Sleep apnoea represents a disorder characterised by recurrent interruption of normal breathing during sleep due to upper airway obstruction.1 Sleep apnoea is generally classified as central, obstructive and mixed. Central sleep apnoea is a rare disorder and it occurs in patients with severe neurological problems affecting the brainstem, neurodegenerative disorders, stroke, complications of surgery of the cervical spine and in primary hypoventilation syndrome. Obstructive sleep apnoea (OSA) is, however, the most common form and occurs when tissues in the upper throat (or airway) collapse at intervals during sleep, thereby blocking the passage of air. These obstructive episodes may last from a few seconds to minutes and lead to severe lack of oxygen. This in turn will cause the lungs to ‘suck air’ at which point the patient makes a loud, snoring sound.
Sometimes these obstructive episodes can only stop if the patient wakes up; however, patients do not remember waking up during the night. In the morning they feel tired, complain of headaches and are irritable.
Obstructive sleep apnoea
It is estimated that OSA affects about 1.5 million adults in the UK; however, it is also predicted that around 85% of those are still undiagnosed. (British Lung Foundation, 2015). Although many people with OSA, particularly women and children, are not overweight,2 OSA occurs mainly in patients suffering from obesity. The patients present with a history of loud snoring and excessive daytime somnolence, are usually middle-aged, have a larger neck circumference, small jaw, large tonsils and tongue, and often suffer from nasal blockage. Other signs and symptoms, as well as diagnosis techniques used to make a positive diagnosis of OSA, are listed in table 1.
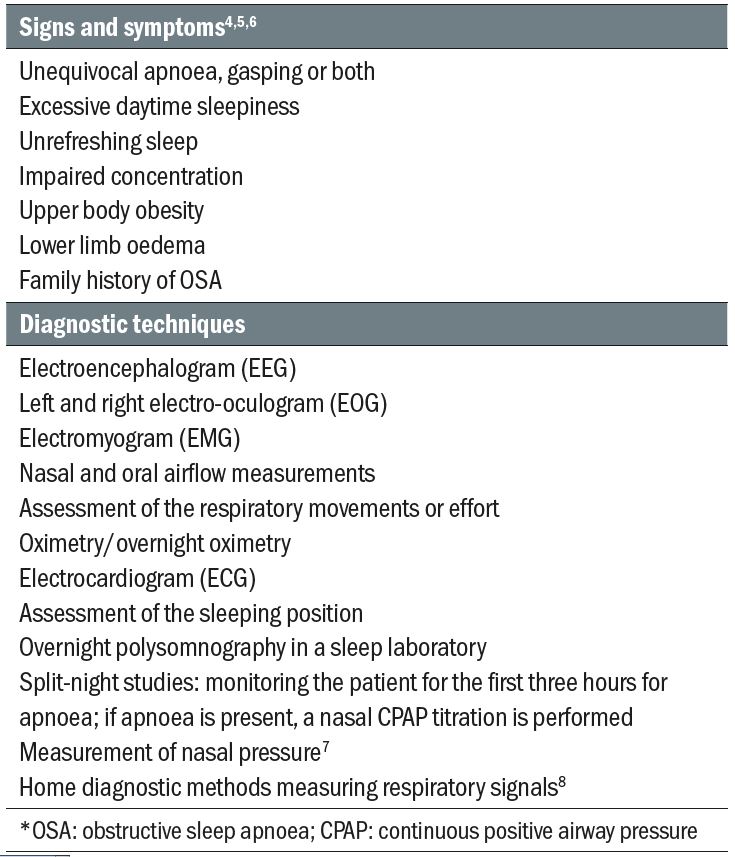
Table1: Diagnosis of OSA*
Common systemic associations of OSA include:
- Systemic hypertension8-10
- Sudden death
- Heart attacks
- Diabetes
- Cognitive deficits
- Neurodegenerative diseases
- Stroke
- Depression
- Epilepsy11
Although OSA has been associated with chronic hypertension,8-10 not all subjects with sleep-related breathing disorders are hypertensive.12,13 It has been demonstrated that some elderly subjects develop nocturnal low blood pressure directly after an apnoeic attack.14 Indeed, it is suggested that there are more episodes of over-dipping in systemic blood pressure, rather than spikes. Nevertheless, this area is still under debate.
The underlying pathogenic mechanisms for the neurological and circulatory problems associated with OSA are still unclear. OSA is accompanied by decreased oxygen saturation and hypercapnia (too much carbon dioxide in the blood).15 Hypoxia causes arteriolar vasodilation by a direct mechanism. The stimulation of the hypoxic peripheral chemoreceptors also leads to changes in heart rate (HR) and peripheral sympathetic outflow.16 Hypoxia results in hyperventilation (increased breath frequency), peripheral vasoconstriction, bradycardia (low heart beat) and cerebral vasodilation.17 In addition, intermittent hypoxia may cause systemic hypertension due to sympathetic activation.16 Hypoxia and acidosis associated with OSA might also result in extreme cardiac arrhythmias and nocturnal sudden death.17
OSA is associated with ocular diseases such as floppy eyelid syndrome, keratoconus,18 ischaemic optic neuropathy,19-20 central serous chorioretinopathy (CSC) and glaucoma. These associations are discussed below.
Floppy eyelid syndrome (FES)
FES is characterised by hyperelasticity of the upper eyelids. It is believed that nearly 100% of patients with FES suffer from OSA and the presence of FES is an indicator of severe OSA. 21 In this condition, the upper eyelid can evert easily, especially when the patient sleeps on one side. During this position, the eyelid turns and exposes the palpebral conjunctiva to the pillow. The patients will usually present with recurrent unilateral red eye that does not respond to any of the conventional treatments. They are often erroneously diagnosed with either infectious conjunctivitis, blepharitis or dry eye and receive various treatments that do not solve the condition.
FES is also associated with ptosis, keratoconus, superficial punctate keratopathy, lower lid ectropion, keratinization of the conjunctival surface of the affected upper eyelid, secondary dry eye and blepharitis (due to inflammation and abnormalities of the tarsal and accessory lacrimal gland), eyelash ptosis and eyelash misdirection.22
When diagnosed, FES patients also need to be referred for sleep studies as well as for surgical correction of their condition. Other management measures can include lid taping/patching, tear substitutes and steroids for the ocular inflammation.
Keratoconus
Keratoconus is a non-inflammatory corneal ectasia that affects younger individuals and results in various degrees of visual disturbances and disabilities. The amount of literature on keratoconus is enormous and a review of this condition is not within the scope of this article.
Keratoconus can be associated with various other ocular conditions, including FES. Therefore, association with OSA seems to be a possibility. Indeed, in a relatively recent study, Gupta et al 23 found that keratoconus patients have a higher probability of being diagnosed with OSA than the general population. Therefore, clinicians should screen for sleep disturbances in keratoconus patients and refer them to their GP for further work when such occurrences are detected.
Anterior ischaemic optic neuropathy (AION)
AION represents an ischaemic disorder of the posterior ciliary artery circulation in the optic nerve head, and is the most common type of acute optic neuropathy in elderly patients. There are two types of AION: arteritic AION, in which case there is an association with temporal arteritis; and non-arteritic AION (table 2).
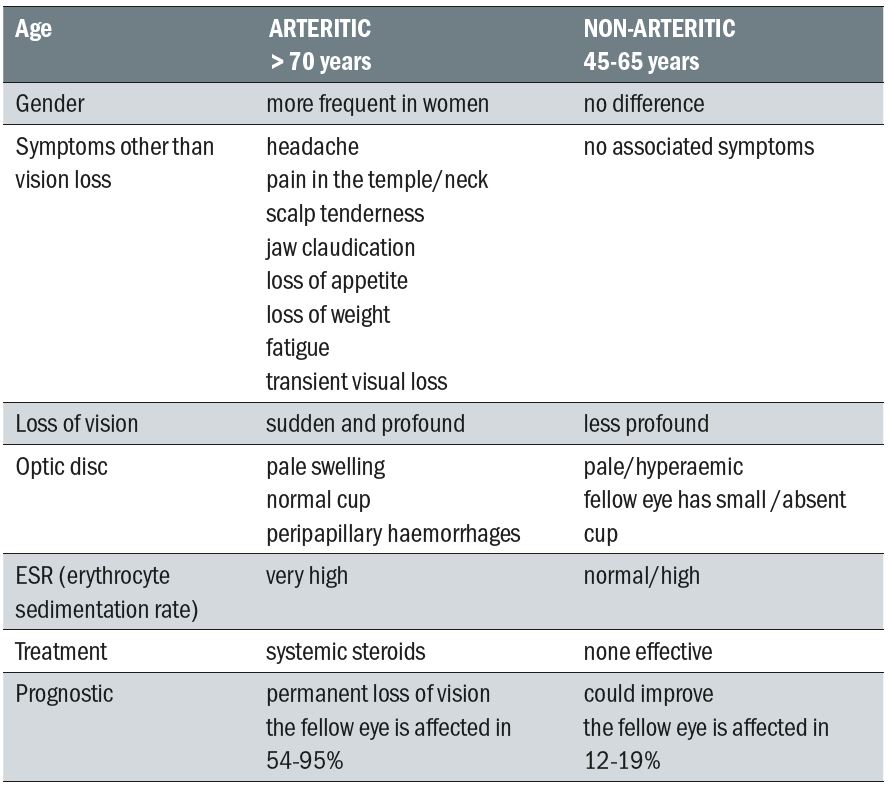
Table 2: Differential diagnosis between arteritic and non-arteritic ischaemic optic
neuropathy (Adapted from the Foundation of the American Academy of Ophthalmology,
Basic and Clinical Science Course Series)
While the arteritic type is associated with various systemic symptoms including pain, the non-arteritic type of AION presents with sudden and painless loss of vision. Patients are usually 55 to 65 years old and there is no gender predisposition. White Europeans are more affected by this disease than any other ethnic group. Risk factors include sleep apnoea, hypertension, diabetes, ischaemic heart diseases, collagen vascular diseases, nocturnal hypotension, atherosclerosis, migraine and other vasospastic disorders, and carotid artery diseases. It is also associated with other ocular conditions, such as small or absent optic disc cup, high IOP, optic disc oedema, ocular vasospasm and other vasculopathies that may affect the local circulation and make the optic nerve susceptible to ischaemic conditions.
A large number of patients with NAION suffer from OSA, with studies publishing percentages as high as 70 to 80%.24 Treatment with continuous positive airway pressure (CPAP) of these patients decreases these numbers, possibly due to the positive effect of CPAP on sleep quality, oxygenation, blood pressure and other haemodynamic changes associated with OSA.25
Any patient suffering from AION (arteritic or not) should be referred immediately to an ophthalmologist for diagnosis and treatment. There is no established treatment for non-arteritic AION; however, early treatment with corticosteroids in all AION cases should begin without delay, even if the exact aetiology (arteritic or not) is not certain. In addition, practitioners should ask NAION patients questions about their sleep and refer patients to their GP to screen for hypertension, obesity, increased neck circumference, large soft palate, and enlarged tonsils as well as to consider polysomnographic studies. Sleep specialists also need to screen for vision disturbances among patients with OSA.26
Central serous chorioretinopathy (CSC)
CSC was first described in 1866 by von Graefe.27 It is defined as a circumscribed serous detachment of the sensory retina, resulting from defective pumping function at the level of the retinal pigment epithelium (RPE), associated with a choroidal vascular hyperpermeability (figure 1). Patients are usually young men, who present with sudden onset of blurred vision that often improves with the addition of a weak plus lens. Micropsia, metamorphopsia, paracentral scotoma, and decreased colour vision may also be present. Hysteria, hypochondria and neurosis are frequently encountered among these patients. Particularly severe CSC may occur in patients who are under immunosuppressive therapy for renal transplants or in pregnant women.28
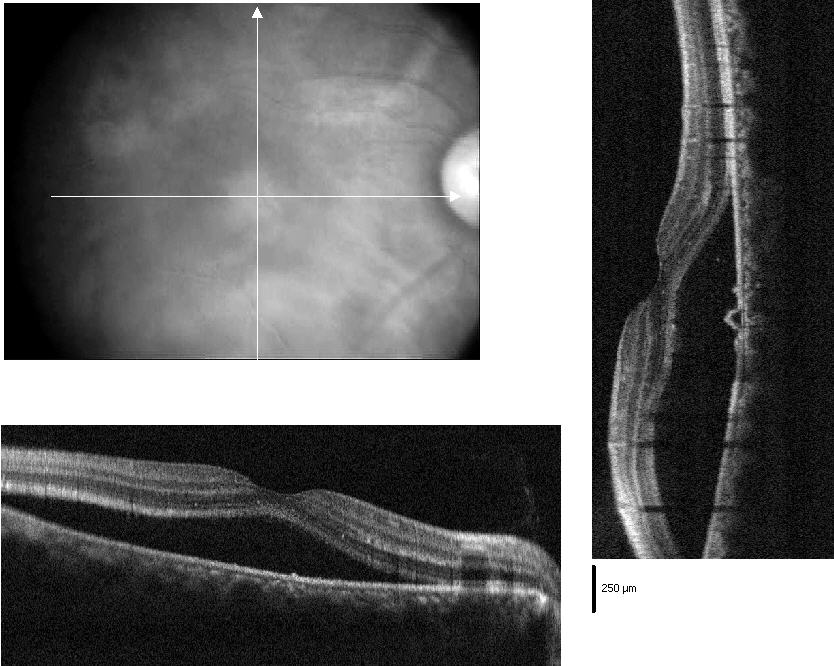
Figure 1: OCT scan of central serous chorioretinopathy
Fundus examination in these patients shows a round or oval serous retinal detachment in the macula, about two disc diameters, associated with pigmentary changes on the surface. The normal foveal reflex is lost.
The visual prognosis for these patients is good, the vast majority recovering to their original visual acuity after three to six months, without any treatment. Photocoagulation at the site of leakage can induce rapid remission. However, since the final Snellen visual acuity after this procedure is not better in the treated compared to untreated eyes, referral for laser treatment is indicated only in certain situations.
The association between OSA and CSC is controversial, with some studies showing an association,29 while others do not.30 The association is based on the fact that patients with OSA have increased levels of circulating adrenalin and noradrenaline, both of which are risk factors for the development of CSC. If an association is suspected, patients with CSC should be screened for sleep disturbances and referred appropriately.
Glaucoma
The association between OSA and glaucoma was first reported in 1982 by Walsh and Montplaisir31 who found a combination of OSA and glaucoma in five members of two generations of a family. Later, Robert et al.32 and McNab et al.33 reported that some patients with sleep disorders screened for floppy eyelid syndrome were also being treated for glaucoma (figure 2). Other authors have also reposted a significantly higher prevalence of sleep breathing disorders (SBD) in POAG patients compared to controls.34-37 These reports have opened a new and interesting path in the study of POAG pathogenesis.
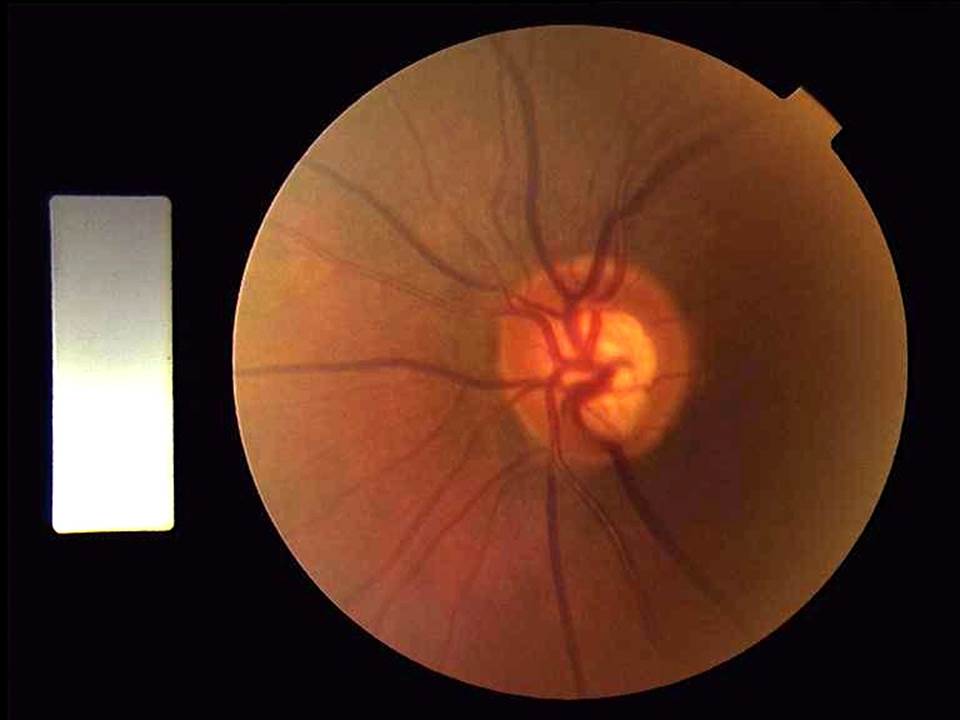
Figure 2: Glaucomatous disc
Decreased arterial blood oxygen saturation is compensated by increased cerebral blood flow (CBF) and consequently the oxygen supply to the brain normally remains unchanged.38 Hypercapnia leads to dilation of the cerebral arteries,39 decreased cerebral resistance, and increased CBF. Changes in blood gas levels influence the retinal and optic nerve blood flow in a manner similar to that in the cerebral circulation. In the eye, hypercapnia results in increased blood flow in the retina and optic nerve.40 Although one can expect that a high blood flow (as a result of hypercapnia) would improve the visual function, it has been demonstrated that contrast sensitivity (CS) may decrease in response to hypercapnia (in studies performed on young normal subjects and on untreated early POAG patients). These findings could suggest that the vasodilatation and consequent ocular blood flow improvement alone are not sufficient to dictate the visual function outcome during gas perturbations and that the harmful effect of acidosis could be more important than the beneficial contribution of the improved perfusion. Moreover, in glaucoma patients with disturbed autoregulation, hypercapnia-induced vasodilatation could potentially redirect blood flow away from the ONH. It seems that the metabolic and vascular effects resulting from elevated blood levels of carbon dioxide together with endothelial damage from apnoea-induced hypoxia could play an important role in the pathogenesis of POAG associated to OSA.
It seems that the effects of the apnoeic episodes on IOP are somewhat less dramatic than those on ocular circulation. It seems that there is no difference between the IOP measured at the end of prolonged apnoeas and the values assessed during periods of normal respiration in patients suffering from both OSA and normal tension glaucoma.41 It can be hypothesized that, in these patients, the optic nerve is likely to be damaged directly by repetitive periods of hypoxia or by an impaired autoregulation of blood flow as a secondary response to repetitive prolonged apnoeas.
Is still unclear if in POAG patients suffering from OSA the occurrence or progression of glaucomatous optic neuropathy (GON) is due to the OSA per se, or to concurrent episodes of exaggerated nocturnal haemodynamic disturbances. In these patients, however, the IOP-lowering therapy may be insufficient and an additional specialized approach may offer a more valuable
contribution. General lifestyle advice, sleep hygiene and weight reduction are just a few measures that could and should be promoted among susceptible patients.41 Appropriate management of conditions such as obesity and abnormal BP, together with an improvement in our understanding of their relationship to POAG, might have a beneficial effect in developing new therapeutic strategies for this disease.
Dr Doina Gherghel is a lecturer in ophthalmology at the School of Life and Health Sciences, Aston University.
References
- Balachandran JS, Patel SR. In the clinic. Obstructive sleep apnea. Ann Intern Med. 2014 Nov 4;161(9):
- Guilleminault C: Clinical features and evaluation of obstructive sleep apnea. In: Karger MH, Roth T, Dement WC eds. Principles and Practice of Sleep Medicine. London: Saunders, 1994:667-677.
- Masood A, Phillips B: Sleep apnea. Curr Opin Pulm Med 2000;6:479-484.
- Castorena-Maldonado A, Espinosa-Morett L, Arrendondo Del Bosque F et al: Diagnostic Value of the Morphometric Model and Adjusted Neck Circumference in Adults with Obstructive Sleep Apnea Syndrome. Rev Invest Clin. 2015 Jul-Aug;67(4):258-65.
- Malhotra A, White DP: Obstructive sleep apnoea. Lancet 2002;360:237-245.
- Ayappa I, Norman RG, Krieger AC, Rosen A, Om RL, Rapoport DM: Non-invasive detection of respitratory effort-related arousals (REras) by nasal cannula/pressure transducer system. Sleep 2000;23:763-771.
- Pittman SD, Tal N, Pillar G, et al: Automated detection of obstructive sleep disordered breathing events using peripheral arterial tonometry and oximetry. Comput Cardiol 2000;27:485-488.
- Eisensehr I, Ehrenberg BL, Noachtar S, Korbett K, Byrne A, McAulley A, Palabrica T: Platelet activation, epinephrine, and blood pressure in obstructive sleep apnea syndrome. Neurology 1998;51:188-195.
- Silverberg DS, Oksenberg A Are sleep-related breathing disorders important contributing factors to the production of essential hypertension? Curr Hypertens Rep 2001; 3: 209-215
- Hoffstein V, Mateika S, Rubinstein I, Slutsky AS: Determinants of blood pressure in snorers. Lancet 1988;2:992-994.
- Ferini-Stambi L, Lombardi GE, Marelli S, Galbiati A: Neurological deficits in obstructive sleep apnea. Curr Treat Option s Neurol 2017; 19: 16
- Duchna HW, Guilleminault C, Stoohs RA, Faul JL, Moreno H, Hoffman BB, Blaschke TF: Vascular rectivity in obstructive sleep apnea syndrome. Am J Respir Crit Care Med 2000;161:187-191.
- Peppard PE, Young T, Palta M, Skatrud J: Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med 2000;342:1378-1384.
- McGinty D, Beahm E, Stern N, Littner M, Sowers J, Reige W: Nocturnal hypotension in older men with sleep related breathing disorders. Chest 1988;94:305-311.
- Coloma Navarro R, Jiménez Caballero PE, Vega G, Ayo-Martín O, Segura Martín T. Cerebral hemodynamics is altered in patients with sleep apnea/hypopnea syndrome. Springerplus. 2016; 5:51
- Fletcher EC, Lesske J, Behm R, Miller III CC, Stauss H, Unger T: Carotid chemoreceptors, systemic blood pressure, and chronic episodic hypoxia mimicking sleep apnea. J Appl Physiol 1992;72:1978-1984.
- Dart RA, Gregoire JR, Gutterman DD, Woolf SH: The association of hypertension and secondary cardiovascular disease with sleep-disordered breathing. Chest 2003;123:244-260.
- Mojon DS, Goldblum D, Fleischhauer J, Chiou AGY, Frueh BE, Hess CW, Gugger M, Basseti C, Boehnke M, Mathis J: Eyelid, conjunctival, and corneal findings in sleep apnea syndrome. Ophthalmology 1999;106:1182-1185.
- Lee AG: Three questions on the role of sleep apnea syndrome in optic disc edema. Arch Ophthalmol 2001;119:1225.
- Muniesa M, Huerva V, Sanchex-De-La-Torre M, et al. The relationship between floppy eyelid syndrome and obstructive sleep apnoea. Br J Ophthal. 2013 Nov;97(11):1387-90
- Roan V. Get taunt on floppy eyelid syndrome. Review of Optometry, October 1 2015
- Walsh JT, Montplaisir J: Familial glaucoma with sleep apnoea: a new syndrome? Thorax 1982;37:845-849
- Gupta PK, Stinnett SS, Carlson AN. Prevalence of Sleep Apnea in Patients With Keratoconus. Cornea 2012;31:595–599
- Aptel F, Khayl H, Pepin JL, Tamsier R, Levy P, Romanet JP, Chiquet C. Association of Nonarteritic Ischemic Optic Neuropathy With Obstructive Sleep Apnea Syndrome: Consequences for Obstructive Sleep Apnea Screening and Treatment. JAMA Ophthalmol. 2015 Jul;133(7):797-804
- Stein JD, Kim DS, Mundy KM, et al, authors. The association between glaucomatous and other causes of optic neuropathy and sleep apnea. Am J Ophthalmol. 2011;152:989–99
- Archer EL; Pepin S. Obstructive sleep apnea and nonarteritic anterior ischemic optic neuropathy: evidence for an association. J Clin Sleep Med 2013;9(6):613-618.
- von Graefe A: Ueber centrale recidivierende Retinitis. Graefe's Arch Clin Exp Ophthalmol 1866;12:211-215.
- Errera MH, Kohly RP, da Cruz L. Pregnancy-associated retinal diseases and their management. Surv Ophthalmol. 2013 Mar-Apr;58(2):127-42
- Leveque TK, Yu L, Musch DC, Chervin RD, Zacks DN. Central serous chorioretinopathy and risk for obstructive sleep apnea. Sleep Breath. 2007 Dec;11(4):253-7
- Brodie FL, Charlson ES, Aleman TS, Salvo RT, Gewaily DY, Lau MK, Farren ND, Engelhard SB, Pistilli M, Brucker AJ. Obstructive sleep apnea and central serous chorioretinopathy. Retina. 2015 Feb;35(2):238-43
- Robert PY, Adenis JP, Tapie P, Melloni B: Eyelid hyperlaxity and obstructive sleep apnea syndrome. Eur J Ophthalmol 1997;7:211-215.
- McNab AA: Flopy eyelid syndrome and obstructive sleep apnea. Ophthalmic Plast Reconstr Surg 1997;13:98-114.
- Onen SH, Mouriaux F, Berramdane L, Dascotte JC, Kulik JF, Rouland JF: High prevalence of sleep-disordered breathing in patients with primary-open angle glaucoma. Acta Ophthalmol Scand 2000;78:638-641.
- Mojon DS, Hess CW, Goldblum D, Bohnke M, Korner F, Mathis J: Primary open-angle glaucoma is associated with sleep apnea syndrome. Ophthalmologica 2000;214:115-118.
- Marcus DM, Costarides AP, Gokhale P, Papastergiou G, Miller JJ, Johnson MH, Chaudhary BA: Sleep disorders: a risk factor for normal-tension glaucoma? J Glaucoma 2001;10:177-183.
- Mojon DS, Hess CW, Goldblum D, Boehnke M, Koerner F, Gugger M, Bassetti C, Mathis J: Normal-tension glaucoma is associated with sleep apnea syndrome. Ophthalmologica 2002;216:180-184.
- Hayakawa T, Terashima M, Kayukawa Y, Ohta T, Okada T: Changes in cerebral oxygenation and hemodinamics during obstructive sleep apneas. Chest 1996;109:916-921.
- Wei EP, Kontos HA, Patterson JLJ: Dependence of pial arteriolar response to hypercapnia on vessel size. Am J Physiol 1980;238:H697-703.
- Maleki N, Alsop DC, Dai W, Hudson C, Han JS, Fisher J, Mikulis D. The effect of hypercarbia and hyperoxia on the total blood flow to the retina as assessed by magnetic resonance imaging. Invest Ophthalmol Vis Sci. 2011 Aug 29;52(9):6867-74
- Goldblum D, Mathis J, Bohnke M, Bassetti C, Hess CW, Gugger M: Nocturnal measurements of intraocular pressure in patients with normal-tension glaucoma and sleep apnea syndrome. Klin Monatsbl Augenheilk 2000;216:246-249.
- McNicholas WT: Obstructive sleep apnea syndrome: who should be treated? Sleep 2000;23 Suppl4:S187-190
