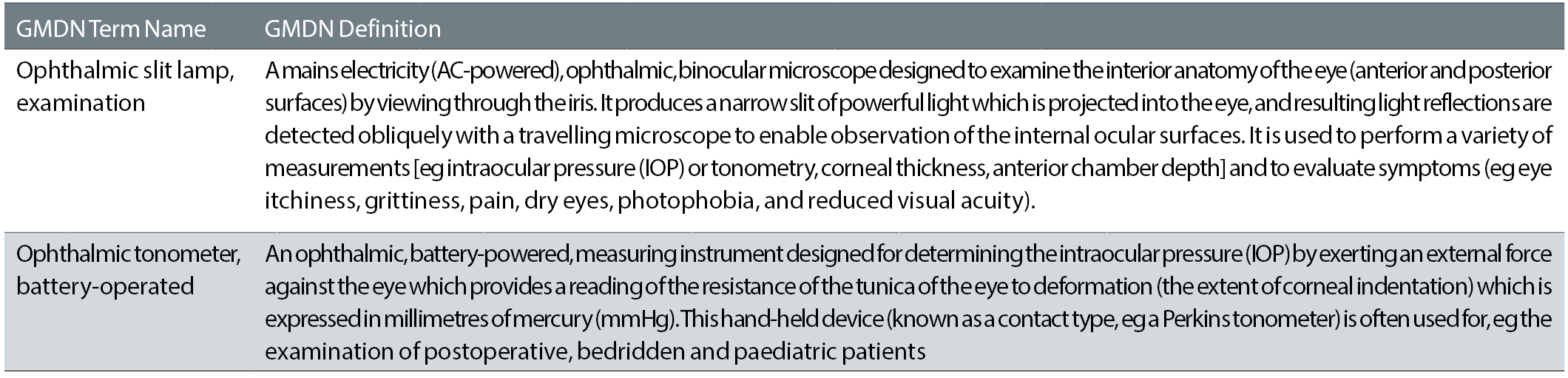
Table 2: Examples of GMDN fields from extract of GUDID data base
(GMDN Term Names and Definitions: Copyright GMDN Agency 2005-2017. Reproduced with permission from the GMDN Agency)
The GMDN codes provide therefore an authoritative source of medical device descriptions/definitions which can act to improve the flow of information between manufacturers, regulators and healthcare authorities and hence improve post market vigilance of medical devices. In addition, the use of such codes can assist in support of inventory control in hospitals and also purchasing and supply chain management.
GUDID and EUDAMED Data Bases
The GUDID data base has been established in the US as an information resource which lists specific classes of medical devices and which is available to the public for read only access (https://accessgudid.nlm.nih.gov). It is a requirement that manufacturers selling medical devices into the US register their products appropriately within GUDID. The regulatory framework outlined by the FDA within its structure is highly complex.5
The equivalent data base within the European Commission is EUDAMED, which is scheduled to go live in an expanded access format by 2020 and will have some function which overlaps with GUDID. Key parts of the revised Medical Device Directive in the EC, however, depend on a timely implementation of EUDAMED. It is with the development and access provision to data bases such as GUDID and EUDAMED that the value of the investment in device identification systems becomes evident.
The Falsified Medicines Directive
(Directive 2011/62/EU)
There is increasing anxiety relating to the growth of ‘fake’ drugs within healthcare.6-8 The Falsified Medicines Directive is designed to tackle issues of ‘fake’ drugs within the European Community but also provides an additional means of verifying the identity of the specific drug within a healthcare facility. Initial details of the UK implementation of the Directive were published in 2013.9 Specific items of information scheduled to be included in a 2D bar code on packaging include a product code which allows the identification of at least the name of the medicine, the common name, the pharmaceutical form, the strength, the pack size, and the pack type, a randomly generated serial number which is a numeric or alphanumeric sequence of a maximum of 20 characters, a batch number and an expiry date. The plan has been scheduled for implementation in the UK by 2019.10 There are perhaps some unanswered questions about how exactly the system will identify ‘fake’ drugs. Will, for example, users be able to interrogate a manufacturer’s data base of ‘valid’ batch and serial numbers for a specific drug and identify if a specific batch/serial number is valid?
Scan4Safety
Within the NHS in England, the Scan4Safety initiative is designed to promote safety in administration, for example, of correct medication or use of implanted medical devices where identification of such elements is verified against planned patient requirements. This allows errors to be detected prior to delivery/administration to the patient and the data trail also allows recall of specific implants to be identified for possible revision (figures 2 and 3). Such an initiative is also identified to save time in processing of manual records.

Figure 2: Patient identification is essential in the secondary care setting
Within ophthalmic practice, there are areas of practice that can benefit from improved process identification/definition. Kelly and Jalil,11 for example, reviewed data within the patient reporting data base for incidents of wrong intraocular lens implant. In a period between 2003 and 2010, a total of 164 reports were identified, though causal effects were only able to be identified for a subset of these cases and which included inaccurate biometry (n=29), wrong IOL selection (n=21), transcription errors (n=10) and handwriting misinterpretations (n=7). In this situation, some level of verification of lens implant would be possible by automatic comparison of digital biometry result and scanned details of proposed IOL implant, though this approach would not remedy other identified system errors. Neilly et al,12 report on similar data from within the Veterans Health Administration in the USA on wrong intraocular lens (IOL) implant. Results indicated that improved scrutiny of biometry data with choice of IOL would reduce errors in selected implants, though the use of ‘reconciliation’ of data between biometry data and IOL implant pack is not specifically referenced.

Figure 3: Establishing a data trail assist in ensuring appropriate care
Wrong Site Surgery
The prevention of wrong site surgery is a continuing theme within all divisions of surgical practice, and a specific focus on such a ‘never event’ in ophthalmic surgery is provided by Fraser and Adams13 where an extensive (but not exhaustive) set of contributory factors is identified. Reference is also made to the worst case of wrong site surgery in ophthalmology ¬ namely removal of the wrong eye.14 Many of the factors indicated by Fraser and Adams13 are related to human factors, where the wrong information can be used or the correct information is misinterpreted. Fraser and Adams also describe a range of approaches based on checking details from a range of viewpoints and also providing equal weighting of opinion to all within the surgical environment. The authors make some reference to systems to match up computer based patient record details with computer read identification of the patient on the operating table.15 With the increasing implementation of electronic patient record systems, this will more and more be seen as the most reliable method of verification of surgical site, though other checks and balances will still probably be required.
Mak16 outlines ‘sentinel events’ within ophthalmology in Hong Kong between 2007 and 2014, including ‘wrong eye’ surgery (five cases: 41%) and ‘wrong patient and surgery’ (two cases: 17%). The author indicates that the remedy for such incidents is to undertake proper time-out procedures to check patient details, but makes no reference to verification of patient details through EPR systems.
Tracking CJD
Most Sterile Service departments in NHS hospitals already operate a tracking systems where individual instruments in procedure trays are laser marked with unique identification codes, to provide the required degree of traceability within instrument trays, for example to track patients subsequently identified with Creutzfeldt-Jakob disease.17
Applications of UDI: Product Recall Management
The Healthcare User Group of GS1 UK has recently published 18 an outline of the framework of use of Unique Device Identification in the management/utilisation of medical devices and where potential benefits are identified in processes of incident reporting, management of product recalls, reduction of clinical errors, improved vigilance related to counterfeit devices and improved effectiveness of purchasing and stock management within hospitals.
In recommendations of the Healthcare User Group, an outline is provided of a specific format of information to be included within two separate EXCEL spreadsheets. The first spreadsheet contains core details of the manufacturer and the text of the field safety notice. The second spreadsheet contains details relative to the specific products identified with inclusion of fields such as the device identifiers (UDI + DI) of products affected, manufacturer’s catalogue number, batch/lot number, serial number, use by date and date of manufacture. The principle within the scheme would be to include a separate line for each unique batch number or appropriate serial number.
The utilisation of this information by the healthcare provider is described essentially as an automated process where field safety notices are checked against purchasing/stock management systems and any implanted devices are similarly checked against inventories of such items. This level of data integration, however, represents potentially significant investment in relevant information systems, but one which should have been on the development horizon of such systems for some time.
The potential open availability of such field safety notice data as essentially digital information gives rise to an altered perspective of the management of medical devices. In the supply chain, for example, automatic distribution of field safety notices to stock holders/distributers could be more effective in prevention of suspect product reaching healthcare providers. Within a healthcare provider, the status of items could be checked as it was scanned and passed into stock and also with the option of verifying devices at the point of use.
Bar Code Data
Two modes of two dimensional bar codes are potentially available for use within healthcare trade items and are known as ‘GS1 DataMatrix’ and ‘GS1 QR Code’. GS1 Uk is keen to indicate that ‘GS1 DataMatrix’19 has already been specified for pharmaceutical products by regulators in countries such as Argentina, Egypt, France, India, Jordan, Korea, Saudi Arabia, Turkey and the US. In addition, the ‘GS1 DataMatrix’ standard is indicated as part of the European Commission’s regulation on medicines verification. It is recommended that packaging should contain a single version of bar code in order to prevent errors in reading diverse codes and also reduce complexity/cost of printing/decoding such code patterns. Also, use of multiple bar code elements takes up valuable space within packaging systems. The data structures of both two dimensional coding systems are identified as having equivalent degrees of ‘error detection’ and ‘error correction’ within their algorithmic structures.
One key feature of the ‘GS1 DataMatrix’ element is the ‘finder pattern’ which defines the orientation of patterns and which is chiefly identified as an ‘L’ shaped feature of dark elements on the edge of the array element. In the remaining sides, the finder pattern includes alternating light and dark elements, so that if the dark ‘L’ shaped pattern is on left and lower sides, the top right element is a ‘light’ square. With such landmarks identified, the various array elements can be read as the data decoded. While ‘square’ elements are more effective for storing data, some print technologies may favour the use of ‘rectangular’ elements where labels are printed at high speed.
In one specific example of a code, a square pattern with a 26x26 pattern array, will have a 24x24 array which contains actual data and which can code numeric 72 codewords (or bytes) which are represented as 44 as actual data and 28 included for error correction. In contrast, while standard linear bar codes include a numeric check digit, they have no error correction facility. Spare characters are identified within a configuration by the character with ASCII value 129. It is apparent, therefore, that a high degree of uniformity of software is required within all ‘GS1 DataMatrix’ software applications to ensure, for example, that whatever is printed anywhere can be read anywhere. Smartphone apps are now available to read GS1 DataMatrix codes and where a demo package can be downloaded from Google Play under title of ‘Healthcare Demo Scanner’.
As data is written into the data matrix, elements of data are identified with tags for ‘Application Identifiers’ which are two to four digit numbers typically enclosed within parenthesis. Table 3 indicates typical identifies utilised. This encoding technique is the same one used with GS1-128 linear barcode.
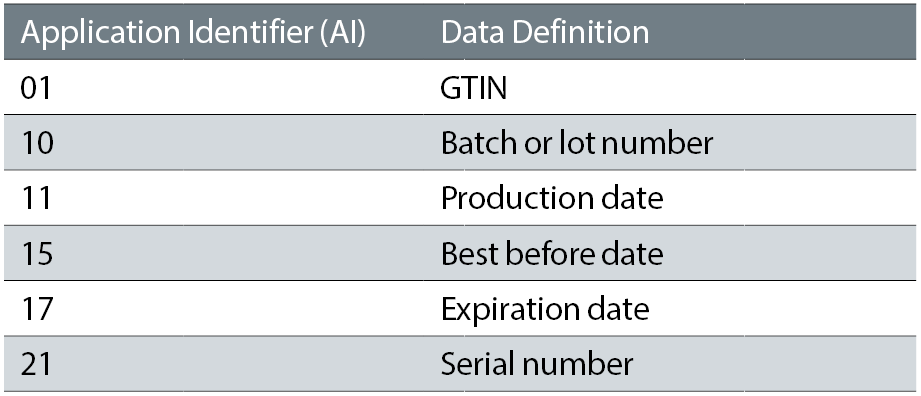
Table 3: Typical application identifier codes
Discussion
The current momentum to move increasing elements of patient information into ‘electronic patient record’ (EPR) systems is designed to provide a safer and more efficient systems of patient care where elements of data are moved from a range of discrete systems into a more inclusive patient record. What then becomes the challenge is to ensure the accuracy of specific entries within such EPR systems to ensure safe patient transactions.
Dr Douglas Clarkson is development and quality manager at the department of clinical physics and bio-engineering, Coventry and Warwickshire University Hospital Trust
References
- Cousins DH, Gerrett D, Warner B. A review of medication incidents reported to the National Reporting and Learning System in England and Wales over six years (2005-10). British Journal of Clinical Pharmacology. 2012;74(4):597-604
- GS1, 2015, GS1 Healthcare GTIN Allocation Rules:GTIN Allocation Rules for the Healthcare Sector: Release 9.0.2, Ratified, Dec 2015. Available at www.gs1.org/docs/gsmp/healthcare/GS1_Healthcare_GTIN_Allocation_Rules.pdf Accessed 05.06.2017
- International Organisation for Standardisation, 2016, ISO 15225:2016, Medical devices ¬ Quality management ¬ Medical device nomenclature data structure
- GMDN Agency, www.gmdnagency.org
- Food and Drug Administration, HHS, Unique device identification system. Final rule, Fed Regist. 2013; 24:78(185):58785-828
- Newton PN, White NJ, Rozendaal JA, Green MD. Murder by fake drugs. BMJ 2002; 324: 800-1
- Johnston A, Holt DW. Substandard drugs: a potential crisis for public health. Br J Clin Pharmacol. 2014;78(2):218-43
- Wang , Yu S, Liu K, Chen FE, Song Z, Zhang X, Xu X, Sun X. Acute intraocular inflammation caused by endotoxin after intravitreal injection of counterfeit bevacizumab in Shanghai, China. Ophthalmology. 2013;120(2):355-61
- The Human Medicines (Amendment) (No. 2) Regulations for the Pharmacovigilance Directive 2012/62/EU, Her Majesty's Stationery Office (HMSO); 08/10/13
- MHRA, Medicines: packaging, labelling and patient information leaflets, Available at https://www.gov.uk/guidance/medicines-packaging-labelling-and-patient-information-leaflets Accessed 06.06.17
- Kelly SP1, Jalil A. Wrong intraocular lens implant; learning from reported patient safety incidents. Eye (Lond). 2011;25(6):730-4
- Neily J, Chomsky A, Orcutt J, Paull DE, Mills PD, Gilbert C, Hemphill RR, Gunnar W. Examining wrong eye implant adverse events in the veterans health administration with a focus on prevention: a preliminary report, J Pat. Saf. 2015 Mar
- Fraser SG, Adams W. Wrong site surgery. The British Journal of Ophthalmology. 2006;90(7):814-816
- Traquair HM.Removal of the wrong eye. Br J Ophthalmol. 1947;31(1):8-12
- Nichols JH, Bartholomew C, Brunton M, Cintron C, Elliott S, McGirr J, Morsi D, Scott S, Seipel J, Sinha D. Reducing medical errors through barcoding at the point of care. Clin Leadersh Manag Rev. 2004;18(6):328-34
- Mak ST, Sentinel events in ophthalmology: experience from Hong Kong, J Ophthalmol. 2015;2015:454096. doi: 10.1155/2015/454096. Epub 2015 Mar 2.
- Nice, 2006, procedure guidance 196, Creutzfeldt–Jakob disease: reducing the risk of transmission by surgical instruments, London, 2006
- GS1 UK, 2017, Recommendations on Medical Device and IVD Field Safety Corrective Actions and Recalls using Unique Device Identifiers & GS1 Standards, www.gs1uk.org/~/media/documents/marketing-documents/gs1_uk_recommendations_on_medical_device_ivd_field_safety_corrective_actions.pdf?la=en Accessed 06.06.2017
- GS1 UK, 2016, GS1 DataMatrix Guideline Overview and technical introduction to the use of GS1 DataMatrix Release 2.3, Ratified, May 2016, www.gs1.org/docs/barcodes/GS1_DataMatrix_Guideline.pdf Accessed 06.06.2017
