The launch of the latest DEWS II report in July 2017 has produced a plethora of new and updated ideas regarding the clinical assessment and diagnosis of dry eye disease (DED). As a budding practitioner, it can be hard to interpret the research in a meaningful way in order to put diagnostic techniques into a realistic practical setting given the equipment and time constraints we all face. It is, however, vital to gain enough clinical information to reach a working diagnosis to aid in the sub-classification of the disease and to develop an effective tailored management plan. No single gold standard sign or symptom exists that correlates perfectly with dry eye disease diagnosis. Instead there is a great range of diagnostic techniques available in our remit to help us reach an accurate diagnosis. This article will focus on each diagnostic, and discuss the best evidence based approach for conducting these tests in practice.
KEY DIAGNOSTIC 1: History
The best diagnostic work up begins with a good history, and the assessment of possible dry eye disease is no exception. Although the relationship between signs and symptoms is variable between individuals, assessing symptoms accurately allows decisions to be made regarding whether further evaluation is important, as well as monitoring response to treatments. Dry eye questionnaires exist to aid the specific diagnosis of dry eye disease and grade the severity to set a baseline for future reference. The OSDI (Ocular Surface Disease Index) is the most widely used but the DEQ-5 (Dry Eye Questionnaire 5) is shorter but still accurate, and can be useful in a practice setting. They both ask a number of questions about visual disturbance and function, as well as discomfort. The final score can be graded to set a baseline and for monitoring treatment.
In addition to this, a list of specific questions to ask during a work up has also been developed, with the intention of allowing the practitioner to differentially diagnose dry eye disease compared to other conditions that can mimic the symptoms (table 1).
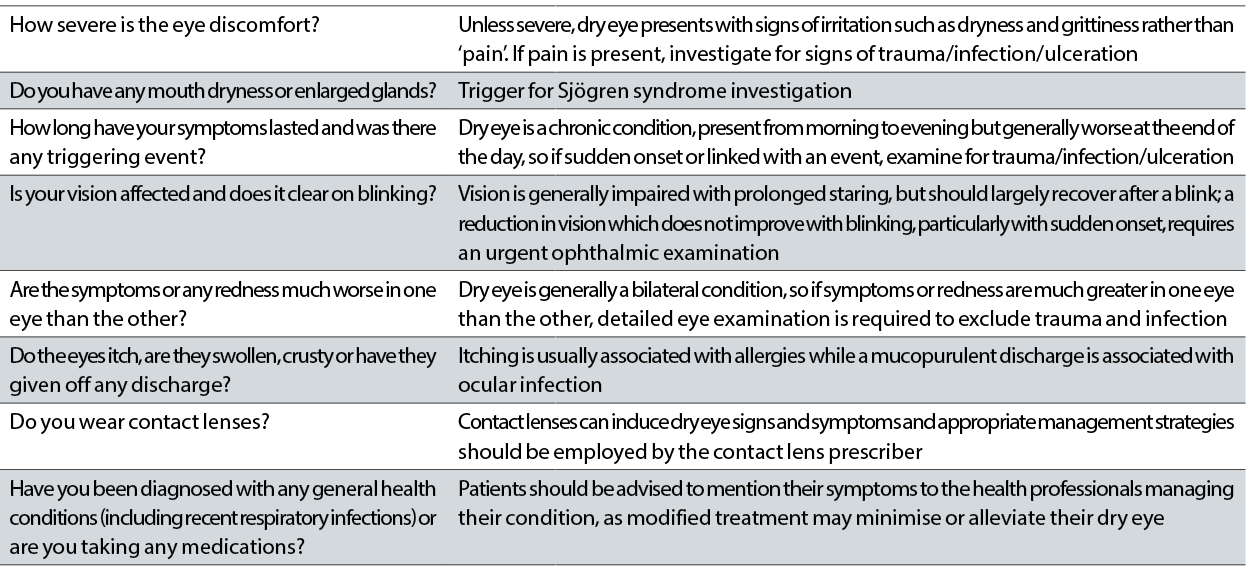 Table 1: Questions to ask to aid differential diagnosis of dry eye disease
Table 1: Questions to ask to aid differential diagnosis of dry eye disease
TOP TESTS
The report has highlighted a number of key tests that are essential in the work up of a dry eye patient. The dry eye diagnostic test battery is summarised in figure 1.
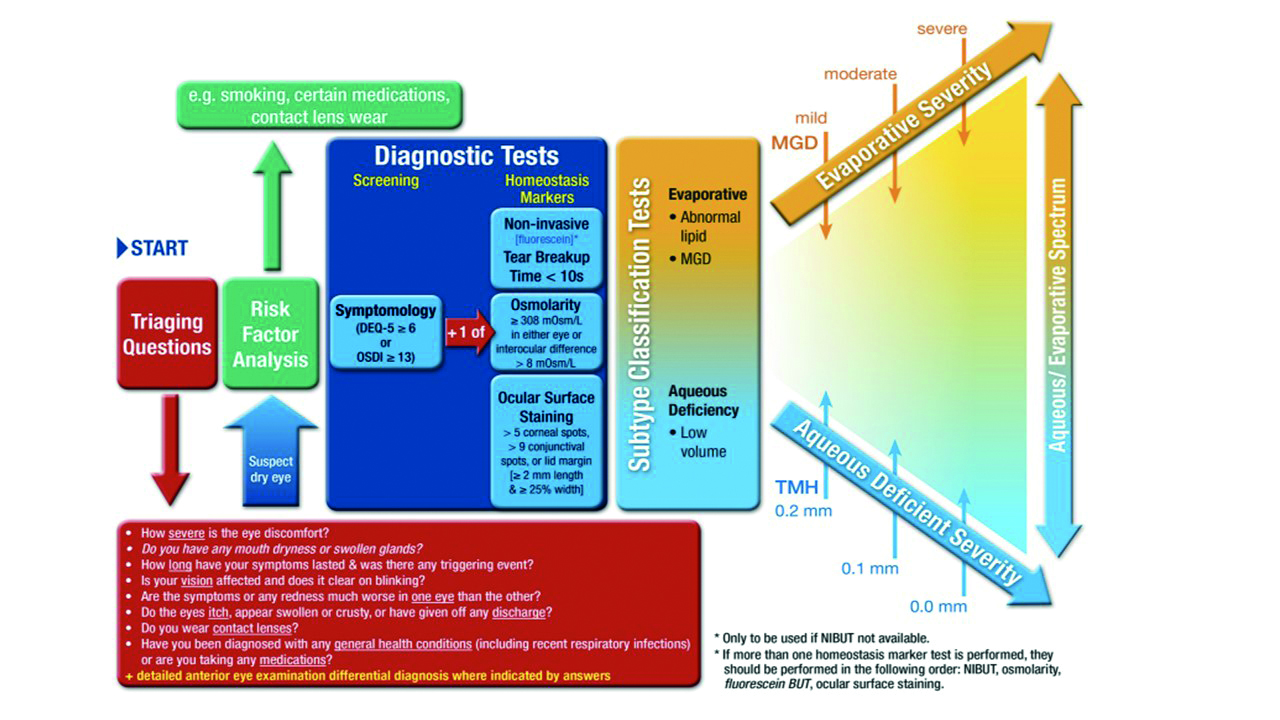
Figure 1: DED Diagnostic test battery: Once dry eye disease is suspected the core diagnostic tests should be carried out; symptomology, tear break up time (TBUT), osmolarity and ocular surface staining. Further tests should then be conducted to look at the possible primary subtype of either aqueous deficient or evaporative. These include MG imaging, observation and expression, lipid thickness and tear volume
CRITICAL DIAGNOSTIC TESTS AND THEIR ORDER:
- Symptoms
- Non-invasive tear break-up time (NITBUT)
- Osmolarity
- Fluorescein break up time (FBUT) if not NITBUT
- Ocular surface staining
TESTS TO INFORM SUBTYPE:
- Meibomian gland imaging (meibography), observation and expression
- Lipid layer thickness measurement
- Tear volume measurement
TOP TESTS WIDELY ACCESSIBLE IN PRACTICE
These are highlighted in table 2, which has been adapted by the author from the report. The table highlights common diagnostic tests and the normal versus dry eye values expected in a practice clinical setting. It also indicates in the right column the amount of change in a test reading required after treatment before a patient will experience a beneficial improvement in symptoms.
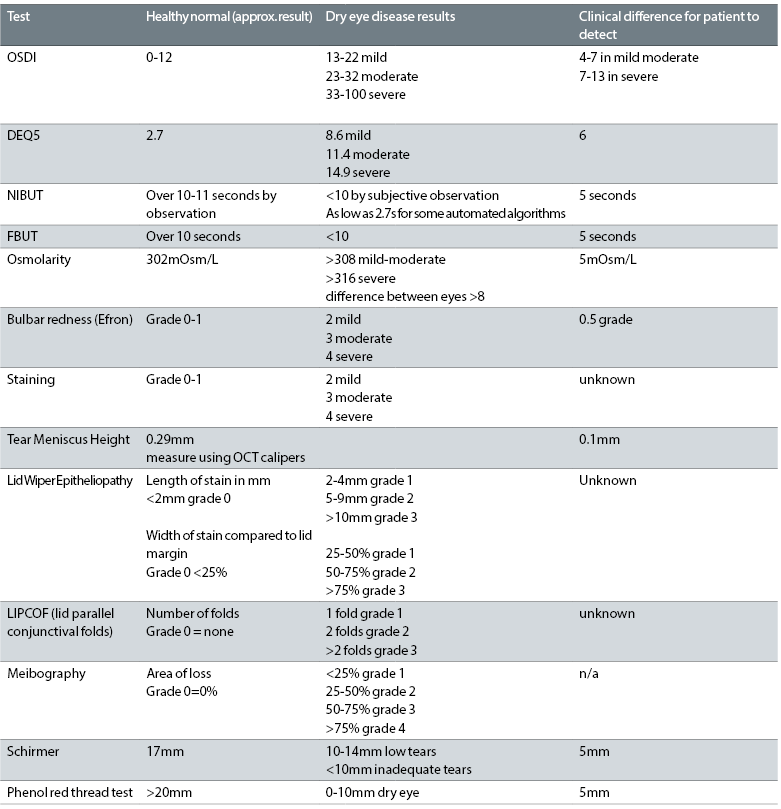 Table 2: Common diagnostic tests and normal and dry eye values
Table 2: Common diagnostic tests and normal and dry eye values
KEY DIAGNOSTIC 2: Tear film stability
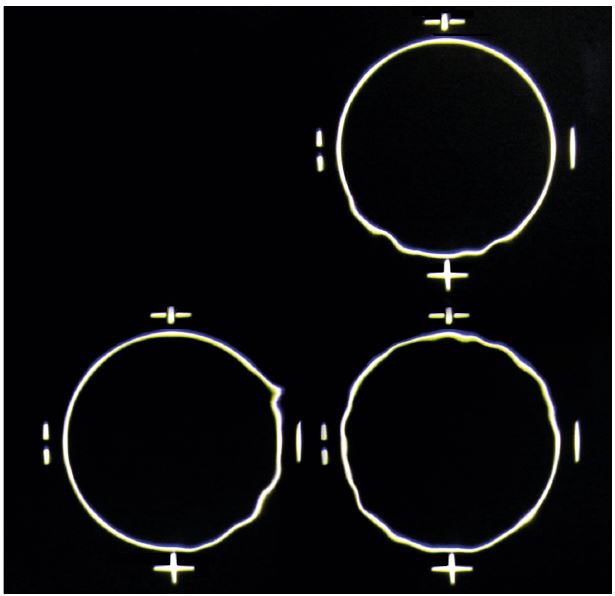 Figure 2: Schematic showing image degradation for the assessment of non-invasive tear break up time
Figure 2: Schematic showing image degradation for the assessment of non-invasive tear break up time
Impaired tear film stability is a key diagnostic feature. Tear break up time (TBUT) is a direct indicator and can be measured with no drop instillation using a tear-scope or automated topography based system (non-invasive NIBUT – figure 2) or using fluorescein (FBUT – figure 3).
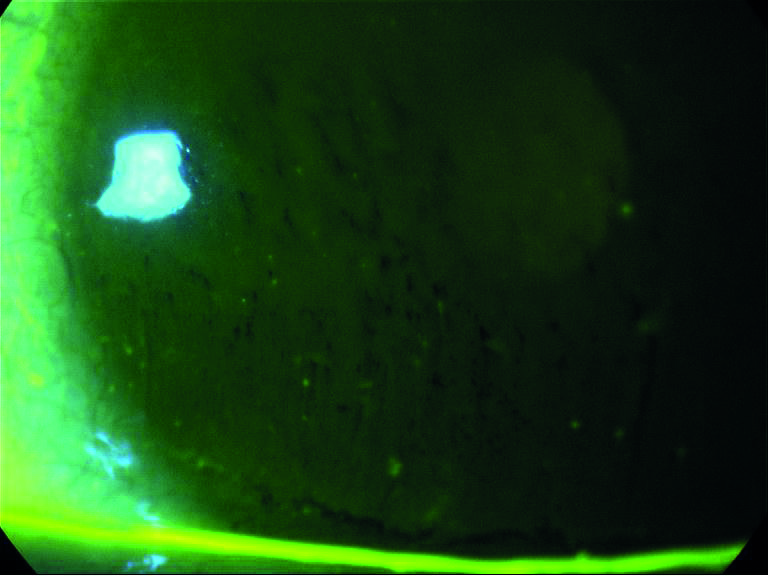
Figure 3: Fluorescein break up
The fluorescein itself reduces the stability of the tears and means the technique is less sensitive and less specific than NIBUT, but its ease of use makes it a very commonly used technique in practice. The fluorescein should be instilled at the outer canthus to avoid surface damage (figure 4), with excess shaken off the strip or a reduced area strip used. Viewing should take place between one to three minutes after installation. A suggested methodology is to instruct the patient to blink naturally three times and then to cease blinking until instructed. If the patient (after instruction) can no longer refrain from blinking before the tear film breaks up, this is typically counted as the breakup time. As long as the approach is consistent between visits, the practitioner will be in a position to potentially gauge change over time.
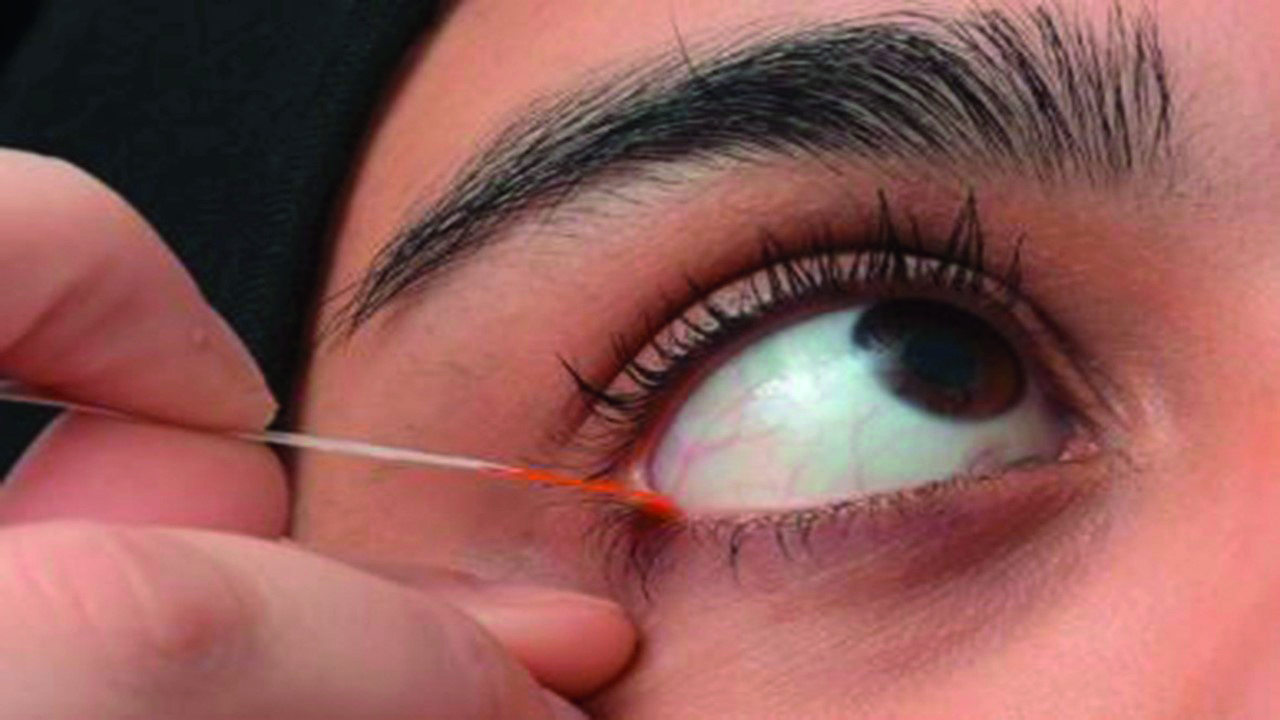
Figure 4: Recommended location to apply ophthalmic dyes in strip form to avoid confounding damage to the conjunctiva and lid margins observed for the diagnosis of DED and its sub-classification. See video on TFOS website for further guidance
KEY DIAGNOSTIC 3: Osmolarity
Tear osmolarity has been shown to have the greatest correlation to disease severity of the clinical DED tests, and has been reported as the single best metric to diagnose and classify DED (figure 5). An osmolarity of 308 or more and or an interocular difference of eight or more is considered positive for dry eye disease. The two values that are important to note are the higher value of the two eyes, which is considered more indicative of the DED process, and the difference in value between the two eyes, which provides insight about the instability of the tear film. There is greater inter-eye variability in osmolarity in dry eye, which increase with severity and decrease with successful management. The tears of DED individuals show increased variability generally due to possible poor mixing of the tears between blinks. Osmolarity measurement is the least variable of all the common signs of dry eye disease over time.
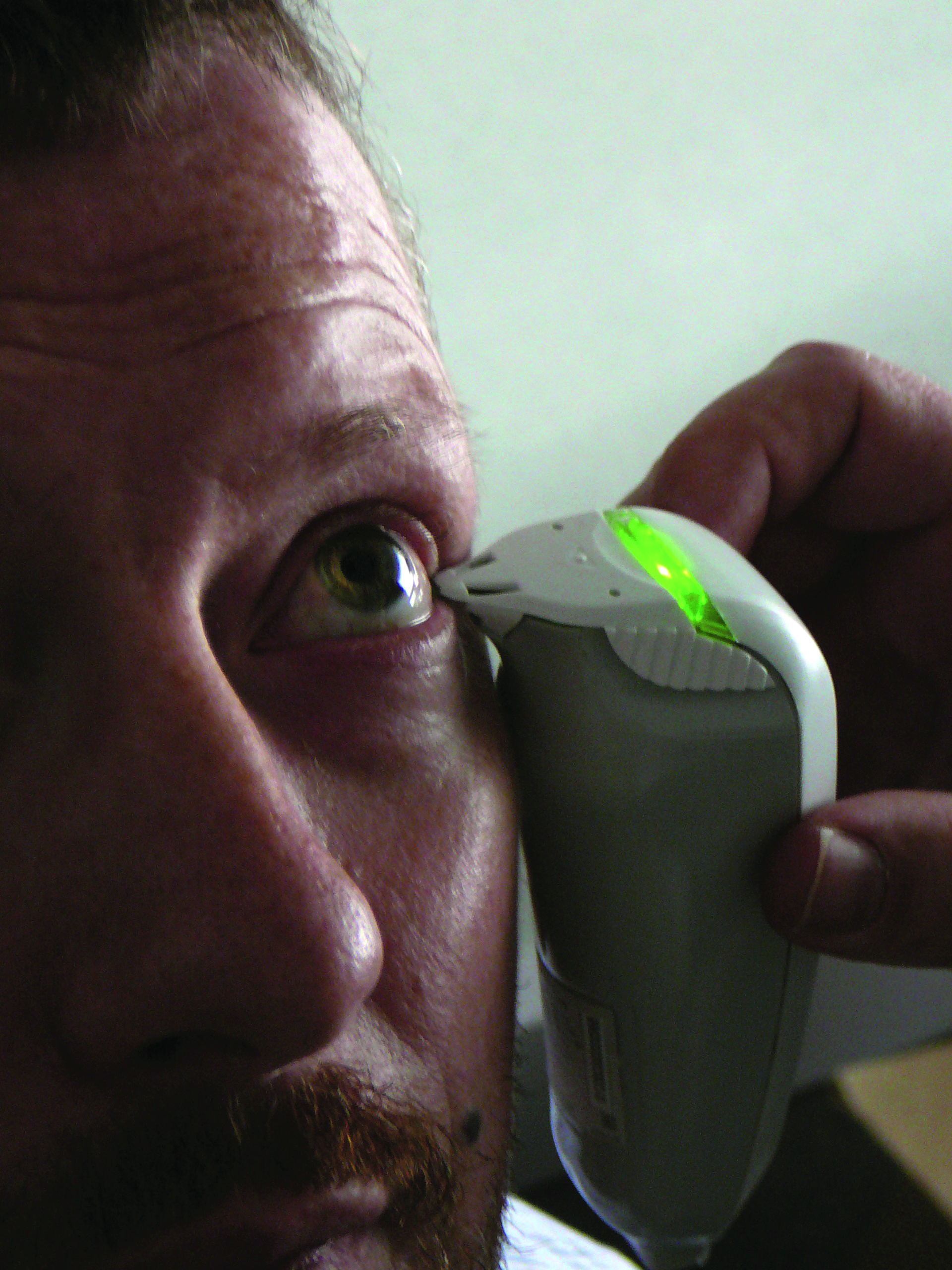 Figure 5: Osmolarity assessment using the TearLab
Figure 5: Osmolarity assessment using the TearLab
KEY DIAGNOSTIC 4: Ocular surface staining
Punctate staining of the ocular surface is a feature of many diseases. In DED, the use of fluorescein and lissamine green is currently advised in the report to highlight corneal and conjunctival/eyelid margin tissue damage respectively. In the UK, lissamine green is a medical device and is an unlicensed product and is currently not recommended for use in practice. Fluorescein stains viable cells with a compromised integrity.
Lissamine green stains epithelial cells only if the cell membrane is damaged (figure 11). When using strips to instil dye, it is recommended to instil a drop from the strip inside the lower temporal lid with the patient in up gaze, with the lid pulled slightly temporally to avoid damage to the conjunctival or lid wiper tissue. Both fluorescein and lissamine green should be observed roughly one to three minutes after instillation. Lid wiper epitheliopathy can be observed with either lissamine green or fluorescein. Corneal staining is considered to probably be a later stage sign of DED, and staining in mild to moderate cases shows poor correlation with disease severity. It is also worth bearing in mind that severity of DED can change with the time of day, so this should be considered when interpreting results and monitoring the condition.
TESTS TO AID SUBCLASSIFICATION
Tear volume
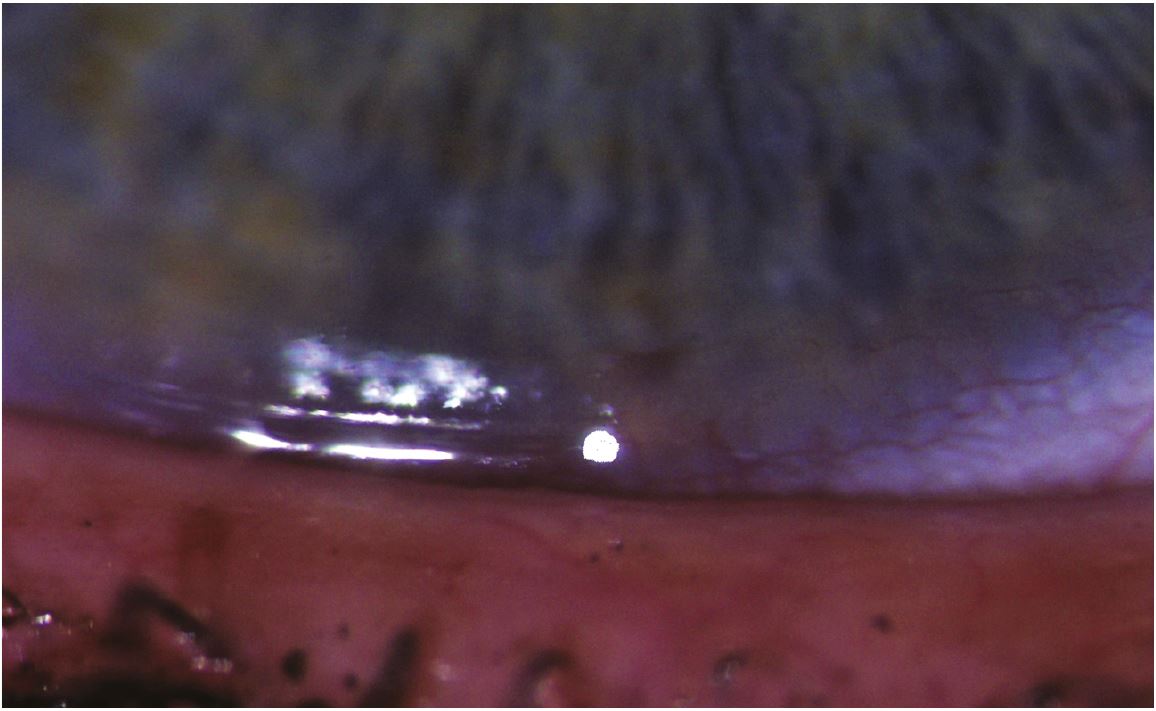 Figure 6 The tear meniscus is measured by gauging its height relative to the known diameter of the slit-lamp projected spot of light
Figure 6 The tear meniscus is measured by gauging its height relative to the known diameter of the slit-lamp projected spot of light
Most of the tear fluid sits in the tear menisci, which serve as reservoirs supplying tears to the pre-corneal tear film. In a practice setting this can be measured using the slit lamp beam height (figure 6), but is poorly repeatable. Schirmer test (figure 7) without anaesthetic remains the recommended diagnostic test for confirming severe aqueous deficiency, but its variability and invasiveness exclude it as a routine diagnostic test of tear volume.
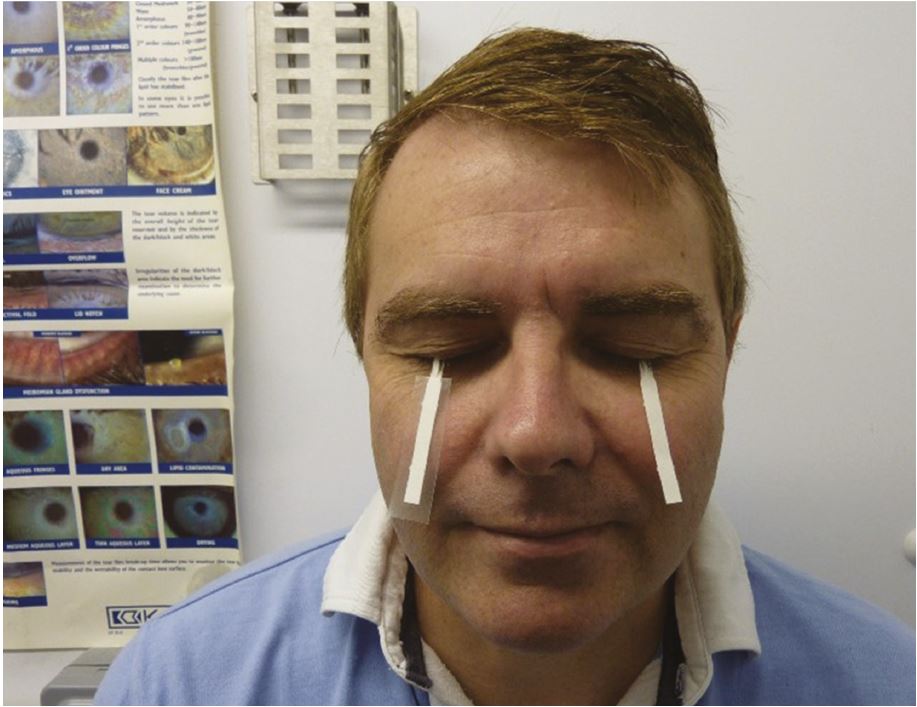 Figure 7: Schirmer test in use
Figure 7: Schirmer test in use
Anterior optical coherence tomography (OCT) assessment involves taking an image in the centre of the lower eyelid without lid manipulation shortly after a blink is advised. The image can then be measured with callipers allowing a non-invasive simple measure (figure 8), but this can easily be disrupted by issues such as LIPCOF (lid parallel conjunctival folds) or conjunctivochalasis.
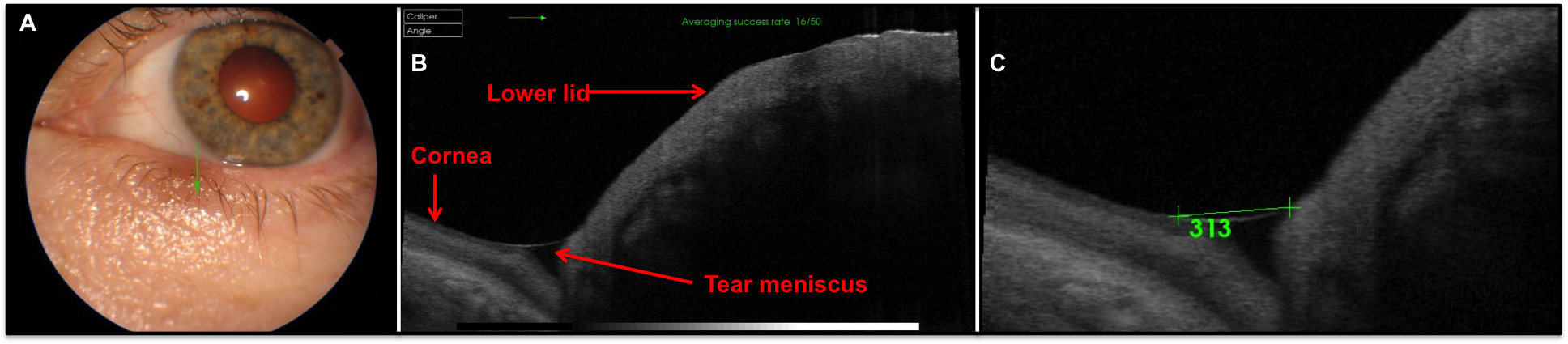 Figure 8: Anterior OCT image showing how tear profile is visible (images courtesy of Topcon)
Figure 8: Anterior OCT image showing how tear profile is visible (images courtesy of Topcon)
LIPCOF
Lid parallel conjunctival folds (LIPCOF) are folds in the lateral lower quadrant of the bulbar conjunctiva (figure 9). They are thought to be associated with decreased mucin production, and correlate with the presence of lid wiper epitheliopathy (lissamine green staining along the lid margin associated with friction). They are observed with white light and can be graded by counting the permanent folds seen.
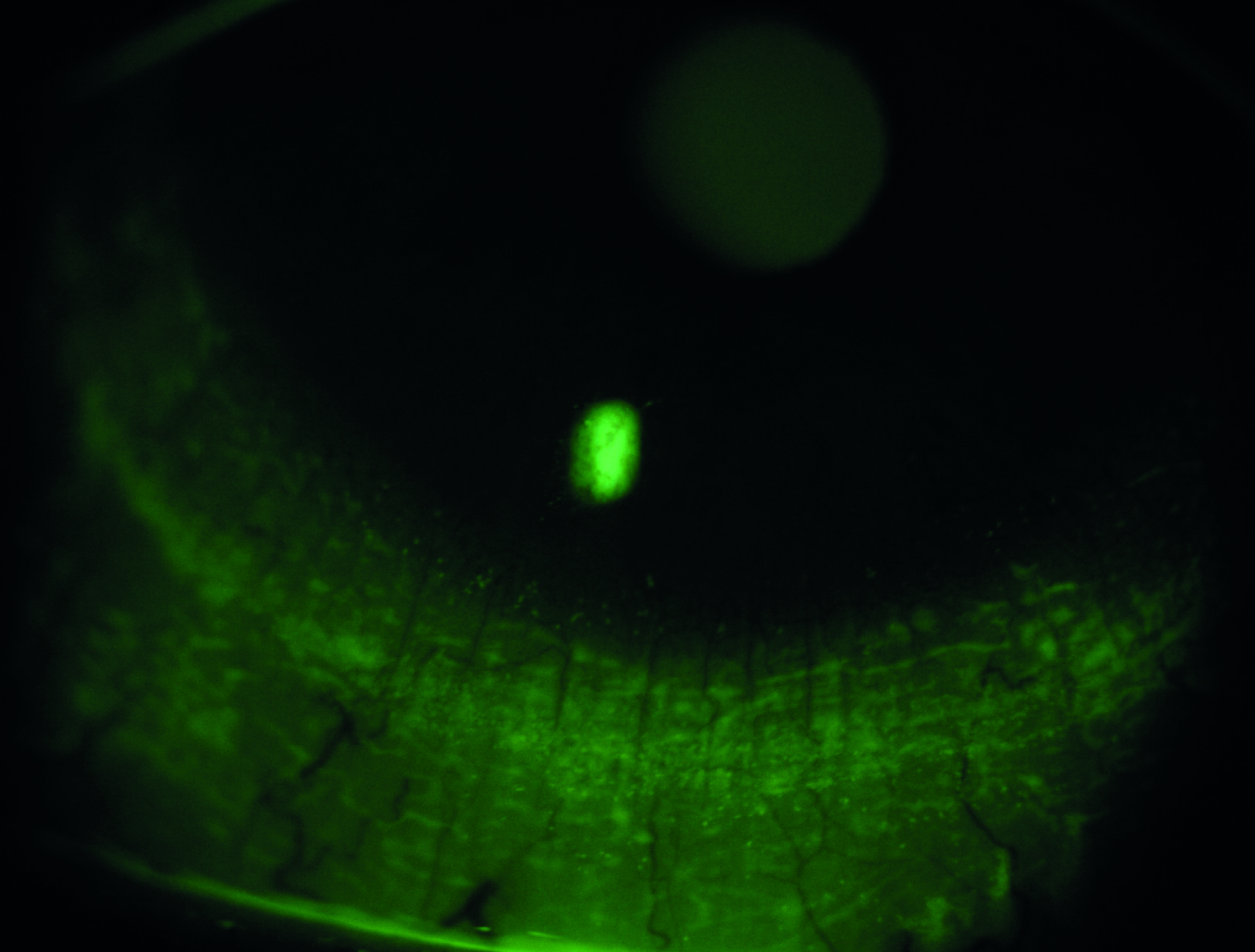 Figure 9: Lid parallel conjunctival folds
Figure 9: Lid parallel conjunctival folds
Inflammation
Inflammation is a recognised component of the mechanism of DED and can indicate its severity. However, inflammation is not specific to dry eye disease and can occur in other ocular or systemic disease. The tests most commonly employed to record it are conjunctival redness grading, either directly by the clinician or using digital image analysis, or directly measuring an inflammatory marker (figure 10), such as matrix metalloproteinases or cytokines, with a commercial device, although it must be considered that these markers tend to increase with age and age specific normal values have not yet been published.

Figure 10: Use of an inflammatory marker
Lid wiper epitheliopathy (LWE)
The lid wiper is the area of the upper and lower lid that wipes the tear film over the ocular surface (figure 11). It appears to be the most sensitive conjunctival ocular tissue. LWE is thought to be related to increased friction throughout blinks, although it may also involve tear film viscosity induced hydrodynamic forces at the start of a blink. It is detected using either lissamine green or fluorescein or both by everting the lid and grading the staining length in mm, and width relative to the lid margin.
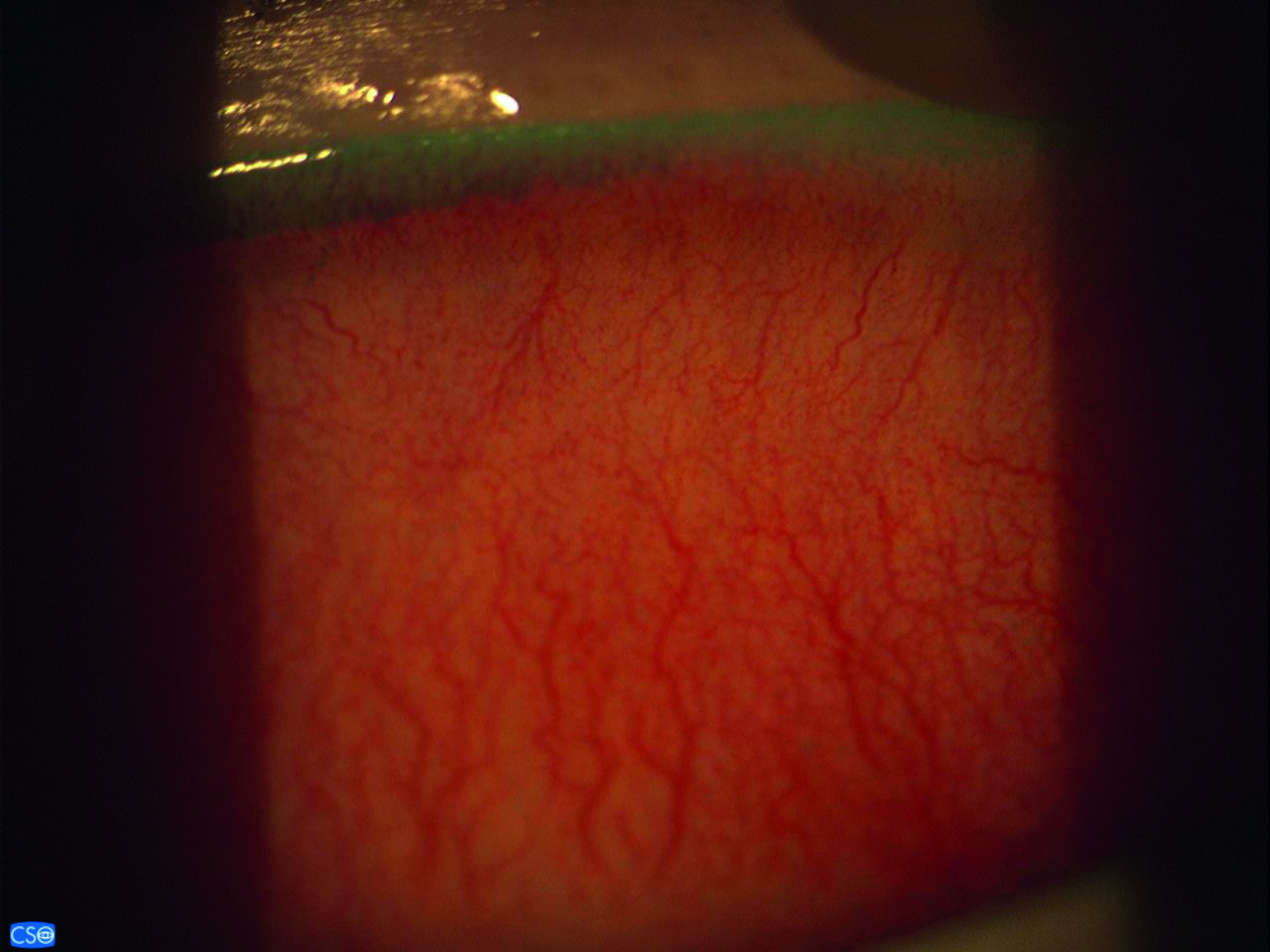 Figure 11: Lid wiper epitheliopathy
Figure 11: Lid wiper epitheliopathy
Meibography
This allows observation of the meibomian gland structure directly (figure 12). It is best carried out using infrared light, either in a mobile device, dedicated device or on a slit lamp. It alone is not sufficient for the diagnosis of MGD, but is a useful adjunct to build up the clinical picture. Unsurprisingly, changes in the glands are less pronounced in aqueous deficiency compared to evaporative issues. The lid is everted and observed. The grading is scored according to the area of loss of defined gland structure.
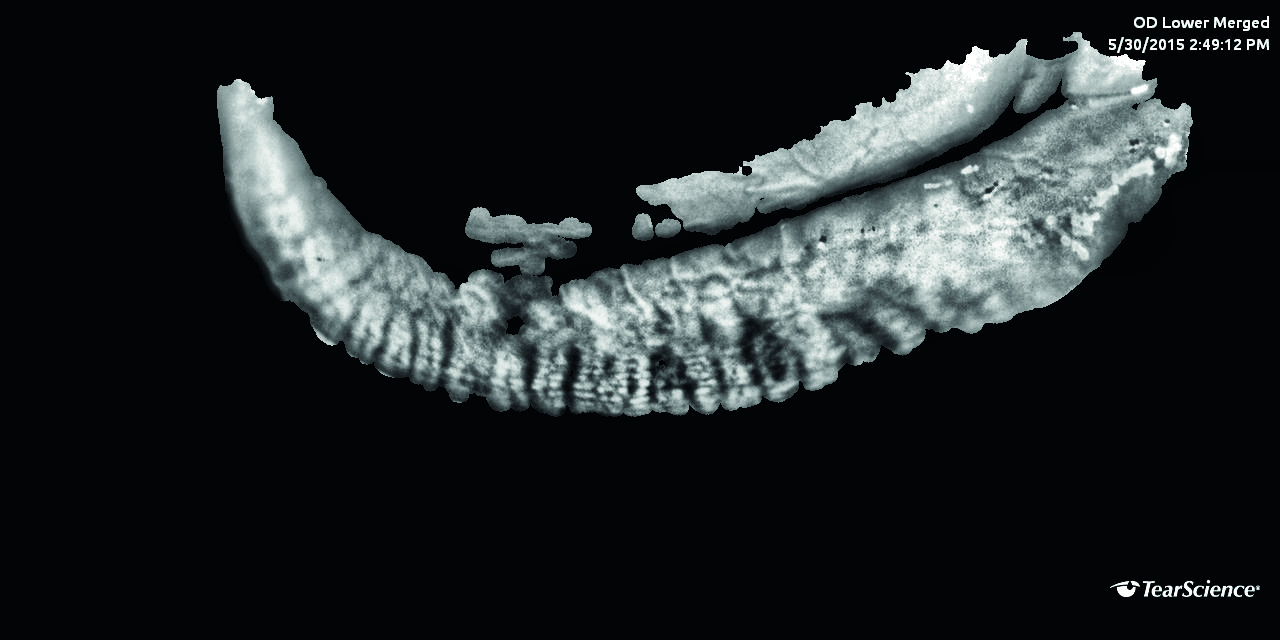 Figure 12: Meibography showing meibomian gland dropout
Figure 12: Meibography showing meibomian gland dropout
MG expression
Meibum quality, quantity and expressibility are thought to reflect gland function. It is carried out in practice by digital expression of the glands along the length of the eyelid, usually with meibomian gland forceps. Normal meibum is clear and readily expressed, but in MGD it loses its clarity and the viscosity increases and becomes increasingly difficult to express in severe MGD. The diagnostic value has not yet been established in DED.
Blink analysis
Blinking action clears debris, provides mechanical protection and reforms the tear film, as well as being vital for meibum distribution. Blink rate and the completeness of the blinks can be evaluated when the patient is performing a task such as questionnaire completion, unaware that they are being observed. The normal blink rate is reported to occur from 10-15 blinks per minute. Incomplete blinking can result in DED symptoms and staining.
According to the report, the best methodical approach during a core work up is as summarised in table 3.

Table 3: Critical diagnostic tests and their order
CO-MORBIDITIES
When diagnosing DED an important part of the work up is to consider co-morbidities that may make the issue more than just the primary DED discussed so far. Typical slit lamp examinations to consider include:
- Eyelashes for anterior blepharitis and Demodex spp
- Eyelid position (ectropion or entropion) and closure
- Eyelid palpebral conjunctiva for MGD, follicles or swelling
- Bulbar conjunctiva for conjunctivitis, redness pattern and swelling
- Cornea for ulceration and staining to detect possible trauma or dystrophies
- Anterior chamber for presence of cells or flare
Blepharitis
Recurrent or persistent blepharitis (figure 13) can cause DED so it is important to examine the eyelid to aid diagnosis. Staphylococcal or seborrheic anterior blepharitis are linked to aqueous deficient dry eye, possibly due to the decreased tear volume supporting less lysozyme or immunoglobulins.
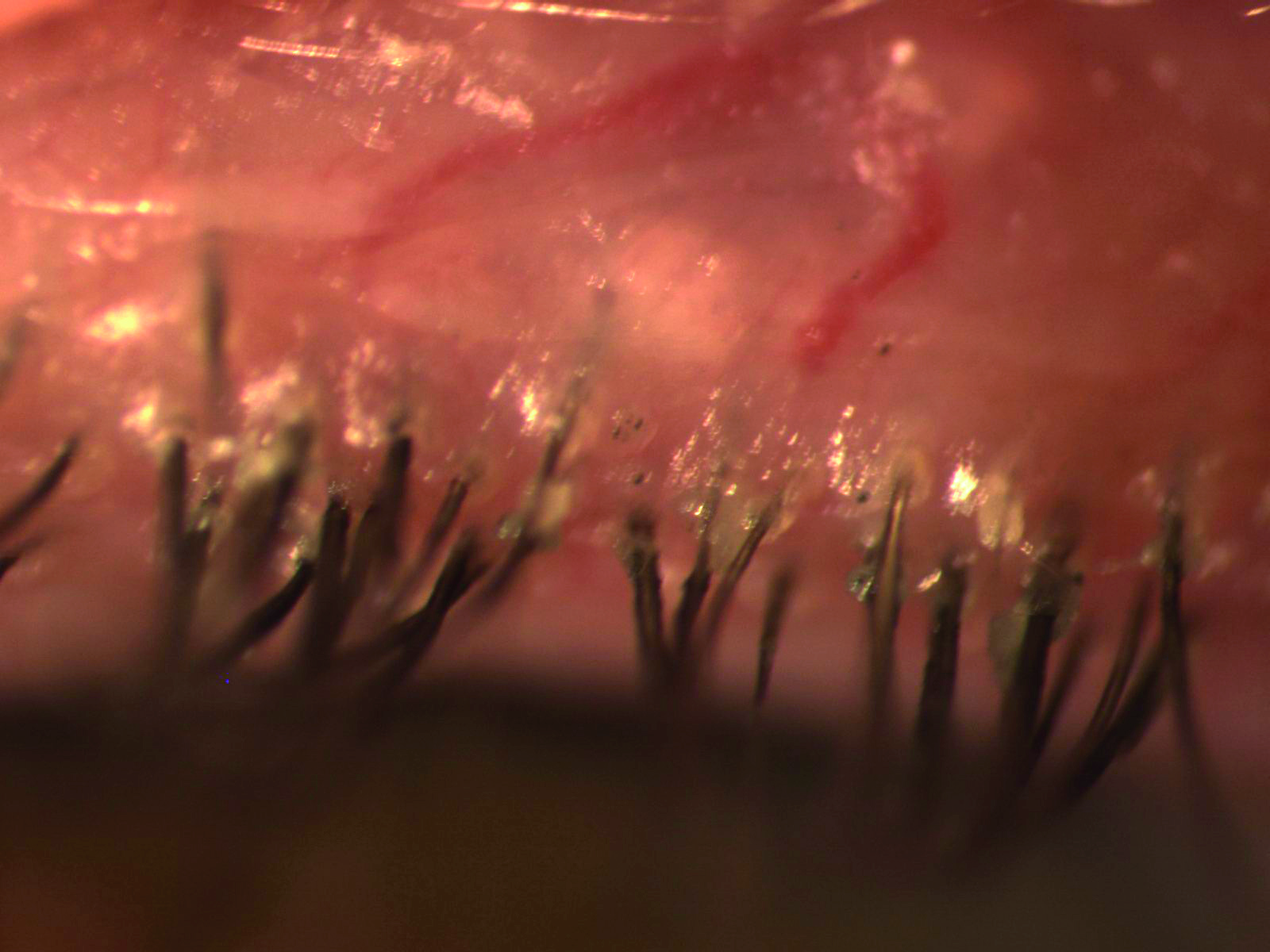
Figure 13: Blepharitis is a cause of DED
Demodex infestation (figure 14) which occurs in 100% of people over 70 can spread from the face to the eyelids, leading to blepharitis and rosacea, although demodex can also be found in asymptomatic individuals. Demodex consume sebum, and may consume follicular and glandular epithelial cells, which could lead to direct damage to the lid margin. They are also thought to cause blepharitis by carrying bacteria on their surface, also the internal protein and their waste products may trigger inflammatory responses.
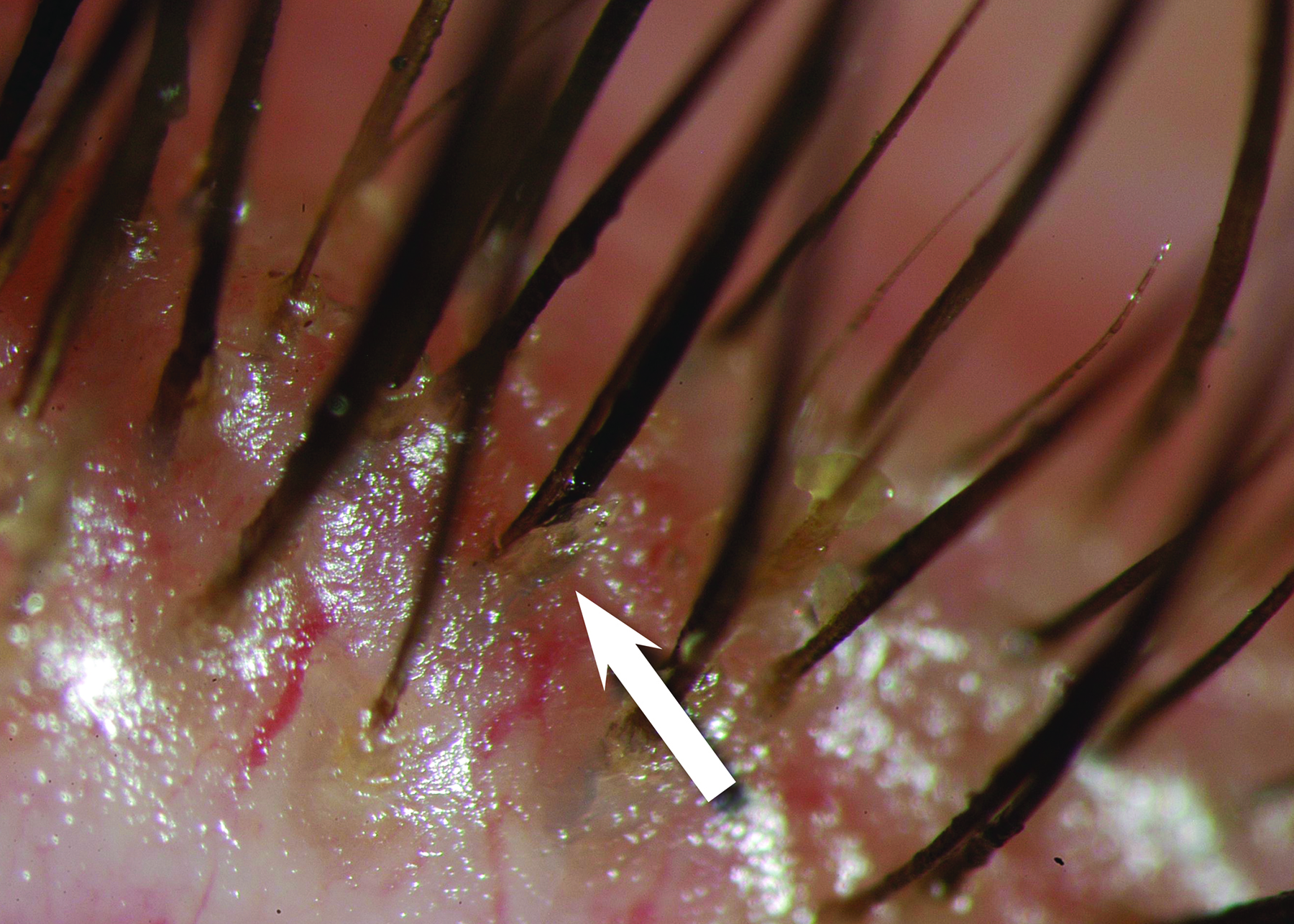 Figure 14: A Demodex mite (courtesy of Professor E Bitton)
Figure 14: A Demodex mite (courtesy of Professor E Bitton)
Iatrogenics
When seeking a diagnosis, it is important to bear in mind that dry eye can be caused inadvertently by many types of intervention. Topical medications can cause DED due to their allergic, toxic and immuno-inflammatory effects on the ocular surface. Preservatives such as benzalkonium chloride may further aggravate DED. Systemic drugs can also induce DED via numerous mechanisms (see table 4).
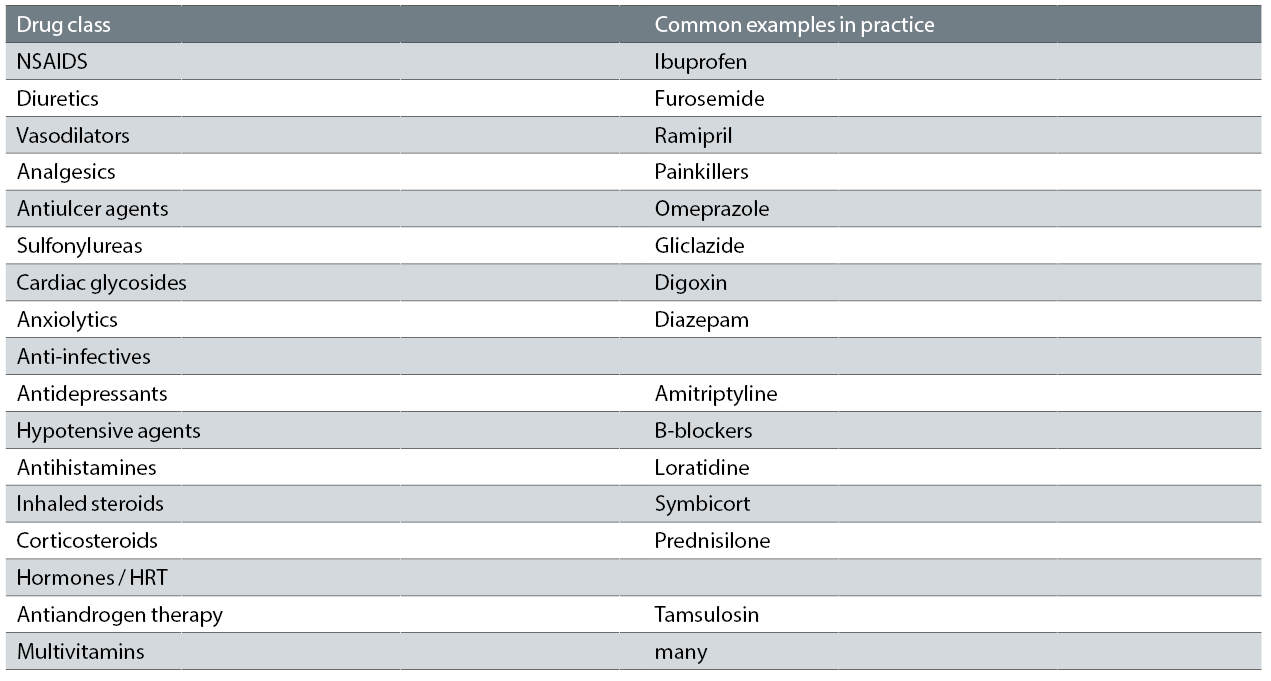
Table 4: Common systemic drugs that possibly cause or contribute to dry eye. A large number have anticholinergic activity and reduce aqueous and mucous and possibly meibomian gland mucin production
The use of contact lenses is also associated with DED. One of the other risk factors is from surgical procedures such as corneal refractive surgery due to corneal nerve cutting, cataract surgery, lid surgery, Botox application and cosmetic procedures. Efforts to aid early diagnosis prior to surgeries, and to better regulate and guide patients in self-medication could help alleviate this common issue.
Summary
The latest DEWS II evidence based consensus has determined for us as practitioners the most appropriate battery of tests to diagnose and monitor DED. A plethora of tests and techniques can be employed when it comes to a dry eye disease work up, but at the core of it, the small number of key tests, now established, will give us as practitioners enough information to make a provisional diagnosis as well as form management decisions that suit the needs of the patient. These tests form the basis of a work up in practice, which can then be refined and modified as further investigations are completed, as well as reviewing the DED response to management over time. The final article in this series will review the DEWS II consensus on how to employ the current best management strategies, once diagnosis has been established, to achieve effective patient outcomes.
Sarah Farrant is a therapeutic optometrist with a specialist interest in dry eye disease practising in Somerset, UK.
The relevant section of TFOS DEWSII can be downloaded from http://dx.doi.org/10.1016/j.jtos.2017.05.001
