Over the last few years, dry eye disease and its management in practice has, fortunately, become one of the hot topics circulating around optometrists in the UK. The increasing prevalence of the condition coupled with advances in effective management strategies mean that it is now possible to grasp dry eye patients by the hands and reassure them that something can be done, and that you are the one that can finally help them, rather than yet another pat on the head and a prescription for hypromellose.
Engaging in such conversations with patients with confidence is reassuringly possible, given the plethora of dry eye management options and treatments now available within our remit. What is equally important in one’s armoury is a working understanding of the latest in evidence based research and consensus regarding the best approaches in diagnosis and management of this complicated umbrella of conditions known as dry eye disease.
TFOS and DEWS
In recent decades the Tear Film and Ocular Surface Society (TFOS) sought to bring together experts from around the world in an international research effort, with the aim of better understanding the composition and regulation of the pre-ocular tear film.1 The launch of the original DEWS (Dry Eye Workshop) report in 2007 began what was to become the latest revolution in our understanding of dry eye disease and its management. This was followed by the MGD (Meibomian Gland Dysfunction) report in 20112 and later the CLD (Contact Lens Discomfort) report in 2013.3
The launch of the DEWS II report in July 2017 brings together the very latest in global consensus regarding many aspects of dry eye disease. It has taken those involved two-and-a-half years to develop and has resulted in the latest ‘DED (Dry Eye Disease) Manual’. Like many manuals, it is a sizable volume containing a lot of evidence based information. This series of articles will attempt to concisely summarise the key elements relevant to an eye care practitioner.
Definition
The original DEWS report definition was the first time dry eye was identified as a disease, with many underlying causes, that was deemed to result in symptoms and signs, in association with tear film hyperosmolarity and ocular surface inflammation.
The latest definition from DEWS II is as follows:
Dry eye is a multifactorial disease of the ocular surface characterised by a loss of homeostasis of the tear film, and accompanied by ocular symptoms, in which tear film instability and hyperosmolarity, ocular surface inflammation and damage, and neurosensory abnormalities play etiological roles.
To pick the definition apart, the main element in the outcome of dry eye disease is the loss of homeostasis, the normal status quo, of the tear film. What it really highlights is that the disease is actually caused by the key elements of inflammation, tear film instability and hyperosmolarity (figure 1).
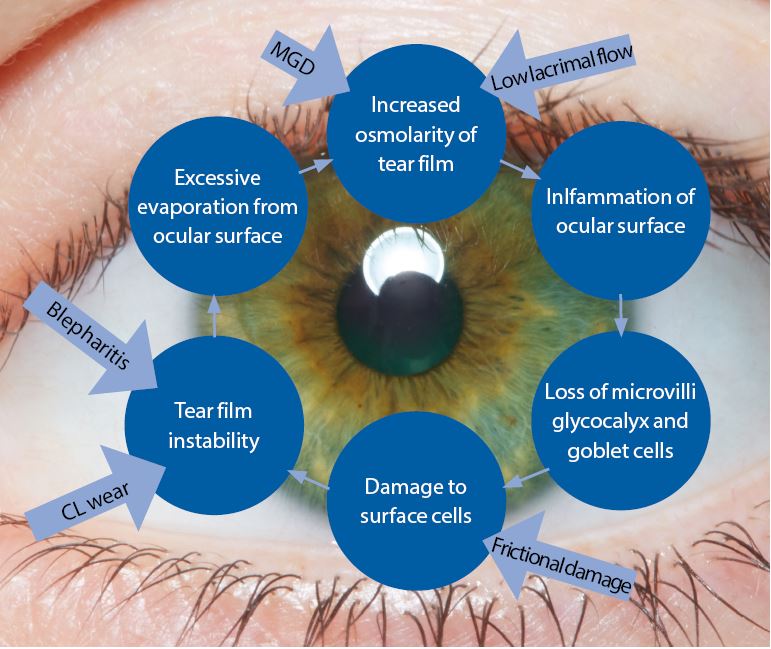
Figure 1: The basic concept of the Vicious Circle (adapted by the author). The core mechanism is hyperosmolarity. The form of dry eye disease determines where on the cycle a patient will enter but, once on the circle, the resultant effect is the same and selfperpetuating
The core mechanism of dry eye disease is tear hyperosmolarity which damages the ocular surface both directly and by initiating inflammation. Tear hyperosmolarity is universally present and ultimately results from excessive evaporation of the water component from the ocular surface. It is this hyperosmolarity that leads to the ‘Vicious Circle’ of dry eye (figure 1). The exact nature of an individual’s dry eye disease determines where they enter the cycle of the disease. The vicious circular nature of the disease means that, once an individual enters the circle, the resulting instability, inflammation and hyperosmolarity further accelerate adverse changes. TFOS DEWS II is the first time the definition has included a signalling role for the disease from issues arising in ocular receptors and nerves.
Prevalence
Looking at existing studies on global prevalence of dry eye disease, the numbers vary enormously depending on the exact criteria used in each study. Studies looking at prevalence of disease involving patient symptoms range between 5% and 50%. Studies looking just at signs alone (ignoring symptoms) generally reported higher and more variable rates of up to 75%. Studies looking for prevalence of both signs and symptoms present ranged between 9% and 30%. Reported prevalence rates specifically for meibomian gland dysfunction, based on clinical signs and expression, ranged from 38% to 68% of individuals over 40. Women consistently have higher prevalence than men.
Classification
The historical classification of dry eye disease has typically considered two distinct disease entities:
- Aqueous deficient
- Evaporative
The latest thinking from the report suggests moving away from this separation and more towards a blurring of the lines between the two classically considered sub-types. In other words, we should consider the disease as more of a continuum of these two sub-types rather than separate entities.
The latest classification scheme encourages a simplified diagnostic strategy to help triage patients (figure 2). The scheme also importantly considers the cases where patients exhibit dry eye symptoms without evidence of obvious signs or, at the opposite end of the spectrum, present with marked signs but have no dry eye symptoms. It also includes a clinical decision guide for practitioners. The classification also recognises the necessity of symptomatic involvement and the need for presence of associated ocular surface signs in making an actual diagnosis of DED.
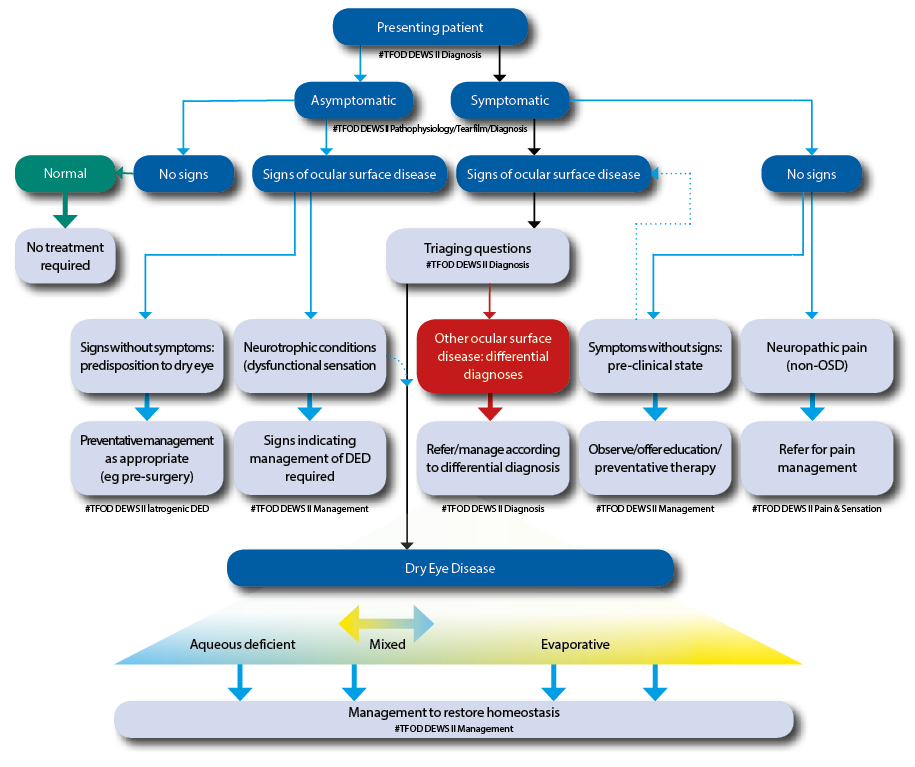
Figure 2: The DEWS II classification of dry eye disease. The upper part of the diagram represents a clinical triage guide, including assessing symptoms, followed by reviewing signs of ocular surface disease. DED exhibits both symptoms and signs and can be treated with appropriate strategies. Symptomatic patients without signs do not fall into the DED group. Asymptomatic patients with signs of DED are then separated into patients with poor corneal sensitivity or those with prodromal signs at risk of developing DED with time or a trigger such as surgery. The lower portion shows the categories of dry eye, now considered as more of a blend or hybrid of the two traditional categories of aqueous deficient (affecting lacrimal gland function) or evaporative (lid related and ocular surface related). More of this sliding scale is devoted to evaporative to reflect the greater proportion of DED attributable to this category. It is acknowledged that, as the disease progresses, it is more likely that both components will become apparent clinically
Pain pathways
Pain in dry eye is caused by tear hyperosmolarity, loss of lubrication, inflammatory mediators and neurosensory factors. Any sort of pain from stimulation of the ocular surface, such as mechanical or chemical is controlled by the ophthalmic branch of the trigeminal ganglion. The detection of wetness, reflex tear production and blink rate is regulated by receptors called cold thermoreceptors. They are receptors in the cornea that are thought to respond to the cooling effect produced by evaporation of the tear film (like sweat evaporating from your skin), by increasing blink rate and basal (base-line) tear secretion. The inflammatory state found in dry eye disease is associated with peripheral nerve and receptor damage, by sensitising the nerve endings and cold receptors and increasing their activity. This increase in activity results in elevated dryness sensation and pain. The long-term effects of ocular surface inflammation and resultant nerve injury cause changes in the neurons which affect their excitability, their interconnection and their firing of signals. These changes in the sensory pathways ultimately cause abnormal sensations of pain from the eye surface.
Symptoms without signs
It is now more widely recognised that often there is a significant mismatch between signs and symptoms in dry eye disease patients. The fact that the latest definition recognises the role of neurosensory abnormalities helps to explain why many patients who present with significant symptoms have very little signs and vice-versa.
The emerging role of neurosensory abnormalities is now considered so important that it is a key part of the new definition. Nociceptors are the receptors designed to detect stimuli that may cause harm to the body. They signal tissue irritation, impending injury or actual injury. On activation, they transmit pain signals via the peripheral nerves to the brain. Nociceptors in the cornea have the potential to be sensitised by repeated physiological stimulation or by noxious stimuli such as hyperosmolarity or inflammation.
Neuropathic pain due to a lesion or disease in the somatosensory system (the sensory system concerned with conscious perception of sensations including pain) results in the scenario where ocular pain symptoms disproportionately outweigh the clinical signs. In these cases, the patient requires pain management and this falls outside the scope of DED therapy (figure 3).
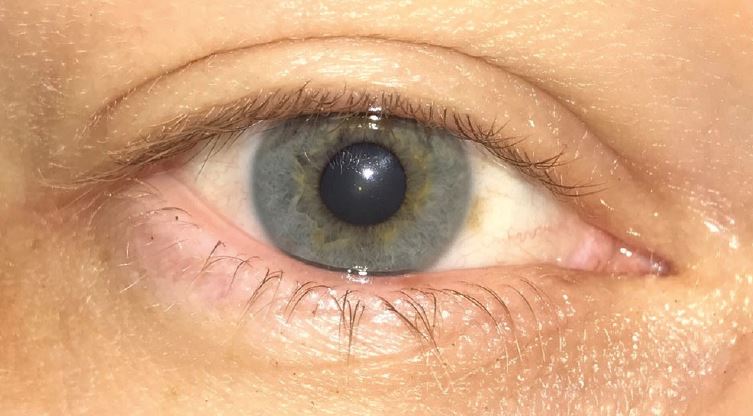 Figure 3: This image shows an example of a 35-year-old male patient who presented in the author’s clinic with significant history of consistently very sore, irritated dry eyes with no specific exacerbating factors. All clinical results were within normal limits including a normal tear osmolarity of R;298 and L;301 measured using TearLab. His complaints of on-going dry eye with no accompanying clinical signs indicated the need for referral for consideration of pain management.
Figure 3: This image shows an example of a 35-year-old male patient who presented in the author’s clinic with significant history of consistently very sore, irritated dry eyes with no specific exacerbating factors. All clinical results were within normal limits including a normal tear osmolarity of R;298 and L;301 measured using TearLab. His complaints of on-going dry eye with no accompanying clinical signs indicated the need for referral for consideration of pain management.
If symptoms of DED are present without signs, it can alternatively indicate a pre-clinical dry eye or episodic dry eye.
Signs without symptoms
A long-debated consideration in managing dry eye disease is for those patients who present in practice with signs of DED and no symptoms. As a practitioner, it can be difficult to decide whether to try and convince the patient to begin a rigorous regime for a problem that they are not aware of. The likely causes are either reduced corneal sensitivity or a predisposition towards dry eye.
Corneal nerve damage secondary to longstanding DED is a recognised issue resulting in reduced sensitivity and will mask patient discomfort. If a patient has early disease, then this might place them at risk of developing symptomatic DED if they undergo ocular surgery such as cataract or refractive surgery. Asymptomatic MGD has recently been reported with a prevalence double that of symptomatic MGD. It is known that symptoms of MGD become more common and more severe with age and, as such, in these cases preventative management should at least be discussed with the patient (figure 4).
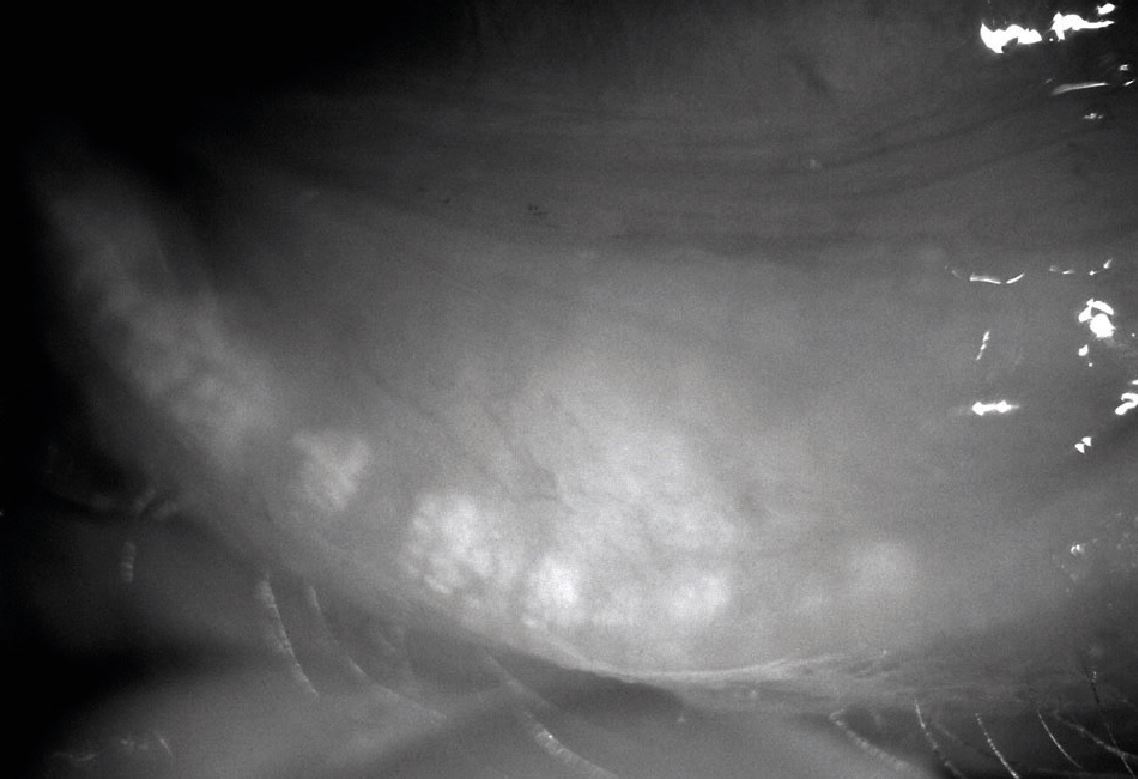 Figure 4: This image is an example of a female patient in her 50s with significant signs of meibomian gland atrophy (grade 4 meibography) with no reported symptoms of dry eye disease on questioning. The patient was advised to treat the condition thoroughly despite the lack of symptoms
Figure 4: This image is an example of a female patient in her 50s with significant signs of meibomian gland atrophy (grade 4 meibography) with no reported symptoms of dry eye disease on questioning. The patient was advised to treat the condition thoroughly despite the lack of symptoms
Sex matters
Being female is unfortunately a significant risk factor for dry eye. A large study in America5 showed the age-related increase in risk for dry eye disease among women to be 70% greater than men. Women have also been shown to report a greater subjective impact of symptoms and visual quality than men. Sex-related differences are present in almost every cell in the body. Many sex-related differences in the eye are attributable to the effects of the sex steroids; androgens, oestrogens and progestins. These steroids have been shown to act on, among others, the meibomian glands, lacrimal glands, conjunctiva and cornea. Gender and biological sex not only affect the DED risk presentation of the disease, the immune response and pain awareness, but also affect the likelihood of an individual seeking help and making use of services available. Women who use postmenopausal hormone therapy have also been shown to be more likely to have DED.
Risk factors
The current understanding of the risk factors likely to be associated with dry eye disease can be split into groups according to how likely the association is (see table 1).
Disease progression?
A theoretical model of dry eye progression 6 proposes the idea that dry eye disease evolves through three stages:
- Initiation of DED
- Reflex compensation
- Loss of the compensatory response
This model suggests that disease may worsen without intervention and over time, and may eventually plateau at a certain stage. Other views also report that not all disease is progressive, although severe disease does appear to progress irrespective of treatment. It is recognised that the disease can wax and wane.
Conclusion
It is clear from the latest DEWS II review that the interest, understanding and attempted management of dry eye disease is ever increasing. As eye care practitioners, we are in the perfect primary care setting to be managing dry eye disease and improving patient symptoms and signs. The need for us as practitioners to have a good understanding of DED, accurate diagnosis and differentiation from other ocular surface diseases is the key to consideration of appropriate management options.
The next article in this series will review the latest strategies in the accurate diagnosis of dry eye disease and how best to approach this diagnostic work up in a practice setting.
Sarah Farrant is a therapeutic optometrist with a specialist interest in dry eye disease practising in Somerset, UK.
References
- www.tearfilm.org
- http://iovs.arvojournals.org/article.aspx?articleid=2126267
- http://www.tearfilm.org/tfoscldreport-english/tfos-cld-report-index.htm
- Schaumberg et al. Prevalence of dry eye syndrome among US women. American Journal of Ophthalmology, 2003;136:318-26
- Bron A et al. Predicted phenotypes of dry eye: proposed consequences of its natural history. Ocular Surface, 2009;7(2):78-92.
- Viso E et al. Prevalence of asymptomatic and symptomatic MGD in the general population of Spain. IOVS, 2012:53(6):2601-6.
