As has been previously outlined in parts one to five of this series, dry eye disease (DED) is a common but complex, multifactorial spectrum of disorders. DED can cause symptoms from mild irritation to severely debilitating reduction in quality of life which can lead to depression. While it can be sub-divided in a number of ways, traditionally it is divided into reduction in tear quality in the form of evaporative dry eye (EDE) or reduction in tear volume in the form of aqueous deficiency dry eye (ADDE). However, due to the complexity of the condition, presenting patients should be examined in a systematic manner to elicit an accurate diagnosis and rule out comorbidity. The optimal order of examinations has been delineated previously in this series.
This article will highlight examinations which may be useful in diagnosing ADDE, and offers a case history of one such patient and how we managed her case.
Examination Techniques – Evaluating Tear Secretion
As eye care professionals, our diagnostic armamentarium contains several methods that can be used to measure tear volume and detect abnormalities.1-3 These include tear meniscus height,4-6 tear osmolarity,7-9 the phenol red thread (PRT) test10-12 and the Schirmer Test.13,14
The Schirmer test
The Schirmer test has long been considered one of the most common methods to evaluate tear volume in clinical practice since its introduction in 1903.15 The test involves the simple process of hooking a sterile strip of filter paper over the lower eye lid. The strip is kept in place for five minutes and then gently removed. The wetted portion of the strip is measured and recorded. A diagnostic cut-off value of 10mm in five minutes has been suggested, with a test specificity of 67.9% and sensitivity of 82.7%.16
Despite its relative ease of use the technique has several disadvantages including patient discomfort, low reproducibility, long testing time of five minutes, difficulty of use in children and the issue of reflex tearing potentially skewing the results. It is ultimately uncertain exactly what the Schirmer test is measuring as the test paper absorbs the tear fluid as it is produced, but also absorbs the tears residing in the eye as the test paper is first applied. The result is that both production and initial volume contribute to the test results. This issue is further compounded by the fact that the test strip causes significant reflex lacrimation, meaning it is impossible to separate the basal lacrimal production rate from the lacrimal system’s ability to produce reflex tears.
Efforts have been made to improve the procedure including changing the type of filter paper, however no significant improvement was achieved.17,18 Performing the test under local anaesthesia to reduce reflex tearing and improve patient comfort has also been explored, however a degree of reflex tearing was still recorded.19-21
Phenol Red Thread Test (PRT)
The PRT test was introduced in 1983 as an alternative to the Schirmer test.22 The test uses a cotton thread treated with phenol red pH indicator.23 The thread changes colour from yellow to light red when moistened by the alkaline tears (pH 7.4). A 3mm length of the thread is folded and inserted one third distance from the temporal canthus of the lower eyelid, with the eye in primary position. The subject is asked to close his/her eyes for the duration of the test. The thread is then gently removed after 15 seconds and the red-coloured portion of the thread assessed and determined in millimetres. A diagnostic cut off value of 10mm in 15 seconds has been defined as dry eye.24 The test has a sensitivity of 86% and specificity of 83%.25
Despite several obvious advantages over the Schirmer test including shorter testing time of 15 seconds, vastly improved patient comfort and tolerability, no topical anaesthetic required and higher test sensitivity, it is again unclear what the PRT test is measuring.26 It is thought that the PRT test measures the residual tears existing primarily in the inferior conjunctival sac, with minimal effect on the secretion rate of the lacrimal gland.20 However, the wetting observed could have contributions from both tear production and tear volume in the eye.21
Tear Meniscus Height
Numerous techniques have been suggested and developed to assess the tear meniscus height including the use of a slit-lamp with an eye-piece graticule, use of fluorescein to aid visualisation,27 optical coherence tomography,28 optical pachymetry29 and various videos systems.30
Around 85-90% of the total volume of the tears reside in the upper and lower tear menisci.31 Using a slit-lamp biomicroscope to estimate tear meniscus height is the simplest and most common method used in clinical practice. This method suggests a mean meniscus height of between 0.25mm32 to 0.35mm33 in the normal population. A diagnostic value below 0.25mm has be suggested for the diagnosis of dry eye.34,35 The major disadvantage of this technique is the inherent subjectivity of the technique, which in turn induces significant inter-observer variability.36 As a result it has been suggested that each clinician establish their own values for normal patients and dry eye sufferers.
Tear Osmolarity
Tear osmolarity has been suggested as a key driver for normal homeostasis in tear film dynamics.37 Elevated tear osmolarity is known to be a central pathogenic cause of ocular surface and nerve damage,38 and has been regarded by some to be medically necessary to assist in the diagnosis of DED.39 Measurement of tear osmolarity may also be necessary to fully understand the severity of dry eye.40 Measurement of tear osmolarity is considered to be an effective objective test that can be used effectively in the diagnosis of dry eye, regardless of the aetiology of the condition and gauge its severity.41-43 Osmolarity values above 308 mOsms/L are indicative of dry eye disease.44 Commercially available products including the i-Pen and the TearLab Osmolarity System measure tear osmolarity by sampling a small amount of the tear film (around 50nL) from the lateral inferior tear meniscus.
Case history
This case history aims to highlight the interplay between tear volume and tear evaporation in a complex aqueous deficient dry eye patient.
History and symptoms: A 74-year-old female attended at the request of her GP complaining of red, gritty and irritable eyelids. She reports mild photophobia and intermittent blurred vision which is significantly affecting her quality of life and work as a professional artist. She also reports symptoms of xerostomia (dry mouth). She has been using baby shampoo to bathe her eyelids twice per day and hypromellose eye drops for around 12 months with no improvement in her symptoms.
Past ocular health: Bilateral pseudophakia.
Family ocular health: Both parents myopic. Age-related cataracts.
General health: Rheumatoid arthritis, hypertension, hypercholesterolemia.
Medications and supplements: Macushield. Simvastatin. Ramapril. Hydroxychloroquine. Methotrexate.
Refraction and visual acuity:
R: -1.00 / +0.50 X 173 6/7.5+ Add: +2.50 (N5)
L: -1.00 / +1.00 X 179 6/7.5- Add: +2.50 (N5)
Examinations
Pupil reactions: Normal
Oculomotor balance: Mild near exophoria. Well controlled
Visual field analysis: Normal
IOPs: R: 21mmHg L: 21mmHg (Using Goldmann applanation Tonometry 10.15am)
CCTs: R: 531µm L: 547µm
Posterior pole:
Right Eye: Unremarkable
Left Eye: Unremarkable
OSDI questionnaire score: 85 (severe)
Initial differential diagnosis:
- Aqueous deficient dry eye
- Evaporative dry eye
- Neuropathic dry eye
- Demodex Blepharitis due to presence of rosacea
Clinical Assessment
Anterior Eye Examination

Figure 1: Right eye – lids and adnexa
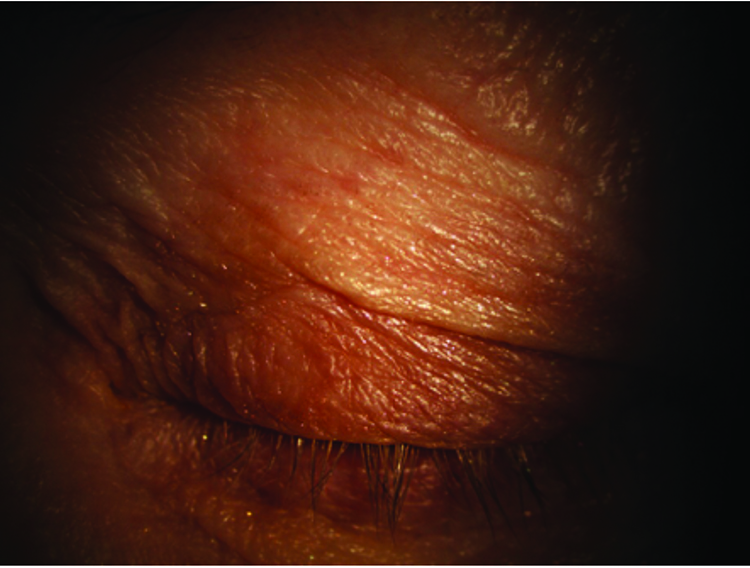 Figure 2: Left eye – lids and adnexa
Figure 2: Left eye – lids and adnexa
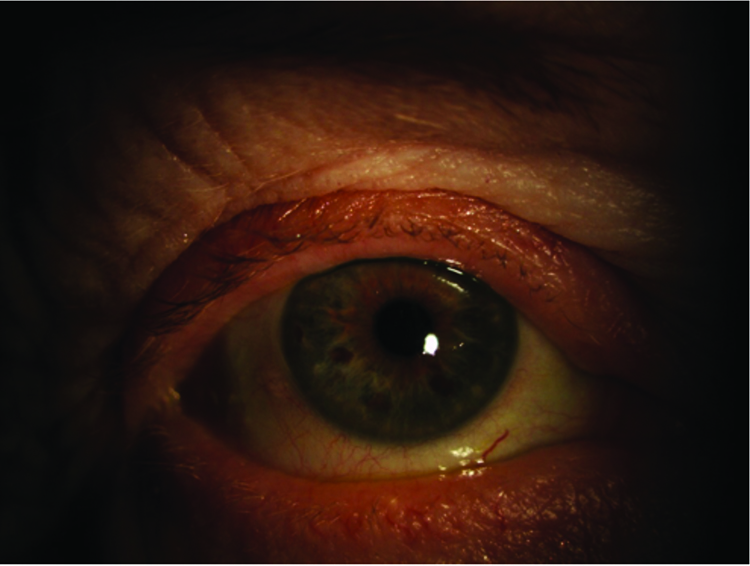
Figure 3: Right eye – external eye
Figure 4: Left eye – external eye
Figures 1-4 show the external eye both open and closed. Results of the slit-lamp examination are summarised in table 1. Moderate meibomian gland dysfunction was noted with around 50% of meibomian glands expressing meibum, mostly cloudy in appearance. Telangiectasia was noted on the lid margin and closed upper eyelid. However, the lids were otherwise healthy and devoid of significant scarring or pitting. The lid margin and eye lashes were clean and devoid of matter. Mild conjunctival and limbal hyperaemia was noted.

Figures 5 and 6 reveal the extent of corneal staining (left eye worse than the right) and poor tear evaporation rates.
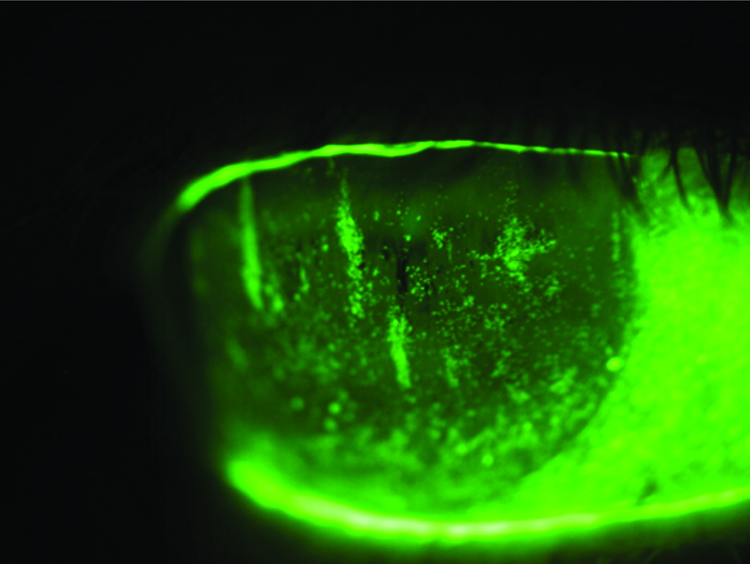
Figure 5: Right eye – fluoroscein staining
 Figure 6: Left eye – fluoroscein staining
Figure 6: Left eye – fluoroscein staining
Tear evaporation measurement
Tear evaporation was measured at 4.0 seconds in the right eye and 3.0 seconds in the left during slit-lamp; assessment aided with fluorescein. Objective non-invasive tear breakup time (NIKBUT) using an Oculus K5 revealed initial evaporation rates of 6.88 seconds in the right eye and 4.63 seconds in the left eye – results shown in figures 9 and 10.
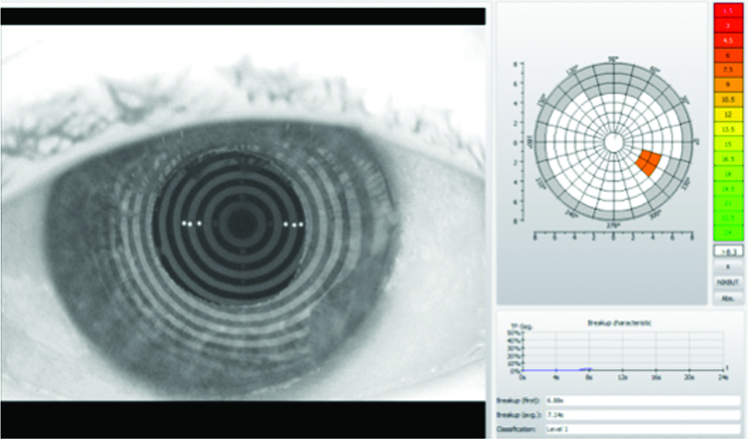
Figure 9: Right eye – objective NIKBUT
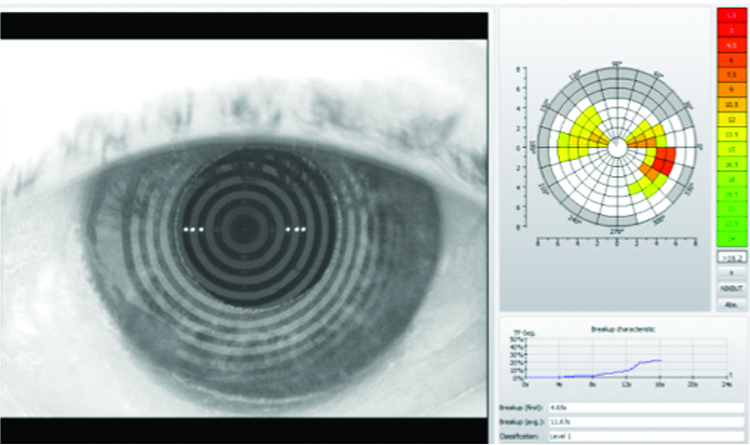
Figure 10: Left eye – objective NIKBUT
Tear volume measurement
Tear meniscus height measured using fluorescein and slit-lamp biomicroscope measured at 0.2mm in both eyes. Non-invasive tear meniscus height measurements achieved again using an Oculus K5 measured at 0.2mm in the right eye and 0.15mm in the left eye, results shown in figures 7 and 8.

Figure 7: Right eye – objective tear volume
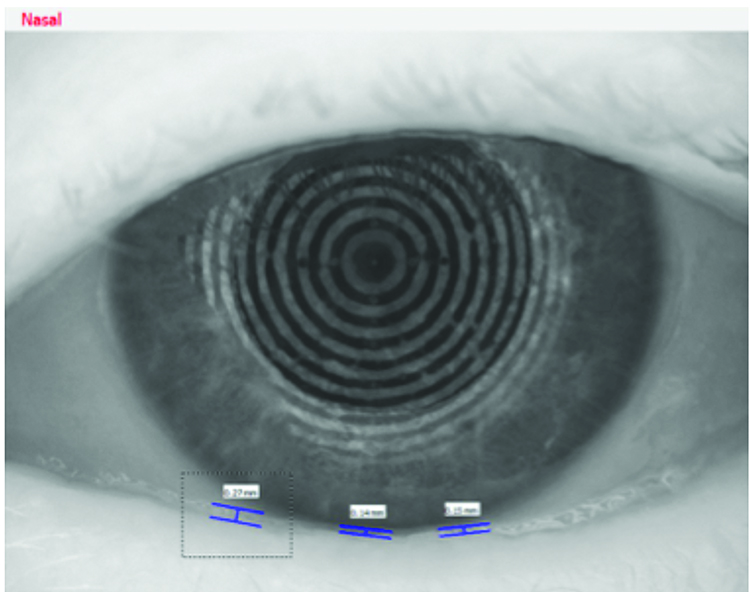
Figure 8: Left eye – objective tear volume
Meibography
Meibography reveals significant shortening of meibomian glands and some localised atrophy and gland drop-off. This is in keeping with chronic meibomian gland dysfunction.

Figure 11: Right eye – meibography Figure 12: Left eye – meibography
Figure 12: Left eye – meibography
Diagnosis
The results of the patient’s eye examination are concisely summarised in the Jenvis Report shown in figure 13. The severity of the patients’ symptoms, history of rheumatoid arthritis, low tear volume, significant corneal staining, poor tear evaporation times and meibomian gland dysfunction are suggestive of keratoconjunctivitis sicca with both ADDE and EDE components potentially secondary to Sjögren’s syndrome.
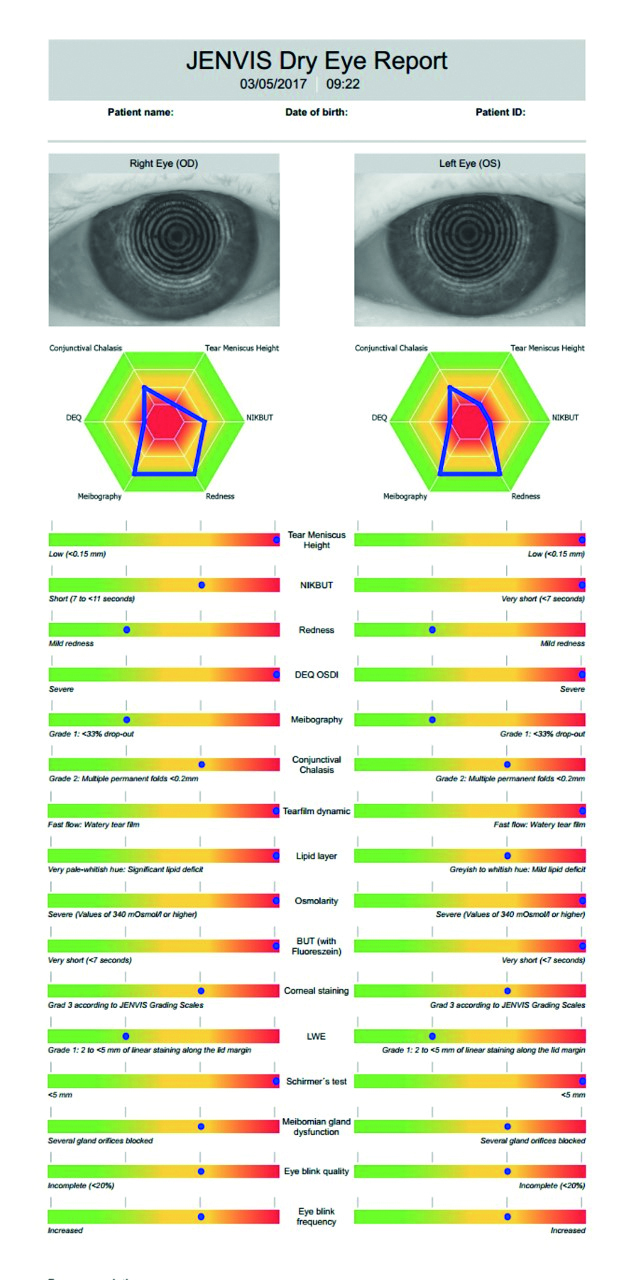
Figure 13: – Jenvis Dry Eye Report
Management
Due to the severity of the patient’s symptoms the initial treatment was aimed at reducing inflammation and increasing the patients comfort.
Initial treatment plan:
- Oral Doxycycline 100mg for three months
- Anti-inflammatory therapy – FML topical steroid eye drops on a 4/3/2/1 basis over a four-week period
- Eyelid therapy at home – heat, lid massage and hygiene via an MGDRx eye bag and Thera Tears Sterilid foam twice per day
- Preservative free artificial tears with lipid component – Emustil eye drops four times per day
- Preservative free gel before bed – Thealoz Duo Gel
- Referral to rheumatology for Sjögren’s testing
- Referral to ophthalmology for consideration for autologous serum eye drops
Treatment plan after three months:
- Preservative free artificial tears with lipid component – Emustil Eye Drops four times per day
- Preservative free gel before bed – Thealoz Duo Gel
- Omega III dietary supplement – Omega Eye from Scope Medical four times per day
- Punctal occlusion – Intracanalicular punctal plugs (see figures 14 and 15)

Figure 14: Right eye - punctual plug before full insertion

Figure 15: Left eye - punctal plugs before full insertion
Outcome
A significant improvement in patient symptoms, OSDI score and resolution of corneal staining was achieved within six months using the treatment planned outlined above. A significant improvement in tear meniscus height was achieved as shown in figures 16 and 17, from 0.2mm to 0.46mm in the right eye, and from 0.2mm to 0.42mm. Similar levels of improvement in non-invasive tear break-up time were also achieved from 6.88 seconds to 9.94 seconds in the right eye, and from 4.63 seconds to 9.75 seconds in the left eye, shown in figures 18 and 19.
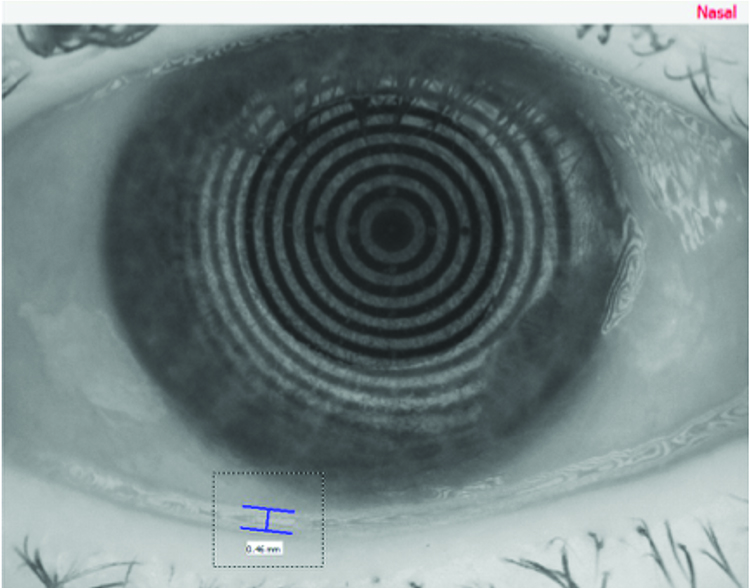
Figure 16: Right eye – objective tear volume (after treatment)
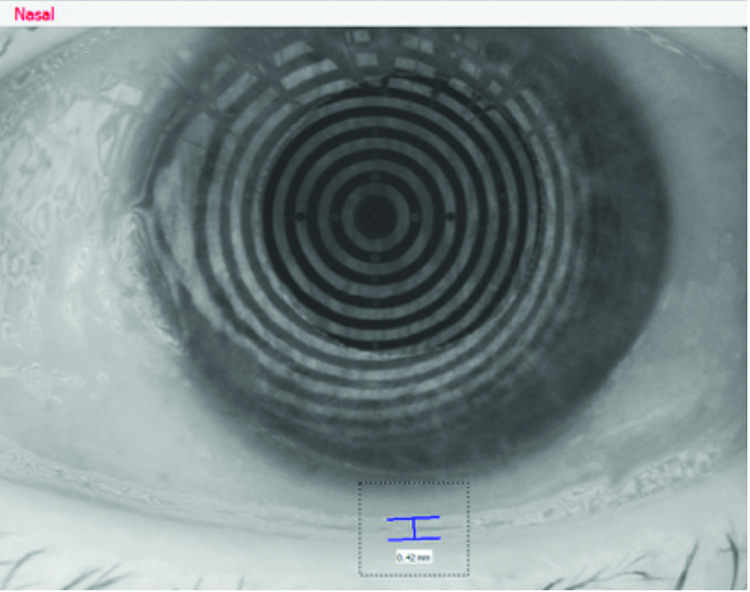
Figure 17: Left eye – objective tear volume (after treatment)

Figure 18: Right Eye – NIKBUT (After Treatment)
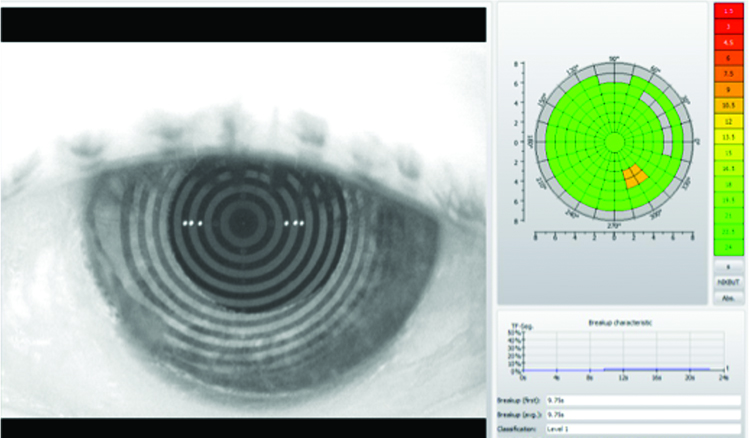 Figure 19: Left Eye – NIKBUT (After Treatment)
Figure 19: Left Eye – NIKBUT (After Treatment)
Partial and temporary punctal occlusion via lower lid absorbable synthetic intracanalicular implants replaced on a three-monthly basis (as shown in figures 14 and 15) in conjunction with use of autologous serum supplied by our local ophthalmology department is currently maintaining a happy patient.
Lacrimal Drainage Occlusion
Blocking tear outflow from the eye is appropriate for a small percentage of patients and is mainly reserved for patient with ADDE where retention of fluid, whether natural or via artificial tears, in the interpalpebral area is desirable. It is not suitable in patients with active lid margin disease due to tear stasis and subsequent increased risk of infection.
Many surgical and thermal options are used within secondary care to achieve lacrimal occlusion including canalicular ligature, canaliculotomy, relocation of the punctum to a position out of the tear meniscus, punctal tarsorrhaphy, the punctal patch, cautery, diathermy and argon laser photocoagulation.
Punctal and intracanalicular occlusion can provide short-term, medium term and long-term occlusion. Their use falls within the skillsets of the community based optometrist. Punctal occlusion can significantly improve the quality of life in appropriate patients suffering from moderate to severe dry eye. There is an extensive range of products commercially available and excellent training available from manufacturers and distributors to help aid practitioners new to this area. Punctual occlusion has been shown to decrease dependency on tear supplements,45 decrease tear osmolarity,46 increase tear volume,47 decrease ocular surface staining,48 increase goblet cell density,49 increase tear film stability and subjectively reduce patient symptoms.50
It is important however that practitioners are aware of not only the benefits but the risks and complications of punctual plugging. Careful patient selection is crucial for the procedure to be successful and it should only be conducted when appropriate. Com-plications can include damage to ocular surface, perforation of the canaliculus,51 loss of plug below punctum,52 epiphora, ocular irritation, infection,53 pyogenic granuloma development,54 spontaneous canalicular mucosal dissection and punctal stretching.55
Autologous Serum
Autologous serum is typically manufactured by centrifugation of the peripheral blood followed by dilution in artificial tears or saline. Autologous serum drops were first used clinically in the 1970s to help treat ocular alkali burns.56 Use of the autologous serum drops in the treatment of dry eye was first described in the literature in 1984,57 however it was not until the 1990s that its use became more widespread.58,59 It is used to treat a variety of conditions including dry eye, dry eye-related disorders such as Sjögren syndrome, persistent epithelial defects, neurotrophic keratopathy, recurrent corneal erosion, superior limbic keratoconjunctivitis, ocular cicatricial pemphigoid, graft-versus-host disease and Stevens-Johnson syndrome.60 Despite having been shown to be a safe and effective treatment, it is not used routinely in part due to potential risk of infection, lack of availability, inconvenience and most importantly cost.
Discussion
As a profession, we have an ever-growing list of diagnostics tests, techniques and equipment at our disposal, in conjunction with an ever-expanding list of potential treatment options for dry eye and dry eye-related conditions. We are ideally situated as primary care givers to tackle a large percentage of dry eye suffers that walk through our doors, saving the patient the time and anxiety involved in visiting a hospital eye clinic. With a carefully planned, systematic approach to our clinical examination and patient history, coupled with the zeal to embrace new diagnostic techniques and treatments for dry eye we should be able to simultaneously ease the burden of the patient, our colleagues in already over-stretched ophthalmology departments and build a thriving and profitable optometric practice. In the next instalment of this series we will explore the dry eye patient journey and how a dry eye clinic can be used to grow your patient database and increase practice profitably.
Craig McArthur is involved in a dedicated anterior eye clinic service at Peter Ivins Eyecare practice in Glasgow.
References:
- Savini G, Prabhawasat P, Kojima T, et al. The challenge of dry eye diagnosis. Clin Ophthalmol 2008;2:31–55.
- Abelson MB, Ousler GW III, Nally LA, et al. Dry eye syndromes: diagnosis, clinical trials and pharmaceutical treatment—“improving clinical trials”. Adv Exp Med Biol 2002;506:1079–1086.
- Abelson MB, Ousler GW III, Lafond A, et al. The pros and cons of dry— eye tests. Rev Ophthalmol 2011;11:62–6
- Johnson ME, Murphy PJ. The agreement and repeatability of tear meniscus height measurement methods. Optom Vis Sci 2005;82:1030–1037.
- García-Resúa C, Santodomingo-Rubido J, Lira M, et al. Clinical assessmentof the lower tear meniscus height. Ophthalmic Physiol Opt 2009;29:487–496.
- Ibrahim OM, Dogru M, Takano Y, et al. Application of visante optical coherence tomography tear meniscus height measurement in the diagnosis of dry eye disease. Ophthalmology 2010;117:1923–1929
- Tomlinson A, Khanal S, Ramaesh K, et al. Tear film osmolarity: determination of a referent for dry eye diagnosis. Invest Ophthalmol Vis Sci 2006; 47:4309–4315.
- Szalai E, Berta A, Szekanecz Z, et al. Evaluation of tear osmolarity in nonSjögren and Sjögren syndrome dry eye patients with the TearLab system. Cornea 2012;31:867–871.
- Lemp MA, Bron AJ, Baudouin C, et al. Tear osmolarity in the diagnosis and management of dry eye disease. Am J Ophthalmol 2011;151:792–798.
- Wee SW, Chun YS, Moon NJ, et al. Clinical usefulness of the phenol red thread test as diagnostic tool in dry eye patient. J Korean Ophthalmol Soc 2012;53:193–199.
- Miller WL, Doughty MJ, Narayanan S, et al. A comparison of tear volume (by tear meniscus height and phenol red thread test) and tear fluid osmolality measures in non-lens wearers and in contact lens wearers. Eye Contact Lens 2004;30:132–137.
- Tomlinson A, Blades KJ, Pearce EI. What does the phenol red thread test actually measure? Optom Vis Sci 2001;78:142–146
- Bawazeer AM, Hodge WG. One-minute Schirmer test with anesthesia. Cornea 2003;22:285–287.
- Cho P, Yap M. Schirmer test. I. A review. Optom Vis Sci 1993;70:152–156
- Schirmer O. Studien zur physiologie und pathologie der tranen-absonderung und tranenabfuhr. Graefes Arch Clin Exp Ophthalmol 1903;56:197–291.
- Vitalli C, Moutsopoulos, H.M et al. The European Community study group on diagnostic criteria for Sjogrens syndrome. Sensitivity and specificity gtests for ocular and oral involvement of Sjogrens syndrome. Ann Rheum Dis, 53, 637-647, 1994
- De Roetth A. Lacrimation in normal eyes. Arch Ophthalmol 1953;49:185–189.
- Halberg GP, Behrens C. Standardized Schirmer tear test kit. Am J Ophthalmol 1961;51:840–842
- Lamberts DW, Foster CS, Perry HD. Schirmer test after topical anesthesia and the tear meniscus height in normal eyes. Arch Ophthalmol 1979;97: 1082–1085
- Clinch TE, Benedetto DA, Felberg NT, et al. Schirmer’s test. A closer look. Arch Ophthalmol 1983;101:1383–1386.
- BaumJL. Clinical implications ofbasal tear flow. In: HollyHJ, Lamberts DW, Mackeen DL, eds. The Preocular Tear Film in Health Disease, and Contact Lens Wear. Lubbock, TX: Dry Eye Institute, 1986:646–652.
- Hamano HM, Hori M, Hamano T, et al. A new method for measuring tears. CLAO J 1983;9:281–289.
- KurihashiK,Yanagihara N,HondaY. A modified Schirmer test: the fine-thread method for measuring lacrimation. J Pediatr Ophthalmol 1977;14:390–397.
- Mainstone JC, Bruce AS, Golding TR. Tear meniscus measurements in the diagnosis of dry eye. Curr Eye Res, 1996; 15, 653-661
- Patel S, Farrell J et al. The value of a phenol read impregnated thread for differentiating between the aqueous and non-aqueous deficient dry eye. Ophthalmic Physiol Opt, 1998;18, 471-476
- Asbell PA, Chiang B. Phenol-red thread test compared to Schirmer test in normal subjects. Ophthalmology 1987;94(suppl):128
- Mainstone JC, Bruce AS & Golding TR. Tear meniscus measurements in the diagnosis of dry eye. Curr Eye Res, 1996; 15, 653-661
- Osama MA et al. Application of Visante Optical Coherence Tomography Tear Meniscus Height Measurement in the Diagnosis of Dry Eye Disease. Ophthalmology. Volume 117, Issue 10, October 2010, Pages 1923–1929
- Patel S, Port MJ. Tear characteristics of the VDU operator. Optom Vis Sci. 1991;68,798-800
- Farrell J, Patel S et al. A clinical procedure to predict the value of temporary occlusion therapy in keratoconjunctivitis sicca. Ophthalmic Physiol Opt, 2003;23,1-8
- Holly FJ. Tear film formation and rupture: an update. In F.J. Holly (Ed.), The Preocular Tear Film in Health, Disease, and Contact Lens Wear (pp. 634-645) Lubbock, Texas: Dry Eye Institute
- Lamberts DW et al. Schirmer test after topical anaesthesia and the tear meniscus height in normal eyes. Arch Ophthalmol, 1979;97,1082-1085
- Tomlinson A et al. What does the phenyl red thread test actually measure? Optom Vis Sci. 2001; 78, 142-146
- Golding TR et al. Relationship between tear meniscus parameters and tear-film breakup. Cornea. 1997;16,649-661
- Oguz H et al. The height and radius of the tear meniscus and methods for examining these parameters. Cornea. 2000;19,497-500
- Johnson ME, Murphy PJ. The agreement and repeatability of tear meniscus height measurement methods. Optom Vis Sci. 2005; 82,1030-1037
- Srinivasan S, Nichols K. Collecting tear osmolarity measurements in the diagnosis of dry eye. Expert Rev Ophthalmol 2009;4:451–453.
- International Dry Eye WorkShop, The definition and classification of dry eye disease. In: 2007 Report of the International Dry Eye Workshop (DEWS) Ocul Surf. 2007;5:75–92
- Hirata H, Mizerska K, Marfurt CF, Rosenblatt MI. Hyperosmolar tears induce functional and structural alterations of corneal nerves: electrophysiological and anatomical evidence toward neurotoxicity. Invest Ophthalmol Vis Sci. 2015;56(13):8125–8140.
- Sullivan BD, Whitmer D, Nichols KK, et al. An objective approach to dry eye disease severity. Invest Ophthalmol Vis Sci. 2010;51(12):6125–6130.
- Khanal S, Tomlinson A, McFadyen A, et al. Dry eye diagnosis. Invest Ophthalmol Vis Sci 2008;49:1407–1414.
- Lemp MA, Bron AJ, Baudouin C, et al. Tear osmolarity in the diagnosis and management of dry eye disease. Am J Ophthalmol 2011;151: 792–798.
- Tomlinson A, Cedarstaff T. Diurnal variation in human tear evaporation. J Br Contact Lens Assoc 1992;15:77–79.
- Foulks GN, Lemp MA, Berg M, Bhola R, Sullivan BD. TearLab™ Osmolarity as a biomarker for disease severity in mild to moderate dry eye disease. American Academy of Ophthalmology PO382, 2009
- Balaram M et al. Efficacy and tolerability outcomes after punctal occlusion with silicone plugs in dry eye syndrome. Am J Ophthalmol. 2001;131,30-36
- Gilbard et al. Effect of punctal occlusion by Freeman silicone plug insertion on tear osmolarity in dry eye disorders. CLAO J. 1989;15,216-218
- Sakamoto A et al. Efficacy and retention rate of two types of silicone plugs in patients with and without Sjögren syndrome. Cornea. 2004; 23, 249-254
- Nava-Castaneda A et al. Effects of lacrimal occlusion with collagen and silicone plugs on patients with conjunctivitis associated with dry eye. Cornea. 2003;22,10-14
- Dursen et al. Ocular surface changes in keratoconjunctivitis sicca with silicone punctum plug occlusion. Curr Eye Res. 2003; 26, 263-269
- Tai MC et al. The clinical efficacy of silicone punctal plug therapy. Cornea. 2002; 21, 135-139
- Fayet B et al. Complications of punctum plug in the symptomatic treatment of dry eye. J Fr Ophthalmol. 1990; 13, 135-142
- Soparkar CN, Patrinely JR. Silicone punctal plug extrusion. Ophthalmology. 2001; 108, 2153-2154
- Gerding H, Kuppers J, Busse H. Symptomatic cicatricial occlusion of canaliculi after insertion of Herrick lacrimal plugs. Am J Ophthalmol. 2003; 136, 926-928.
- Kim BM, Osmanovic SS, Edward DP. Pyogenic granulomas after silicone punctal plugs: a clinical and histopathologic study. Am J Ophthalmol. 2005; 139, 679-684
- Inagaki K et al. Study of change of size of the punctum before insertion and after extrusion of a punctal plug and selection of an appropriate plug for reinsertion. Nippon Ganka Gakkai asshi. 2005; 109, 274-278
- Ralph RA, Doane MG, Dohlman CH. Clinical experience with a mobile perfusion pump. Arch Ophthalmol 1975;93:1039–1043.
- Fox RI, Chan R, Michelson JB, et al. Beneficial effect of artificial tears made with autologous serum in patients with keratoconjunctivitis sicca. Arthritis Rheum 1984;27:459–461.
- Tsubota K, Goto E, Shimmura S, et al. Treatment of persistent corneal epithelial defect by autologous serum application. Ophthalmology 1999;106:1984–1989.
- Tsubota K, Goto E, Fujita H, et al. Treatment of dry eye by autologous application in Sjogren’s syndrome. Br J Ophthalmol 1999;83:390–395.
- Geerling G, Maclennan S, Hartwig D. Autologous serum eye drops for ocular surface disorders. Br J Ophthalmol 2004;88:1467–1474
