Viral keratitis is a common infection of the cornea and not always associated with contact lens use. The most prevalent viruses are Herpes simplex, Varicella zoster and Adenovirus.1 These viruses are found ubiquitously and their transmission is either through contact (Herpes simplex) or through droplets in the air (Varicella zoster and Adenovirus) and contaminated contact lenses (Adenovirus); therefore, infection is common.2 Viral keratitis differs from other microbial keratitis as viruses must invade host cells and utilise their host’s machinery to replicate. The immune response to viruses differs from that of bacteria, amoebae or fungi, as viruses are obligate intracellular infectious agents.3 In addition, although these viruses are associated with keratitis, they cause other common conditions. Herein, the host-virus relationship will be explained, as well as details of each of these viruses.
What are viruses?
Viruses are small infectious agents that replicate inside living cells (bacteria, protists, plants and animals). There is much debate as to whether they can actually be classified as living organisms as they are simply nucleic acid (either DNA or RNA) wrapped in protein and, in some cases, also lipid. However, it is estimated that their origins are with those of the first living organisms on earth 4 and there are several hypotheses to explain their curious life style.5 The idea of their existence came about in late 1800s with Louis Pasteur suggesting that the causative agent of rabies was ‘smaller than a bacterium’ and therefore could not be visualised through a microscope.6 The invention of the Chamberland filter, which could filter particles smaller than bacteria 7 enabled the first studies of viruses as in the 1890s Dmitri Ivanovsky used this filter to isolate and study the tobacco mosaic virus.8
Host-viral relationship and Life cycle
Viral replication and survival is dependent on a living cell as the virus does not possess replication machinery (enzymes, ribosomes, Golgi apparatus –necessary for replication and packaging of viral particles). In layman’s terms they actually ‘hijack’ a living cell (figure 1).
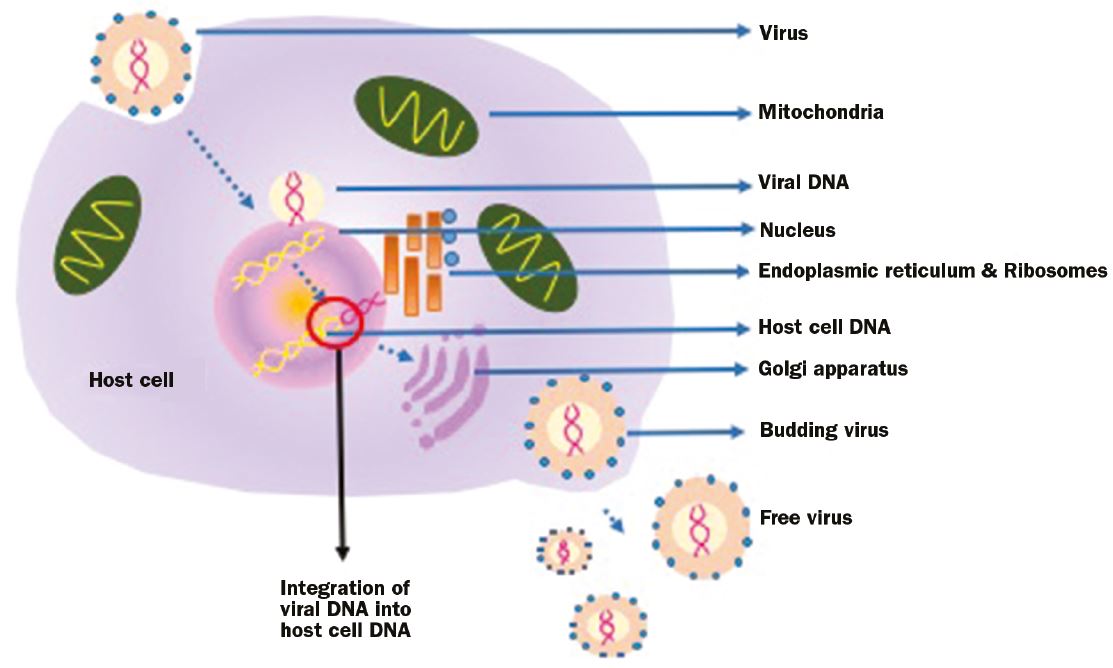 Figure 1: Viruses must ‘invade’ a host cell and utilize its DNA replication and protein translation machinery to produce more virus. This happens through viral DNA integration into the host cell
Figure 1: Viruses must ‘invade’ a host cell and utilize its DNA replication and protein translation machinery to produce more virus. This happens through viral DNA integration into the host cell
This is achieved through attaching to the host cell membrane and inserting viral nucleic acid and some viral protein into the host cell. The nucleic acid then integrates in the host cell genome and viruses will then be produced as the host cells undergoes DNA replication and protein translation.9 The life cycle is complete when viruses bud from the host cell membrane and attack another host cell. In some cases, the full life cycle is not complete for some time. The virus can integrate into the host cell genome and can remain dormant for extensive periods. This is called ‘latency’ and it is a characteristic of Herpes simplex and Varicella zoster that can cause viral keratitis.10 Latency is a way for the virus to also evade the host’s immune response.10
Immune response
Immune responses to viral infections involve not only the elimination of the viruses themselves, but also the elimination of infected host cells. Therefore, immune responses to viruses can often result in extensive tissue destruction. The effector cells are namely the natural killer (NK) cells of the innate immune response and CD8+ T cells of the adaptive immune response.11 These cells respond to virally infected cells through differences of the HLA class I molecules on the host cell surface. Once these cells have been identified both cell types release cytotoxic granules that kill the infected cell, thus eliminating the viral reservoir.
Immune response in the cornea
The exposed position of the cornea means that it is an important tissue to protect against external elements, making it extremely vulnerable to microbial invasion.12 However, in the case of viruses, invasion can also come from the internal environment (discussed later in this article). Due to its protective nature, the cornea has some degree of separation from the rest of the eye as it lacks vasculature and during an inflammatory reaction immune cells come from surrounding vascularised tissues. Some resident innate immune cells (such as dendritic cells) will infiltrate the cornea. Corneal epithelial cells can secrete cytokines,13 which are inflammatory molecules that attract immune cells. In particular, IL-1α and TNF-α are released upon corneal injury and damage. These influence the corneal keratocytes to produce IL-6 and defensins that also have antimicrobial activities.12
In contrast, too great an immune response can be detrimental to the cornea. Long-term effects of IL-1α can lead to neovascularisation (formation of new blood vessels), which can lead to a loss of corneal transparency and IL-6, defensins and other cytokines are involved in corneal transplant rejection. To oppose the risk of uncontrollable immune responses leading to high levels of inflammation, the cornea secretes an IL-1α antagonist and TGF-β that also has a role in wound healing.14
Herpes simplex virus (HSV)
There are two types of HSV. Type 1 infects that oropharynx tract and can cause ocular disease, whereas type 2 infects the genital area. Transmission of type 1 is through close contact and typically it causes herpes labialis, the common cold sore.15 It is estimated that approximately 67% of the world’s population is infected with this virus 16 and its pathogenesis provides an understanding as to why it is the most prevalent cause of viral keratitis. During a primary infection, it enters the cells through the attachment to host cell receptor on epithelial cells 17 and its DNA is injected into the nucleus, where it replicates. Typically, the HSV type 1 virus persists in a quiescent form during latency in neural ganglia, specifically in the trigeminal ganglia via the peripheral sensory nerves. This is called retrograde transport. Latency is allowed through the expression of latency associated transcript (LAT), which interferes with natural cell death.18 Triggering factors, such as secondary infection, stress, UV light, heat can reactivate the virus, which travels backs through the sensory nerves to reactivate in the epidermis. This results in recurrent infection and the reason why cold sores appear time and time again in certain affected individuals.
Herpes simplex keratitis
Ocular infection could also happen from a retrograde transport from an oral infection 19,20 (figure 2).
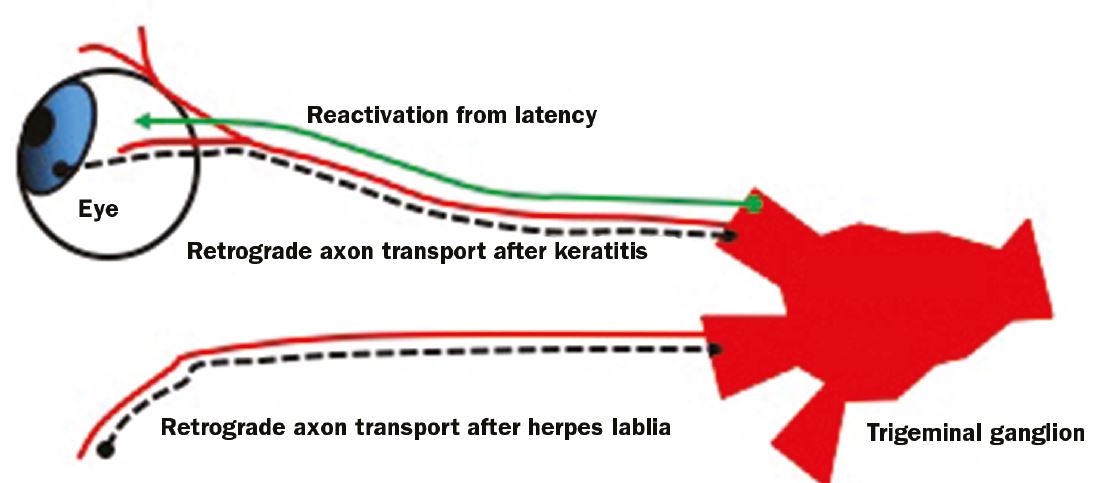 Figure 2: HSV can ‘hide’ in the trigerminal ganglion and reactivate upon stress signals in the patient. HSV keratitis can be from a primary infection or reactivation from a keratitis infection or an extraocular infection (for example herpes lablia or cold sore)
Figure 2: HSV can ‘hide’ in the trigerminal ganglion and reactivate upon stress signals in the patient. HSV keratitis can be from a primary infection or reactivation from a keratitis infection or an extraocular infection (for example herpes lablia or cold sore)
Suspected keratitis caused by HSV is characterised by associated skin lesions, stress-induced recurrence, an immunocompromised status, the presence of superficial dendrites and loss of corneal sensation. However, patients can also present with a primary infection, which is characterised by a diffuse punctate keratitis. The recurrence of Herpes simplex in the cornea is recognisable by blepharoconjunctivitis (inflammation of eyelid and conjunctiva), episcleritis or scleritis (inflammation of the sclera), corneal vesicles and ulcers (corneal, dendritic, amoeboid and limbal). It may also result in neurotrophic keratopathy (degenerative corneal disease induced by an impairment of trigeminal nerve), stromal or disciform keratitis (swelling of the cornea), endotheliitis (inflammation of the endothelial layer), iridocyclitis (inflammation of the iris and ciliary body) and trabeculitis (inflammation of the trabecular meshwork).21
Varicella zoster virus (VZV)
The VZV is a member of the herpes virus family and commonly known to cause chickenpox (or varicella) affecting children to young adults and the cause of shingles in older adults. As with HSV, VZV can undergo retrograde transport to remain in a latent state in neural ganglia. Reactivation of VZV can cause recurrent infection can present as a primary and recurrent infection.22
Herpes zoster ophthalmicus keratitis (HZO)
Recurrent infection is caused by reactivation of VZV that is residing in the ophthalmic nerve (the first division of the trigeminal nerve) and it is known as Herpes zoster ophthalmicus keratitis (HZO) or ophthalmic zoster. It can be transmitted via direct contact or droplets. In the cornea it presents as punctate epithelial erosions that appear two to three days after onset and can last for up to two weeks, but it can also cause nummular keratitis, which is the presence of stromal granular deposits. Disciform keratitis can also develop up to several years after the typical varicella rash onset and there can also be corneal nerve damage.23
Adenovirus
The adenovirus is a common virus that can cause a wide range of symptoms including the common cold, sore throat, bronchitis, pneumonia, diarrhoea, urinary tract inflammation, gastroenteritis, and rarely, neurological disease. It can also infect the eye, where is manifests as keratoconjunctivitis (inflammation of cornea and conjunctiva) and/or pharyngoconjunctival fever (characterised by high fever, pharyngitis, acute follicular conjunctivitis). Adenoviruses are highly contagious and physicians have coined the term ‘epidemic keratoconjunctivitis (EKC)’ to reflect the fact that it tends to occur in closed institutions (schools, camps, hospitals, etc).24 There are more than 50 serotypes of adenovirus and they are transmitted by close contact, sneezing and coughing and touching contaminated objects. Other possible routes of transmission include swimming pools. Since, environmental contamination with adenovirus is a common source of infection, contact lens use is a particular risk factor as lenses could easily come into contact with the virus. Contact lenses of patients should be discarded as the adenovirus can survive both heat and disinfectants, such as hydrogen peroxide.25
Diagnosis
Laboratory identification is important to understand the origin of the infectious agent, in particular for microbial keratitis as clinical presentation is similar across all types. For the diagnosis of viral keratitis, taking a swab or a scraping from an infected eye and adding it to a cell culture flask containing cultured human cells is considered the gold standard for diagnosis.26 Polymerase Chain Reaction (PCR) is also often used to identify specific viruses together with Giemsa stain.27 PCR is the amplification of specific viral DNA sequences from a corneal swab or scraping. The advantage of PCR over culture is the speed of diagnosis (two hours for PCR versus 24 hours minimum for viral in vitro culture). In addition, PCR will have to be achieved after in vitro culture in any case to identify virus and serotype.28 Serology and ELISA (enzyme-linked immunosorbent assay) can also be performed to identify specific antibodies to specific viruses. Diagnosis is highly successful even after a delay as viruses can be detected for long periods of time. For example, adenovirus can be recovered from the eye and throat for as long as 14 days after the onset of clinical symptoms.
Treatment and Management
Most cases of viral keratitis resolve spontaneously within three to four weeks. However, treatment is essential to minimise severe damage to the cornea. For primary infection of HSV keratitis ganciclovir, acyclovir or trifluridine are recommended.29 However, antiviral resistance is emerging a vidarabine is recommended to those non-responsive to treatment. Acyclovir is also used to treat herpes zoster ophthalmicus keratitis, along with topical steroids to reduce the inflammatory response and appropriate pain relief. For adenovirus infection cidofovir is the antiviral of choice, but the patient should be treated in isolation and contact lenses discarded safely. Artificial tears and cold compresses may relieve discomfort and cycloplegic agents may help to relieve severe photophobia.30
Prevention
Viral keratitis is difficult to prevent, especially if it is herpetic as it may be caused by a recurrent infection. If a patient has a history of cold sores, herpes blisters, chicken pox or shingles then it is recommended to avoid touching eyes, eyelids and surrounding areas. As for adenoviral infections, prevention of transmission is critical and patient isolation is highly recommended, followed by discarding contact lenses.
Recommendations for Professionals
If keratitis is suspected, professionals should take a detailed medical history of the patient, paying particular attention to cold sores. Herpes zoster ophthalmicus is evident from the outset as scabbing in the area surrounding the eye is often present. In the case of multiple cases of keratitis in a family or individuals who are in close proximity to each other, then an adenovirus should be suspected and great care to avoid spread and transmission should be employed. This must always include a careful sterilisation of anything used during the consultation which may have been contaminated by the patient. If the patient has recurrent episodes of viral keratitis the discontinuing the use of contact lenses should be discussed.
Professor Fiona L Henriquez is Professor of Parasitology, Infection and Microbiology Group Leader, Institute of Biomedical and Environmental Health Research, University of the West of Scotland.
References
- McGill J, Scott GM, Viral Keratitis, British Medical Bulletin, Volume 41, Issue 4, 1 January 1985, Pages 351–356,
- http://www.who.int/mediacentre/factsheets/fs400/en/
- Braciale TJ, Hahn YS. Immunity to viruses. Immunological reviews. 2013;255(1):10.
- Iyer LM, Balaji S, Koonin EV, Aravind L. Evolutionary genomics of nucleo-cytoplasmic large DNA viruses. Virus Res. 2006 Apr;117(1):156-84.
- Mahy WJ & Van Regenmortel MHV. Desk Encyclopedia of General Virology 1st Edition, Academic Press, 2009
- Bordenave G. Louis Pasteur (1822-1895). Microbes Infect. 2003 May;5(6):553-60.
- Gamgee Arthur. The Pasteur-Chamberland Filter Br Med J 1886; 1 :464
- Lecoq H. [Discovery of the first virus, the tobacco mosaic virus: 1892 or 1898?]. C R Acad Sci III. 2001 Oct;324(10):929-33.
- http://www.hhmi.org/biointeractive/viral-lifecycle
- Grinde B. Herpesviruses: latency and reactivation – viral strategies and host response. Journal of Oral Microbiology. 2013;5:10.
- Koszinowski UH, Reddehase MJ, Jonjic S. The role of CD4 and CD8 T cells in viral infections. Curr Opin Immunol. 1991 Aug;3(4):471-5.
- Akpek EK, Gottsch JD. Immune defense at the ocular surface. Eye. 2003. 17, 949-956.
- Niederkorn JY, Peeler JS, Mellon J. Phagocytosis of particulate antigens by corneal epithelial cells stimulates interleukin-1 secretion and migration of Langerhans cells into the central cornea. Reg Immunol. 1989 Mar-Apr;2(2):83-90.
- Saika S. TGF-beta signal transduction in corneal wound healing as a therapeutic target. Cornea. 2004 Nov;23(8 Suppl):S25-30.
- http://www.nhs.uk/conditions/Cold-sore/Pages/Introduction.aspx
- http://www.who.int/mediacentre/factsheets/fs400/en/
- Birkmann A, Mahr K, Ensser A, et al. Cell Surface Heparan Sulfate Is a Receptor for Human Herpesvirus 8 and Interacts with Envelope Glycoprotein K8.1. Journal of Virology. 2001;75(23):11583-11593.
- Nicoll MP, Hann W, Shivkumar M, Harman LER, Connor V, Coleman HM, et al. (2016) The HSV-1 Latency-Associated Transcript Functions to Repress Latent Phase Lytic Gene Expression and Suppress Virus Reactivation from Latently Infected Neurons.
- Ohara PT, Chin MS, LaVail JH. The Spread of Herpes Simplex Virus Type 1 from Trigeminal Neurons to the Murine Cornea: an Immunoelectron Microscopy Study. Journal of Virology. 2000;74(10):4776-4786.
- Antinone SE, Smith GA. Retrograde Axon Transport of Herpes Simplex Virus and Pseudorabies Virus: a Live-Cell Comparative Analysis. Journal of Virology. 2010;84(3):1504-1512.
- Azher TN, Yin X-T, Tajfirouz D, Huang AJ, Stuart PM. Herpes simplex keratitis: challenges in diagnosis and clinical management. Clinical Ophthalmology (Auckland, NZ). 2017;11:185-191.
- Pergam S, Limaye A, the AST Infectious Diseases Community of Practice. Varicella Zoster Virus (VZV). American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2009;9(Suppl 4):S108-S115.
- Catron T, Hern HG. Herpes Zoster Ophthalmicus. Western Journal of Emergency Medicine. 2008;9(3):174-176.
- http://emedicine.medscape.com/article/1192751-overview?pa=ajsnNZK73u6k8EDHPz0n7jWeVqQdV8HbrC7uCNukYruGliIAwT4lFS%2FNwZVoDYL7jvBe91%2FCLS7rVenEu5xzNqMMvXjUcyZ8uhFNFUtVAb4%3D
- Kowalski RP, Romanowski EG, Waikhom B, Gordon YJ. The survival of adenovirus in multidose bottles of topical fluorescein. Am J Ophthalmol. 1998 Dec;126(6):835-6.
- Sharma S. Diagnosis of infectious diseases of the eye. Eye. 2012;26(2):177-184.
- Farhatullah S, Kaza S, Athmanathan S, Garg P, Reddy SB, Sharma S. Diagnosis of herpes simplex virus-1 keratitis using Giemsa stain, immunofluorescence assay, and polymerase chain reaction assay on corneal scrapings. The British Journal of Ophthalmology. 2004;88(1):142-144.
- Marangon FB, Miller D Alfonso E. Laboratory results in ocular viral diseases: implications in clinical-laboratory correlation. 2007 Arquivos Brasileiros de Oftalmologia, 70(2), 189-194.
- https://www.aao.org/clinical-statement/herpes-simplex-virus-keratitis-treatment-guideline
- Shaikh S, Ta CN. Evaluation and management of herpes zoster ophthalmicus. Am Fam Physician. 2002 Nov 1;66(9):1723-30.
