Pigmented fundus lesions are often noted on routine optometric examination. They range from lumps and bumps that are perfectly harmless, where the patient remains asymptomatic throughout their life, to sight- and life-threatening conditions that are cause for urgent referral. They are also often difficult to discern from each other because of the variety of types seen and there are many differentiating characteristics. It is therefore imperative that optometrists working in primary care have a sound understanding of their differential diagnoses. This article will discuss the various pigmented lesions encountered by optometrists on examination of the fundus.
Melanin
Melanin is a biological pigment which is present in most organisms, of which there are three types: eumelanin (brown and black pigments found in the skin and hair), pheomelanin (reddish pigments found in greater abundance in red-haired people) and neuromelanin (melanins found in the nervous system).1 Melanin is produced within organelles known as melanosomes in specialised cells known as melanocytes. The process begins with the amino acid tyrosine which is oxidised (by tyrosinase) to dopaquinone, which then goes through cyclization to produce indole-5,6-quinones (chemicals which are also found in the browning of bananas) and then polymerisation reactions to form melanins.2
Melanin has several functions in the human body, the most important of which being protection against ultraviolet (UV) radiation, which is borne out in the 70-fold increased risk of skin cancers in those with white skin versus black skin, the latter containing more eumelanin specifically. This protective effect appears to be achieved by both scattering and absorbing UV radiation in the epidermis.3 Melanins also have anti-oxidative and free radical scavenging capabilities,4 as well as influencing cytokine production, thus enhancing immune response.1
Within the eye, melanin is found in the iris and ciliary body stromata as well as the ciliary, iris and retinal pigment (RPE) epithelia.5 While the pigmentation (primarily eumelanin) of pigmented epithelium is dense in all races and eye colours, pigmentation of uveal melanocytes varies greatly between humans, and the relative proportions and total amount of eumelanin and pheomelanin determines iris colour.6 Like in the skin, the primary role of melanins in the eye appears to be one of light absorption, especially in the iris and RPE. The increased rates of age-related macular degeneration (AMD)7,8 and uveal melanoma9 in individuals with lighter coloured skin hints at this. These may also be explained by choroidal melanin’s antioxidant properties fading with age, which is a lesser problem in melanocyte-rich darker-coloured eyes.5
What to ask and what to record
As the majority of pigmented fundus lesions will be discovered serendipitously, the practitioner will usually have conducted their normal history taking procedure prior to examination of the eyes. However, once a lesion is discovered, practitioners should take a more closed line of enquiry and ask specifically about symptoms that may indicate malignancy. These include vision loss, scotoma, floaters or photopsia.
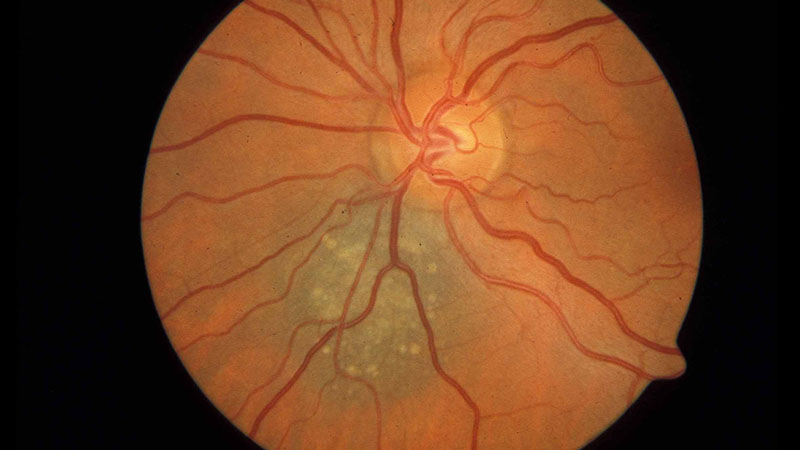
Figure 1: What should you record when presented with this lesion?
Where imaging is possible this should be done, however, all cases should be thoroughly described in the patient’s clinical record too (figure 1). The description should include:
- Colour
- Size (in disc diameters or using slit beam height adjustment)
- Shape
- Distinctness of the margin
- Lesion thickness
- In which structure the lesion is located (eg choroid, RPE, retina)
- Position in the fundus
- Presence or absence of drusen
- Presence or absence of lipofuscin
- Presence or absence of sub-retinal fluid
The list below summarises some useful terminology associated with the description of pigmentary lesions.
- Atrophy: a decrease in cell size
- Carcinoma: a type of cancer of epithelial cells
- Dysplasia: abnormal changes in cell shape, size and organisation
- Hamartoma: a non-neoplastic benign exaggeration of hypertrophy and hyperplasia of mature cells
- Hypertrophy: an increase in cell size, the number of cells remains the same
- Hyperplasia: an increase in the number of cells
- Malignant: a tumour that is destructive and may spread to other locations via the blood and lymphatic system
- Metaplasia: a change to a different cell type
- Metastasis: the spread of a malignant tumour to other sites
- Migration: a movement of cells
- Neoplasia: commonly called a tumour, an abnormal proliferation of cells, which may be benign or malignant
Lesions of the RPE and retina
RPE hypertrophic lesions
CHRPE
Congenital hypertrophy of the retinal pigment epithelium (CHRPE) is a relatively common condition, found in just over 1% of the population. They typically appear as a flat, round and well-demarcated lesion that is brown or black in colour, often with central depigmented areas known as lacunae (figure 2). They usually reside in the mid-peripheral fundus, are unilateral, solitary and 2mm to 5mm in diameter, although they may grow slightly over the patient’s lifetime.10 While benign in the vast majority of cases, they have occasionally lead to neoplastic changes.11

Figure 2: Examples of CHRPEs showing distinct lacuna (a) and no lacunae (b)
Histologically, the RPE cells appear up to twice as tall, with an increased melanin content. The retina above this shows loss of outer nuclear layer and photoreceptor cells.12 As expected, optical coherence tomography (OCT) findings correlate with the histological findings, and include a brighter, thickened RPE band, thinning or absence of the outer nuclear layer and ellipsoid zone, posterior shadowing through pigmented areas and transmission through lacunae (figure 3).
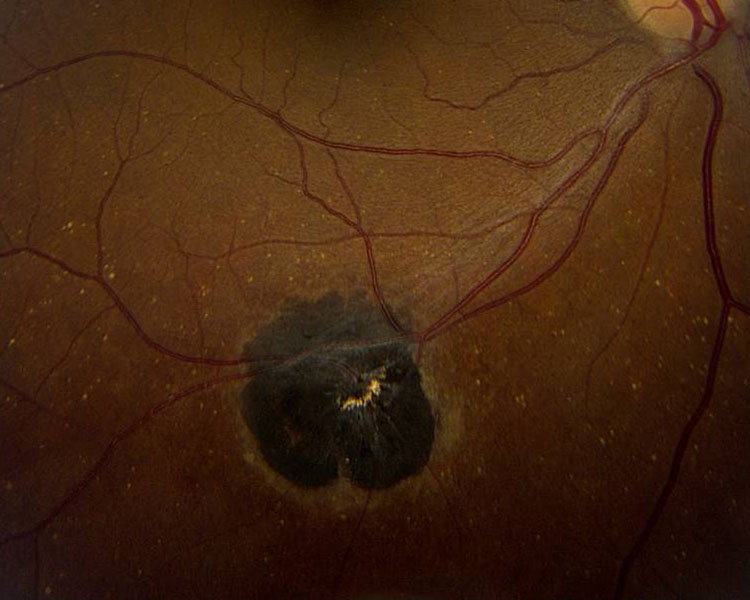
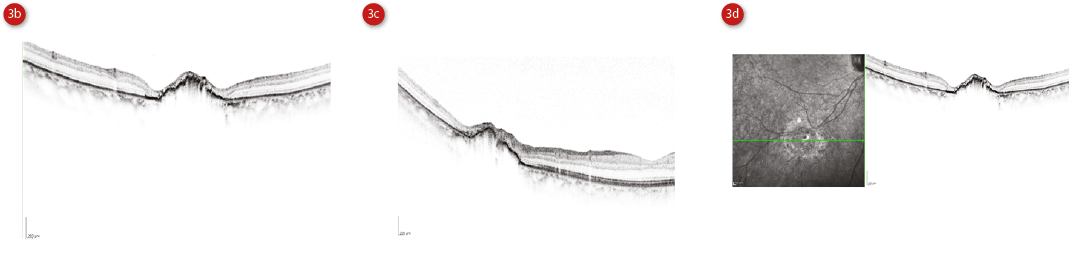
Figure 3: Multicolor image of a CHRPE (a), single OCT scans through the lesion (b and c) and scan next to infra-red image (d). Courtesy of Heidelberg Engineering
OCT may also show a sub-retinal cleft (hyporeflective space).13 Fundus autofluoresence (FAF) is an emerging technology within primary optometric practice in the UK and CHRPE appear as hypoautofluorescent lesions, whereas lacunae are hyperautofluorescent. Visual fields may show an absolute defect.10
Congenital grouped pigmentation of the RPE
A variant of typical CHRPE includes congenital grouped pigmentation of the RPE (CGP-RPE), also known as ‘bear tracks’ because of their paw print-like appearance (figure 4).
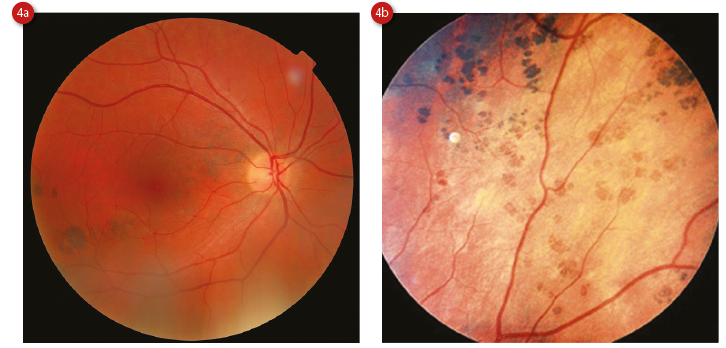
Figure 4: Congenital grouped pigmentation of the RPE (a) and showing more ‘bear track’ appearance (b)
Clinically, they are similar to CHRPEs but smaller (less than 2mm and usually less than 300μm, enlarging towards the periphery) and multiple (up to 30), clustered in single quadrant.11 Histologically, they are somewhat different, with RPE cells that are normal in size but with an increased concentration of melanin. They do not show any changes on OCT, and FAF shows uniformly hypoautofluorescent lesions.10 A variant of CGP-RPE is ‘polar bear tracks’, also known as congenital grouped albinotic spots, which are similar in appearance to CGP-RPE but white in colour. It is postulated that the RPE cells accumulate a whitish material, which is a precursor to melanin.14
Pigmented ocular fundus lesions in familial
adenomatous polyposis
CHRPE-type lesions, known as pigmented ocular fundus lesions (POFL), are also present in familial adenomatous polyposis (FAP), an inheritable condition characterised by the development of colorectal polyps in adolescence. If untreated, the polyps undergo malignant transformation to colorectal cancer, and as a prophylactic measure patients require a colectomy. POFLs are the most frequently encountered lesion of FAP outside of the colon, occurring in about two thirds of patients and is therefore a reliable indicator of disease, especially in those too young to undergo diagnostic sigmoidoscopy or colonoscopy.
FAP with extracolonic lesions, for example skeletal hamartomas and soft tissue lesions (such as lipomas), is often termed Gardner syndrome. Turcot syndrome is the term for FAP in conjunction with brain lesions such as medulloblastomas and astrocytomas. Lesions are usually bilateral and multiple, although not clustered, and a diagnostic criteria of at least four lesions or two lesions, one of which being large has been suggested.15 POFLs may be pisciform (fish-shaped) or oval lesions with irregular pigmentation and a hypopigmented halo, or punctiform round, dark spot-like (less than 0.1 disc diameter) lesions which act as satellite lesion to larger ones.16 On OCT they show hyperreflectivity of the RPE band and thinning of the outer retinal layers.10
RPE adenoma and adenocarcinoma
The RPE does not have a tendency to neoplasia despite a tendency to become hyperplastic, and care should also be taken to discern CHRPE from RPE adenomas (benign) and adenocarcinoma (malignant) which do require treatment, with excision, radiotherapy or enucleation. These appear as oval, raised, deeply pigmented lesions often with concurrent vitreous cells and blood vessel development.17
RPE hyperplastic lesions
RPE hyperplasia is a very commonly seen lesion in optometric practice, whose aetiology may be inflammation, trauma, haemorrhage, retinal detachment or idiopathic (figure 5).
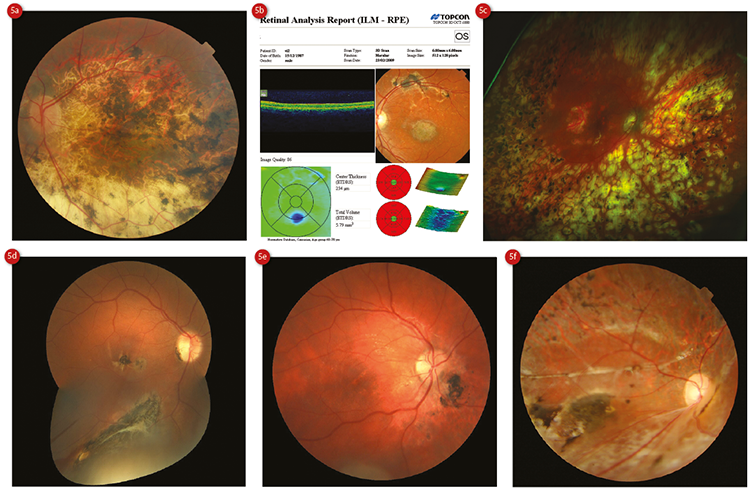
Figure 5: RPE hyperplasia resulting from (a) myopic degeneration, (b) cone dystrophy, (c) panretinal photocoagulation, (d) foreign penetrating body, (e) and (f) separate incidents of traumatic commotion retinae
Ophthalmoscopically, they appear as flat, irregular pigment clumping.10 These lesions may be large and difficult to differentiate from neoplastic lesions, and rarely neoplastic lesions may arise from them.18 OCT shows superficial hyperreflectivity and posterior shadowing, with uniform hypoautofluorescence with FAF (figure 6).15
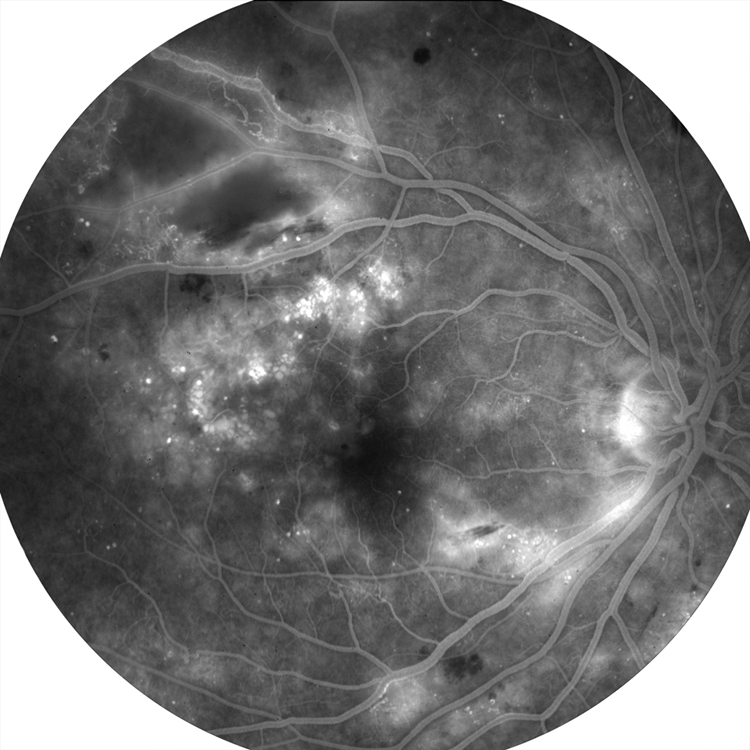
Figure 6: Fluorescein fundus angiography of a patient with diabetic maculopathy – small defined black patches correspond to RPE hyperplasia
Combined hamartoma of the retina and RPE
Combined hamartoma of the retina and RPE (CHR-RPE) is a rare and benign lesion which appears as a charcoal grey, elevated and ill-defined10 with vascular tortuosity and a superficial whitish sheen. They are usually located in the juxtapapillary region but sometimes in the peripheral fundus. They are not usually linked to any other condition but have been associated with neurofibromatosis (especially type 2). Patients usually present in childhood with blurring, metamorphopsia, leukocoria and/or strabismus.11 Histologically CHR-RPE appears as hyperplastic RPE and glial cells.10
Lesions of the choroid and retina
Chorioretinal scars of infectious aetiology
Ophthalmoscopically, resolved chorioretinitis secondary to infections such as those caused by the parasite Toxoplasma gondii appear as pigmentary lesions (figure 7). The pigmentation is heterogeneous, and the lesions span the retinal and choroidal layers.19 Obviously, care should be taken to ensure that there is no evidence of activity such as symptoms (photopsia, floaters, vision loss) or any evidence of retinitis, vitritis or uveitis across the whole uveal tract.11 This will be discussed in more detail in a later Optician article.
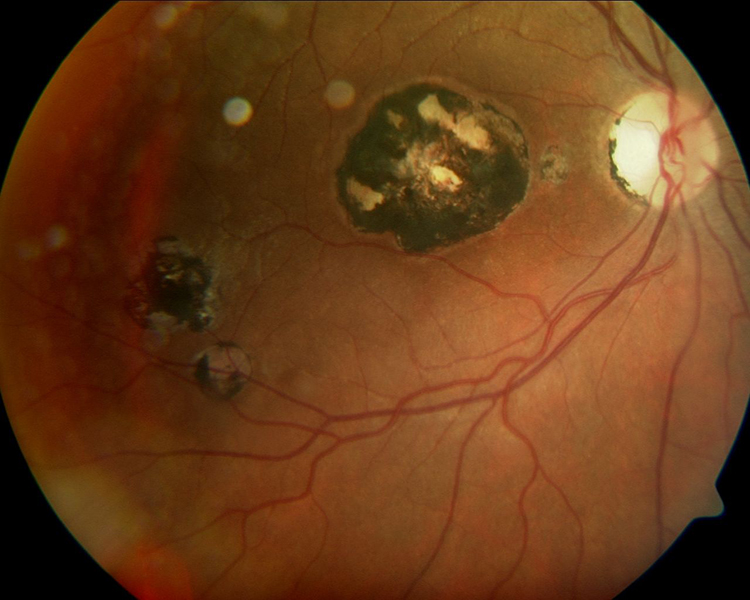
Figure 7: Multiple chorioretinitis scar pigmentation subsequent to Toxoplasma gondii infection
Torpedo maculopathy
This is a congenital hypo-pigmented chorioretinal lesion, typically unilateral and horizontally oval with a pointed-headed nasal margin (pointing towards the fovea), always located in the temporal macula area.20 Visual acuity remains unaffected and the patient is usually asymptomatic, although there is often a corresponding visual field defect. No treatment is required.21
Choroidal naevus
Choroidal naevi are relatively commonly seen neoplasias. The recent National Health and Nutrition Examination Survey,22 a large population-based study in the US, found the prevalence of choroidal naevi in 45° disc-centred and macula-centred photographs to be 4.7%, although the actual prevalence could be as high as 28% when the entire fundus is taken into account. Most patients with choroidal naevi are asymptomatic throughout their life. However, some develop vision loss, visual field loss, photopsia and floaters.23
Histologically, naevi are comprised of so-called naevus cells which are thought to be modified melanocytes. Within the choroid, melanocytes are found in the suprachoroid lamellae, around blood vessels and in the outer stroma. Naevus cells are larger than normal choroidal melanocytes, but also have other morphological variations. These varieties are classified as polyhedral, spindle, intermediate and balloon. In the region of the naevus the choriocapillaris is thinned and drusen may form on Bruch’s membrane, and, although more common in melanomas, lipofuscin may form in the RPE.24
Choroidal naevi are usually well-defined, flat or slightly raised (less than 2mm thick), small (less than 5mm in diameter) lesions which are stable in size.25 In 91% of cases they are posterior to the equator.23 With time, they develop surface drusen (which suggests chronicity, and are present in 98% of naevi13), and display RPE atrophy, hyperplasia and fibrous metaplasia, or even a pigment epithelial detachment (PED).26 The visual acuity of an eye with a naevus tends to be normal, especially when the lesion is peripheral. However, it has been reported as reduced in as many as 26% of those with a subfoveolar lesion,27 which accounts for 6% of lesions (figure 8).23
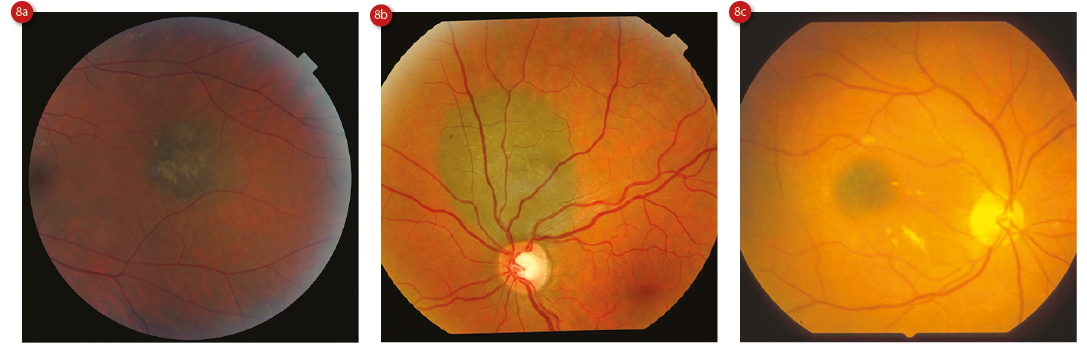
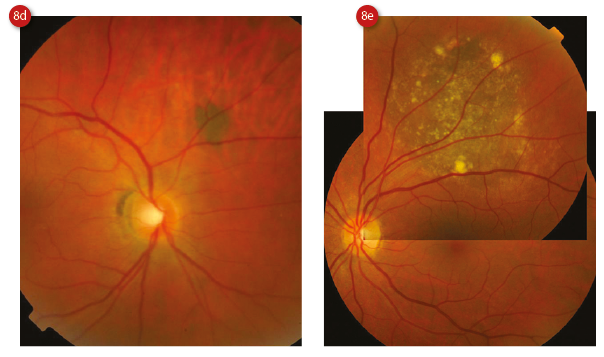
Figure 8: Choroidal naevi showing (a) some drusen, (b) apposition to the disc, (c) subfoveal location, (d) darker hue and (e) large size with multiple surface drusen. Courtesy of Heidelberg Engineering
Amelanotic naevi are those which lack the pigmentation typically seen, and although are thought of as being quite rare, comprise up to 10% of lesions. Histologically, these lesions contain slender spindle naevus cells.24 Lack of pigment in a lesion may cause more diagnostic uncertainly for the practitioner, and care should be taken to discern it from its differential diagnoses which include choroidal metastasis, amelanotic melanoma, choroidal osteoma and posterior scleritis. Practitioners should refer to ophthalmology if there is any diagnostic uncertainty whatsoever.23
Halo naevi are rare, and are named for the yellowish ring of depigmentation surrounding the central naevus. Histologically, this halo is comprised of balloon naevus cells. It has been suggested that these patients should be investigated for skin melanoma due to an associated increased prevalence (3.3% of patients).28
While sub-retinal fluid in choroidal naevi is usually due to leakage at the level of the RPE (which occurs in 15% of young to middle-aged adults with naevi managed at a tertiary referral centre23), eyes with choroidal naevi may develop a choroidal neovascular membrane too. This may be treated using anti-VEGF agents or photodynamic therapy, although other treatment methods have been suggested.26
Optical coherence tomography (OCT) has been suggested as a useful adjunct in the monitoring and diagnosis of choroidal tumours.29 The advent of enhanced depth imaging, as well as swept source OCT, with its longer wavelength and greater penetration through the RPE is of particular use. OCT features of naevi include a flat or dome-shaped elevation of the RPE and retina, sometimes with drusen and sub-retinal fluid.
The choroid shows compressed choriocapillaris as well as diffuse hyperreflectivity, which correlates with the lesion itself, and there is often posterior shadowing.13 Choroidal naevi tend to be hypoautofluorescent on examination with FAF because of chronic RPE atrophy,30 whereas drusen, lipofuscin and sub-retinal fluid appear as hyperautofluorescence (figure 9).13 Compare this picture to a choroidal melanoma, which appears as brightly hyperautofluorescent on FAF.26
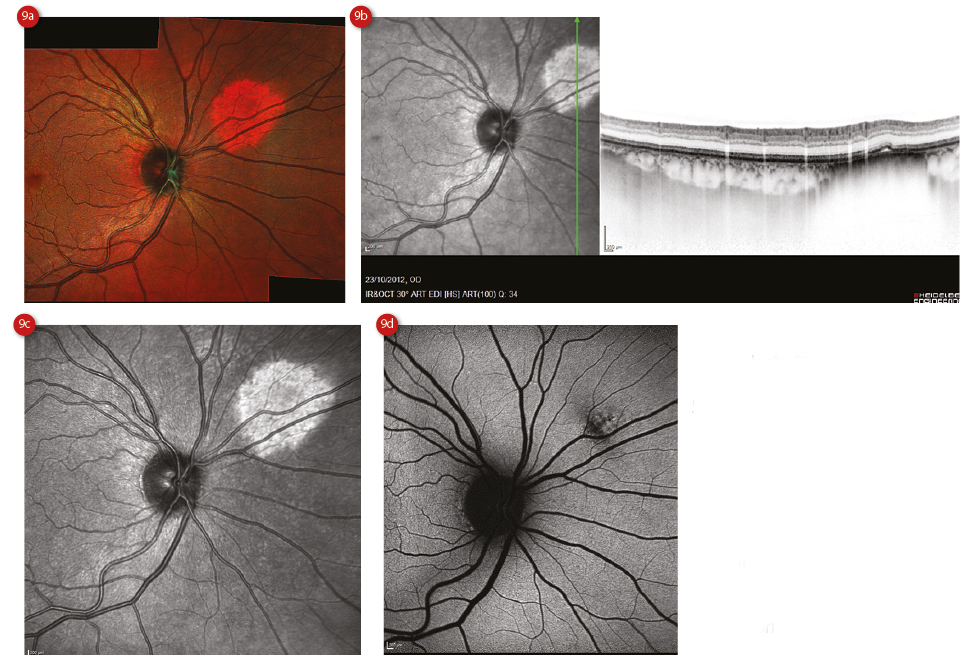
Figure 9: Spectralis assessment of a choroidal naevus showing (a) multicolour representation, (b) OCT scan and infra-red image, (c) enlarged infra-red image and (d) autofluorescent image showing hypofluorescence
It is advisable to monitor these patients annually with imaging13 (and dilation if required), due to the potential for malignant transformation, which is thought to occur in 1 in 8,845 cases.31 Early detection of transformation improves prognosis significantly.32 The introduction of the TFSOM mnemonic in the 1990s greatly aided the detection of transformation. Table 2 shows the key features to consider.
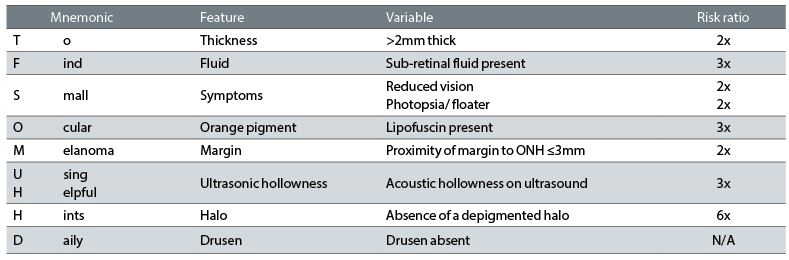
Table 2: The TFSOM-UHHD mnemonic features and risk ratio
Patients with three or more of these features showed a 50% chance of growth in five years (versus 3% chance in lesions with none of the features).33 The features highlighted in this mnemonic are those naevi with presence or absence of lipofuscin pigment or sub-retinal fluid, location in relation to the optic nerve head (ONH), size and symptoms. In 2009, Shields and colleagues32 added the last three features, namely presence or absence of drusen or an amelanotic halo on the lesion, and its ultrasonic characteristics.
Ceri Probert is an optometrist practising in Wales, a tutor at Cardiff University Department of Optometry and is College of Optometrists council member, Wales.
References
1 El Obeid AS, Kamal-Eldin A, Abdelhalim MAK, Haseeb AM. Pharmacological Properties of Melanin and its Function in Health. Basic & Clinical Pharmacology & Toxicology 2017;120(6):515-522.
2 d’Ischia M, Wakamatsu K, Cicoira F, Di Mauro E, Garcia-Borron JC, Commo S, Galvan I, Ghanem G, Kenzo K, Meredith P and others. Melanins and melanogenesis: from pigment cells to human health and technological applications. Pigment Cell & Melanoma Research 2015;28(5):520-544.
3 Brenner M, Hearing VJ. The protective role of melanin against UV damage in human skin. Photochemistry and Photobiology 2008;84(3):539-549.
4 Meredith P, Sarna T. The physical and chemical properties of eumelanin. Pigment Cell Research 2006;19(6):572-594.
5 Hu DN, Simon JD, Sarna T. Role of ocular melanin in ophthalmic physiology and pathology. Photochemistry and Photobiology 2008;84(3):639-644.
6 Wakamatsu K, Hu DN, McCormick SA, Ito S. Characterization of melanin in human iridal and choroidal melanocytes from eyes with various colored irides. Pigment Cell & Melanoma Research 2008;21(1):97-105.
7 Friedman DS, Katz J, Bressler NM, Rahmani B, Tielsch JM. Racial differences in the prevalence of age-related macular degeneration - The Baltimore eye survey. Ophthalmology 1999;106(6):1049-1055.
8 Ambati J, Ambati BK, Yoo SH, Ianchulev S, Adamis AP. Age-related macular degeneration: Etiology, pathogenesis, and therapeutic strategies. Survey of Ophthalmology 2003;48(3):257-293.
9 Hu DN, Yu GP, McCormick SA, Schneider S, Finger PT. Population-based incidence of uveal melanoma in various races and ethnic groups. American Journal of Ophthalmology 2005;140(4):612-617.
10 Ly A, Nivison-Smith L, Hennessy M, Kalloniatis M. Pigmented Lesions of the Retinal Pigment Epithelium. Optometry and Vision Science 2015;92(8):844-857.
11 Denniston AKO, Murray PI. Oxford handbook of ophthalmology. 2014. xxxiv, 1069 pages p.
12 Lloyd WC, Eagle RC, Shields JA, Kwa DM, Arbizo VV. Congenital hypertrophy of the retinal-pigment epithelium - electron-microscopic and morphometric observations. Ophthalmology 1990;97(8):1052-1060.
13 Kaur G, Anthony SA. Multimodal imaging of suspicious choroidal neoplasms in a primary eye-care clinic. Clin Exp Optom 2017.
14 Kim DY, Hwang JC, Moore AT, Bird AC, Tsang SH. Fundus autofluorescence and optical coherence tomography of congenital grouped albinotic spots. Retina-the Journal of Retinal and Vitreous Diseases 2010;30(8):1217-1222.
15 Tiret A, Taiel Sartral M, Tiret E, Laroche L. Diagnostic value of fundus examination in familial adenomatous polyposis. British Journal of Ophthalmology 1997;81(9):755-758.
16 Traboulsi EI, Krush AJ, Gardner EJ, Booker SV, Offerhaus GJA, Yardley JH, Hamilton SR, Luk GD, Giardiello FM, Welsh SB and others. Prevalence and importance of pigmented ocular fundus lesions in gardners-syndrome. New England Journal of Medicine 1987;316(11):661-667.
17 Shields JA, Eagle RC, Shields CL, Brown GC, Lally SE. Malignant Transformation of Congenital Hypertrophy of the Retinal Pigment Epithelium. Ophthalmology 2009;116(11):2213-2216.
18 Shields JA, Shields CL, Slakter J, Wood W, Yannuzzi LA. Locally invasive tumors arising from hyperplasia of the retinal pigment epithelium. Retina-the Journal of Retinal and Vitreous Diseases 2001;21(5):487-492.
19 Butler NJ, Furtado JM, Winthrop KL, Smith JR. Ocular toxoplasmosis II: clinical features, pathology and management. Clinical and Experimental Ophthalmology 2013;41(1):95-108.
20 Trevino R, Kiani S, Raveendranathan P. The Expanding Clinical Spectrum of Torpedo Maculopathy. Optometry and Vision Science 2014;91(4):S71-S78.
21 Golchet PR, Jampol LM, Mathura JR, Jr, Daily MJ. Torpedo maculopathy. British Journal of Ophthalmology 2010;94(3):302-306.
22 Qiu M, Shields CL. Choroidal Nevus in the United States Adult Population Racial Disparities and Associated Factors in the National Health and Nutrition Examination Survey. Ophthalmology 2015;122(10):2071-2083.
23 Shields CL, Furuta M, Mashayekhi A, Berman EL, Zahler JD, Hoberman DM, Dinh DH, Shields JA. Clinical spectrum of choroidal nevi based on age at presentation in 3422 consecutive eyes. Ophthalmology 2008;115(3):546-552.
24 Lewis DA, M AD. Choroidal Nevi. In: Ryan SJ, editor. Retina. 5 ed. London: Elsevier; 2013. p 2219 - 2230.
25 Cheung A, Scott IU, Murray TG, D SM. Distinguishing a Choroidal Nevus From a Choroidal Melanoma. EyeNet 2012 2/1/2012 1.
26 Chien JL, Sioufi K, Surakiatchanukul T, Shields JA, Shields CL. Chereidal nevus: a review of prevaence, features, genetics, risks, and outcomes. Current Opinion in Ophthalmology 2017;28(3):228-237.
27 Shields CL, Furuta M, Mashayekhi A, Berman EL, Zahler JD, Hoberman DM, Dinh DH, Shields JA. Visual acuity in 3422 consecutive eyes with choroidal Nevus. Archives of Ophthalmology 2007;125(11):1501-1507.
28 Shields CL, Maktabi AM, Jahnle E, Mashayekhi A, Lally SE, Shields JA. Halo Nevus of the Choroid in 150 Patients The 2010 Henry van Dyke Lecture. Archives of Ophthalmology 2010;128(7):859-864.
29 Filloy A, Caminal JM, Arias L, Jordan S, Catala J. Swept source optical coherence tomography imaging of a series of choroidal tumours. Canadian Journal of Ophthalmology-Journal Canadien D Ophtalmologie 2015;50(3):242-248.
30 Almeida A, Kaliki S, Shields CL. Autofluorescence of intraocular tumours. Current Opinion in Ophthalmology 2013;24(3):222-232.
31 Singh AD, Kalyani P, Topham A. Estimating the risk of malignant transformation of a choroidal nevus. Ophthalmology 2005;112(10):1784-1789.
32 Shields CL, Furuta M, Berman EL, Zahler JD, Hoberman DM, Dinh DH, Mashayekhi A, Shields JA. Choroidal Nevus Transformation Into Melanoma Analysis of 2514 Consecutive Cases. Archives of Ophthalmology 2009;127(8):981-987.
