The story of OCT probably begins in 1971 with Professor Michel Duguay’s successful attempt to use femtosecond (10–15 seconds) optics to image ‘light in flight’. Interestingly, at the time, Prof Duguay went as far as suggesting ‘seeing inside human tissue’ as a possible application of his research, an outcome that took some 20 years of R&D to bring to fruition.
Further foundations of the technology were presented in a 1986 paper by Fujimoto et al. using femtosecond optical ranging in biological tissue. Professors James Fujimoto and David Huang – who was a combined MD (ophthalmology), PhD graduate student at the time (see Huang et al, 1991) – are recognised as the fathers of ophthalmic OCT.
OCT is the light analogue of ultrasound imaging – the harnessing of reflected and back-scattered light emanating from tissue that may or may not be homogenous optically. Figure 1 shows some possible fates of light incident on a simple two-media optical system.

Figure 1
A so-called A-scan is a single-axis, axial determination of depth utilising back-scattered light. The same terminology is also used in an ultrasound context. A B-scan is an areal assembly of numerous A-scans to form an ‘image’ of an area of interest (horizontal and vertical) viewed from the front (the term en-face is sometimes used to describe such a presentation).
If coherent, monochromatic light is incident on such a system (figure 2), the exiting light rays resulting from reflection and/or refraction are delayed by amounts of time that are dependent on: the depth of the section; the refractive index of the media traversed; and the presence or absence of optical inhomogeneities within the media that can reflect, refract, and/or scatter the incident light.
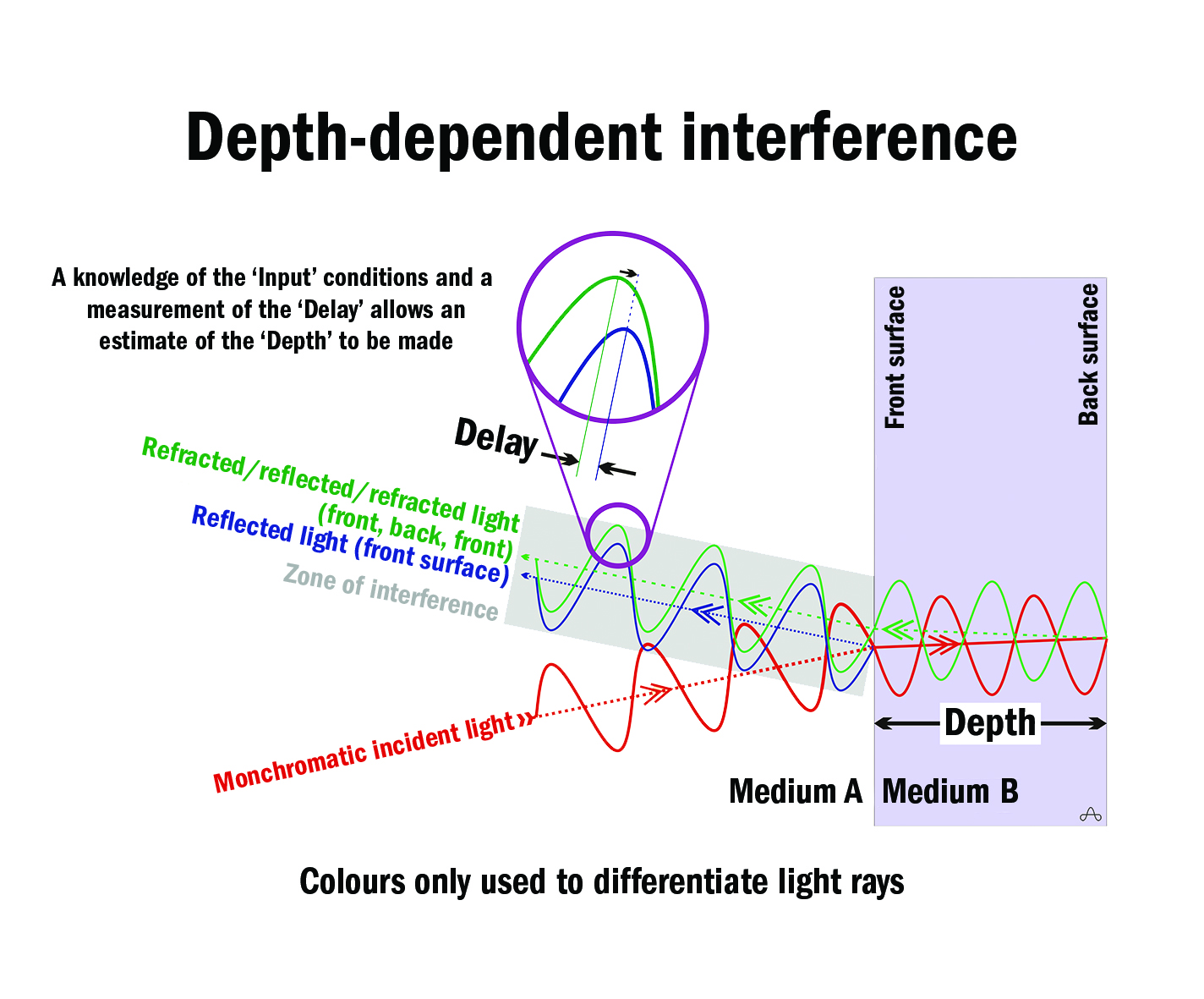
Figure 2
The exiting light rays can interfere with one another constructively, destructively (figure 3), or partially. Analysis of such delay-dependent interference, when combined with a knowledge of the incident light, allows an estimate of the depth of tissue under examination to be made.
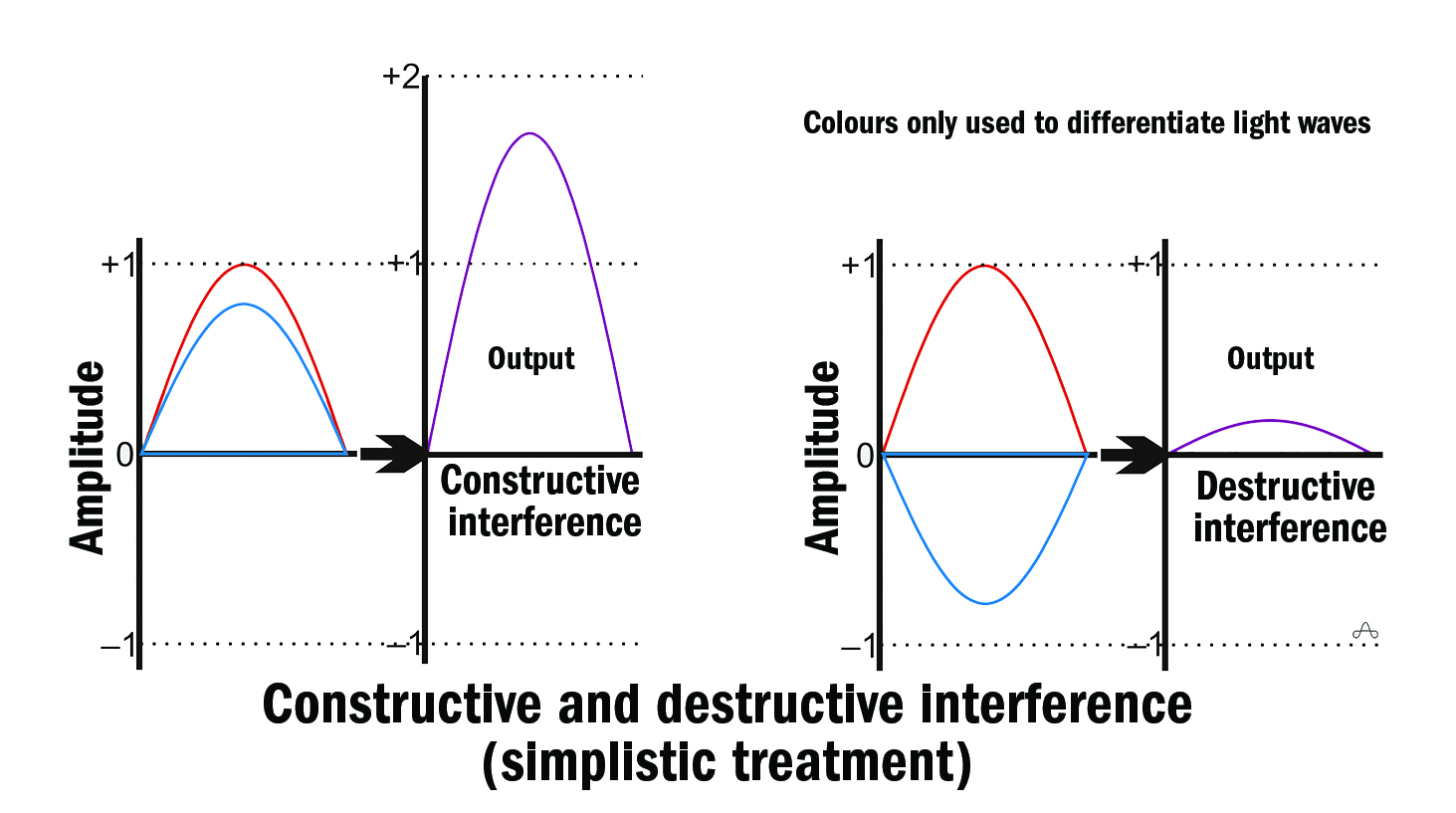
Figure 3
In practice, it is difficult to make such estimates over long distances (eg, the axial length of the eye), so instead of continuous incident light, femtosecond pulses of partially coherent light over a relatively narrow range of wavelengths are used and their delays assessed (figure 4).

Figure 4
Low-coherence interferometry measures the echo time delay and intensity of back-scattered light by interfering it with light that has travelled a known reference path length and time delay in a Michelson-type interferometer (figure 5).
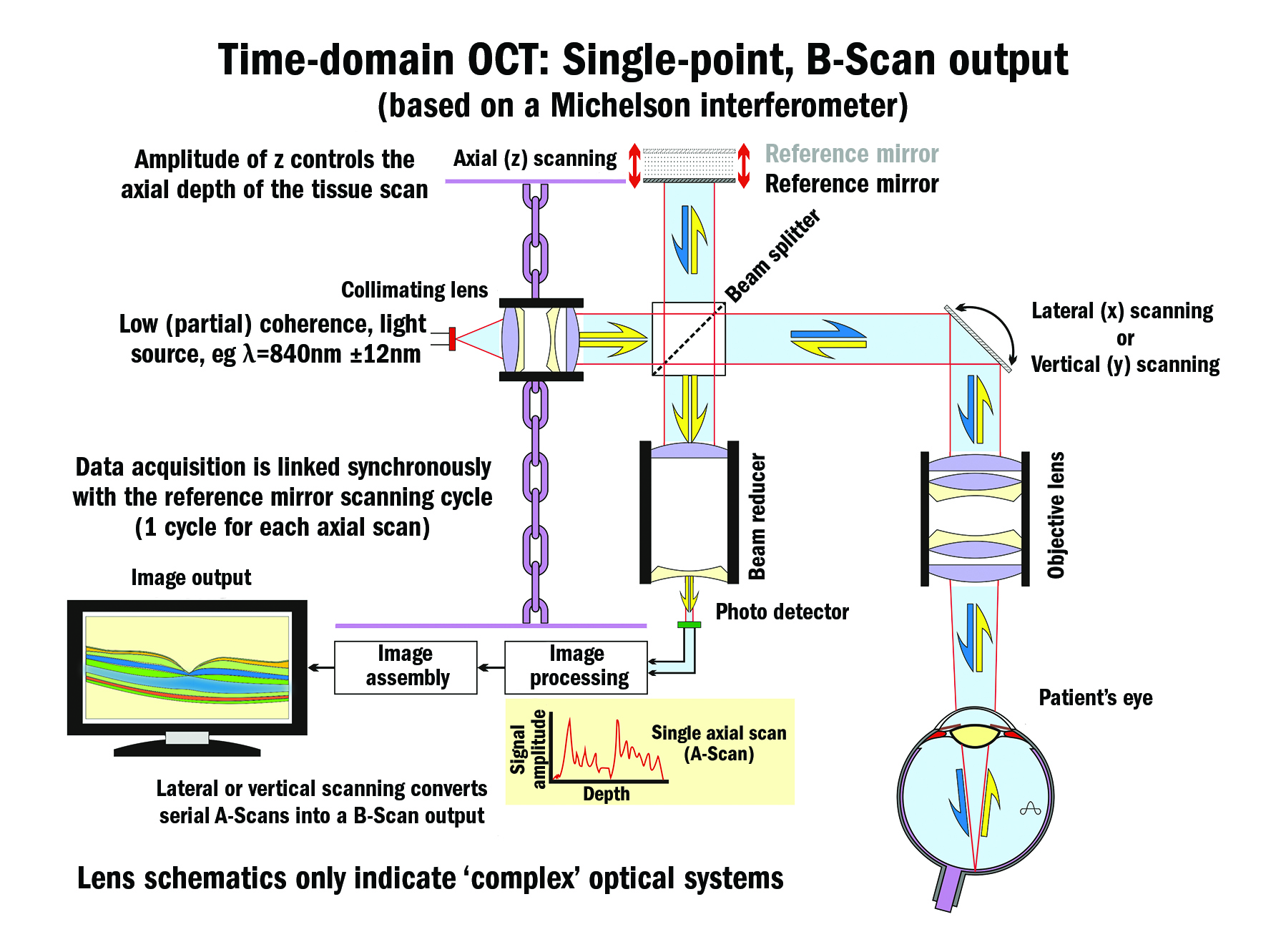
Figure 5
Development
Although others were also pursuing the concept, especially in Japan, it was not until 1991 (patent date) that Profs Huang and Fujimoto and colleagues at MIT created the first successful ocular OCT device based on a Michelson interferometer (840 nm source, axial resolution 15 µm).
It was a further two years (1993) before in vivo ocular images were possible. Zeiss – through its Humphrey Instruments, San Leandro, California acquisition – was the first company to bring a practical instrument to market in 1996. It was a time-domain-based instrument (figure 5, first generation), a technology that was later surpassed by Fourier-domain (spectral-domain, second generation, 2006) technology (figure 6), and later still by swept-source, spectral-domain (SS SD) OCT (figure 7, third generation, 2006 to present).

Figure 6
Fourier-domain instruments, especially swept-source-based ones, are now offered by all major instrument companies as well as some specialised OCT companies because they offer greater resolution (axial and lateral), much higher scanning rates, wider fields, better signal-to-noise ratios, shorter ‘take’ times, and, depending on the central wavelength used, greater depth of penetration (longer wavelengths – eg 1,050 nm, penetrate deeper, experience less attenuation from ocular opacities and optical inhomogeneities, but at slightly lower axial resolution). Hardware-wise, swept-source instruments have the advantage of not requiring a spectrometer sub-system including its special line-scan camera.
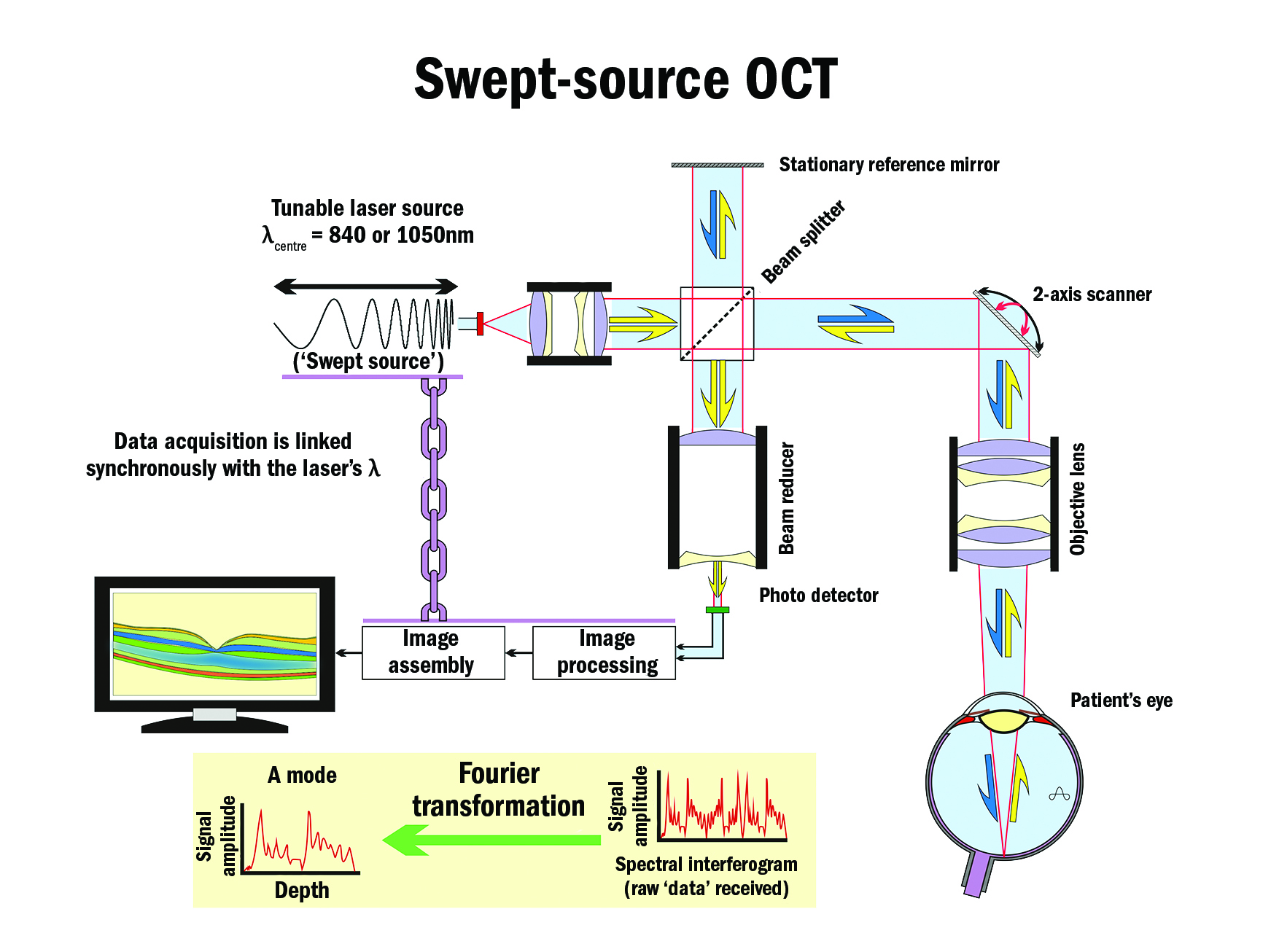
Figure 7
Light sources include lasers, tuneable lasers (over relatively limited wavelength ranges, typically <150 nm but up to 246 nm has been reported), and so-called superluminescent light-emitting diodes. Despite the almost universal use of colour in OCT images, the colours are added during image processing to indicate the different reflectance levels of the ocular tissues to help differentiate features of interest. Generally, red, orange, and yellow colours represent features of greater optical reflection and back-scatter, while blue and black colours represent minimal returning signals.
All current OCTs use light sources beyond the visible spectrum (beyond the red end – ie near-IR). That means that the patient being examined is unaware of the instrument’s optical machinations with the exception of a fixation device that can be internal or external. However, OCT instruments may offer the examiner near-IR illumination of the fundus for on-screen alignment and targeting purposes.
More recently, manufacturers have added anterior segment modules to their instruments’ range of options, and many specialty scleral and miniscleral contact lens fitters are using the technology to refine their fitting techniques and to monitor changes at after-care visits subsequently. Such imaging is also of interest to glaucoma and cataract specialists as well as other ophthalmological sub-specialists. The main barrier to wider adoption of SS SD OCT currently appears to be its higher costs.
Fundus auto-fluorescence (FAF) is also widely available and imaging field sizes up to 45° are offered. The most recent addition to a clinician’s armamentarium is the addition of a Doppler function to OCT devices that allows blood flow velocities to be assessed using added colour coding of fast-flowing, slow-moving, and stagnant blood.
Experimental OCT devices using dual-beam and polarisation-sensitive optics have also appeared in the literature. The latter allows investigations of birefringent and anisotropic structures, especially of the cornea.
Resolution
The resolution of OCT depends largely on optical factors that are fixed for particular instruments. Axial resolution is a direct function of the square of the centre wavelength of the light source used (shorter is better) and is related inversely to the bandwidth (∆λ) of the light source (broader is better).
Transverse resolution depends on the numerical aperture of the objective lens (larger is better) and the ‘spot’ size of the focus light beam (smaller is better). Transverse resolution is usually in the 10-15 µm range.
Axial resolutions in the range of 1-3 µm are now being realised experimentally but current instruments offer axial resolutions in the 3-6.5 µm range. By way of contrast, time-domain devices offered 10-15 µm resolutions axially.
Depth of penetration is usually in the 2-3 mm range. Most instruments use wavelengths of around 850 nm or 1,050 nm with claims of up to 13 mm square scanning fields (B-scans). The scanning rates for Fourier devices range from 20,000-200,000 scans per second (per sec) whereas time-domain technology managed 400 scans per sec due largely to the need to scan the reference mirror axially to vary the instrument’s reference arm’s path length.
At the 2016 ARVO meeting in May, several advances were detailed. An astonishing scan rate of 1.7 million A-scans per sec was claimed for a limited field (5° x 5°) instrument by one group and a functional, full eyeball-length SS SD OCT was detailed by another. That instrument offered simultaneous full-depth, anterior chamber and posterior eye imaging with a 30° retinal field using dual optical channels at 1,045 nm (∆λ was 100 nm).
At the same meeting, a long-range SS SD OCT instrument specialised to imaging the crystalline lens in 3D was described. A depth range of 17 mm was claimed over a 7 mm x 7 mm iris area. Several papers covering OCT-based ocular angiography in various conditions were also delivered.
OCT is also now making inroads into vascular and dermatological specialties and that list of users is likely to grow.
Significance of OCT
The invention of the direct ophthalmoscope in the mid-19th century was by far the greatest boost to eye care at the time and probably of all time. While the evolution of the slit-lamp biomicroscope was also a significant advance, it is probable that the arrival of ophthalmic OCT runs a close second to the ophthalmoscope. OCT has changed almost every aspect of eye care, especially in the medical retina field. As the technology advances, its significance can only grow.
References
- Duguay MA, 1971. Light photographed in flight. American Scientist 59: 551-556.
- Fujimoto JG et al, 1986. Femtosecond optical ranging in biological systems. Opt Lett. 11: 150-153.
- Huang D et al, 1991. Micron-resolution ranging of cornea and anterior chamber by optical reflectometry. Lasers Surg Med. 11: 419-425.
