In the area of personal healthcare humans often do not act in their own interests. In 2008, Gordon Brown announced the NHS ‘Health Check’ initiative to provide nationwide assessments for common diseases such as diabetes, heart disease and dementia.1 Despite the generally easy access of the community to these free services, uptake is low. When over 5,000 people at high risk of cardiovascular disease in London were offered a screening assessment, fewer than 50% attended, an outcome which is sufficiently low to question the potential impact of the scheme on the health of the population.2
When we are provided some form of clinical treatment which we control (as distinct from that administered by a healthcare professional), the situation becomes more complex and worse. It is evident that the extent to which a person’s behaviour is in line with healthcare advice provided – the definition of compliance adopted for this article – is often poor.
In general medicine for example, compliance with treatment for chronic diseases stands at around 50% only.3 A well studied area is compliance with medication for hypertension. The 1998 Health Survey for England found that 42% of adult men and 33% of adult women were hypertensive. The dangers of hypertension are well known; if systolic blood pressure is maintained at less than 140mmHg, there would be tens of thousands fewer related deaths in the UK each year, and an even greater reduction in non-fatal events. Yet, despite readily accessible, low cost drug therapy for this well understood condition, fewer than 10% of hypertensives are able to maintain their blood pressure below this threshold.4 There are numerous factors which lead to this poor clinical outcome, but the key reason is patient non-compliance. Of course, drug treatment may be confusing to some patients and there can be concerns around side-effects and other issues, but an overall compliance rate of only 20-50% with hypertensive medication4 – treatment which may prevent death by a stroke or heart disease – reveals the challenge faced by healthcare professionals in having their patients act in their own best interests.
Perhaps more surprisingly, fewer than 80% of patients correctly adhered to advice to take tamoxifen, 12 months after its recommendation as a therapy for breast cancer.5
In these examples, when personal morbidity and even mortality are put at risk when compliance is poor, patients fail to change their behaviour. More immediately relevant to eye care practitioners is the situation with glaucoma treatment. In common with hypertension, compliance here is generally poor. In a 2009 study, Okeke and colleagues assessed the use of glaucoma drops which were instilled using a dosing device which contained a memory chip which recorded each time the bottle of drops was squeezed. The drops were prescribed to be instilled once per day, at night-time, and 282 glaucoma patients were tracked over a three-month period. Fifty six per cent of the group applied their drops on 75% or more of the treatment days and around one in five patients used drops on half of the days, or fewer.6
Against the backdrop of low compliance with prescribed treatment for systemic and ocular diseases, it is clear that eye care professionals face some significant challenges when outlining the appropriate use of contact lenses and associated care products to their contact lens patients. Furthermore, perhaps in common with hypertension medication and glaucoma drops, there are minimal immediate gains when correctly adhering to prescribed contact lens care. Certainly on a day-to-day basis, vision and comfort will usually be consistent, so helping patients adhere to appropriate lens care is principally related to the ability of the practitioner conveying messages about best practice to their patients.
As outlined below, however, it is clear that there are steps which patients can take to reduce their own likelihood of an eye infection during lens wear and eye care practitioners have the opportunity – and the responsibility – to help their contact lens wearers minimise this negative consequence of contact lens wear. To that end, this paper considers the key areas of patient behaviour on which we can focus, so that compliance can be improved to help patients reduce the likelihood of corneal infection or inflammation.
Contact lens adverse events which may be related to wearer compliance
There is a range of adverse events that may arise during contact lens wear but the issues which are most worthy of consideration when discussing wearer compliance are those that appear to be related to the presence of micro-organisms at the ocular surface during lens wear. In turn, the most important example here is the range of adverse events that fall under the umbrella term of ‘corneal inflammatory event’ (CIE) or keratitis. These range greatly in severity from an asymptomatic form in which small clusters of white blood cells (‘infiltrates’) aggregate in the peripheral cornea (‘asymptomatic infiltrative keratitis’) to a sight-threatening infection of the cornea with ulceration (‘microbial keratitis’).7
CIEs are generally associated with the presence of micro-organisms. This is obviously the case for corneal infections, but there is good evidence that the presence of bacteria is important for non-infectious CIEs such as a ‘contact lens peripheral ulcer’ which requires the presence of the bacterium Staphylococcus aureus at the ocular surface with the ulcer a response to the release of toxins from the bacteria rather than a frank infection of the cornea.8
Normally, of course, the ocular surface is remarkably good at defending itself from microbial infection. Most adults report that they have never had an eye infection or can only remember an occasional incident, perhaps as a child. Such an observation reflects the potency of the ocular defensive systems, which are numerous. A key element in the defensive process is the blinking, tearing and the drainage system which are able to rapidly remove micro-organisms from the ocular surface. This system is rendered less potent when bacteria are trapped between a contact lens and the cornea. In this scenario, bacteria – and indeed other micro-organisms – benefit from a longer residence time at the corneal surface than would usually be the case.9 Furthermore, the contact lens acts as a vehicle for micro-organisms reaching the ocular
surface10 as they can be introduced to the eye via the hands of the contact lens wearer,11 or perhaps sourced from the case in which the lenses have been stored.12 This is an unnatural situation; the eye has not evolved to defend itself from the introduction of microbes in this way.
Contact lens wear, therefore, shifts the balance in the eye’s ability to avoid infection. A greater number of micro-organisms reach the ocular surface in contact lens wearers and the eye’s ability to clear a contaminated tear film is compromised by the presence of the lens itself.
It is in this context in particular, therefore, that contact lens care systems are required. While they have a role to play in enhancing comfort and maintaining good vision, their primary purpose is a simple one – to minimise the number of micro-organisms which reach the eye when a contact lens is applied. The evidence for the important role of contact lens care systems has been clearly highlighted by epidemiology studies of contact lens infections. Over 20 years ago, Radford and others found that the likelihood of an Acanthamoeba keratitis was more than 50 times greater in contact lens wearers who did not disinfect their reusable soft lenses compared with those properly storing their lenses.13 More recently, Stapleton reported a three fold increase in microbial keratitis in patients with poor lens case hygiene.14
Which lens care behaviours are associated with an increase in CIEs?
The study of the specific causes of CIEs is not straightforward. The more serious forms, such as microbial keratitis, are rare and cannot readily be studied in prospective clinical trials of different types of contact lenses. Large numbers (many tens of thousands) of wearers would be required over long study periods to the point where the work becomes unjustifiably expensive. Instead, ‘case control’ experiments are conducted. Rather than fitting patients and counting the number of people who develop CIEs and other adverse events, the work is done the other way around. Researchers base themselves in hospital eye departments or major contact lens clinics – ideally large centres with sizeable catchment populations Ω and gather information about patients attending with microbial keratitis or CIEs. A wide range of information is captured about these patients; from the types of lenses worn and solutions used, through demographic features such as age and genders, to various behavioural traits such as swimming with lenses, hand washing, smoking and case care. Then, a matching group of contact lens wearers without any ocular problems is identified and the same set of questions asked. The areas where there are significant differences in the two groups (the contact lens wearers with CIEs, and the matched ‘controls’) are identified and are termed risk factors. A good example here is gender. Typically, in a group of contact lens wearers with CIEs, about half of the sample is male and half is female. Initially, this might seem correct given the equal balance of the genders in the overall population. However, the split of genders in a contact lens population is not equal. Only one third of contact lens wearers is male, so men are over-represented in a CIE group and as such, being male is a risk factor for the disease.
Being male is a non-modifiable risk factor – as is being young or someone’s socio-economic status. These are interesting outcomes from an epidemiology point of view, and can help in the selection of an initial lens choice – extended wear might be a poor choice for an average young male compared to, say, an older female.
However, these non-modifiable risk factors are less helpful when advising wearers about their ongoing use of contact lenses. Modifiable risk factors are much more relevant here, and a number of these relate to compliance with contact lens wear and care.
In fact, of course, there are numerous actions which are undertaken by a contact lens wearer during each day of wear related to lens wear and care. Indeed, Young listed 49 daily steps for someone using reusable lenses with solutions.15 All can be scrutinised and potentially improved upon.
Some of these daily steps of lens care have been associated with an increased likelihood of CIEs. In a 2011 report on contact lens compliance in different countries, Morgan and colleagues identified eight key factors which have been proven to be associated with CIEs: inadequate hand washing, non-prescribed overnight wear, excessive duration of overnight wear, excessive lens replacement interval, inadequate case cleaning, failure to use the correct disinfecting solution, failure to rub and rinse lenses and ‘topping off’ solution.16 In each case, epidemiological studies have shown that inappropriate use of contact lenses and solutions in these ways is associated with an increase in microbial keratitis and/or other types of CIEs. When the behaviours of 4,021
contact lens wearers were evaluated, only 0.2% of patients using reusable lenses were deemed to be fully compliant compared with 14.7% of daily disposable wearers.
On which behaviours should we focus?
Having established a range of modifiable risk factors which are associated with CIEs, we cannot simply conclude that these are all equally important as there is a large range of their frequency within contact lens wearers. Four poor behaviours – inadequate hand washing, excessive lens replacement interval, failure to rub and rinse and poor case care – occur in a majority of wearers (figure 1). This last behaviour type, poor case care, is demonstrated by almost all contact lens wearers.
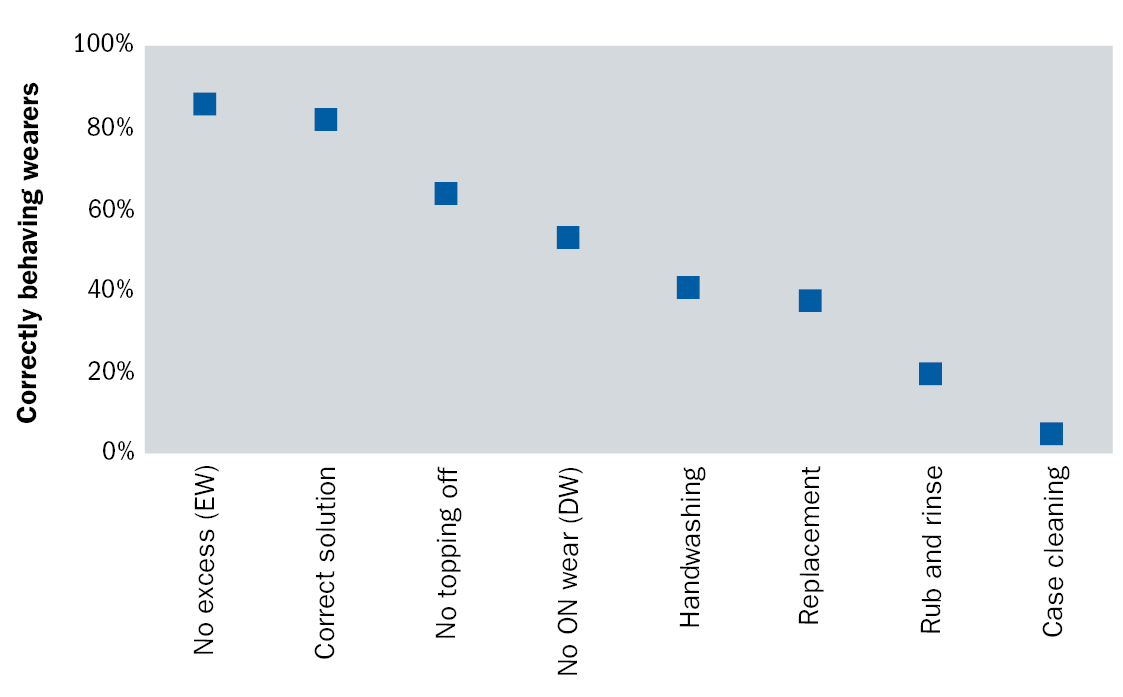 Figure 1: Proportion of contact lens wearers compliant with a range of lens care steps. (From Morgan et al, 2011).16
Figure 1: Proportion of contact lens wearers compliant with a range of lens care steps. (From Morgan et al, 2011).16
It is of course noteworthy that daily disposable lenses are associated with better levels of compliance primarily driven by their simplicity of use; that is, there is less to ‘go wrong’. Interestingly, this lens category is not associated with reduced levels of contact lens-related infections (at least when earlier generations of daily disposable lenses were assessed),14,17 which reinforces the requirement for practitioners to be conscious of the possibility of infections with all lens types. That said, more recent information suggests that lower rates of CIEs are associated with daily disposable lenses.18
Overall, then, four of these compliance-related behaviours are seen in most contact lens wearers and are all associated with an increase in risk of CIEs. However, one further factor needs to be considered: the potential for these behaviours to improve with further training and direction. In the past, it has been shown that improvement in compliance is difficult despite the repeated provision of information.19 A more recent assessment confirmed that a number of poor behaviours were resistant to improvement; however, both these studies noted some exceptions to this trend and identified behaviours which can, in fact, be improved. These are hand washing,20,21 case cleaning and lens rinsing.21 Here are three behaviours which (a) are associated with CIEs, (b) are performed poorly by most contact lens wearers and (c) have been shown to be improved with repeated instruction.
It seems logical, therefore, that these three areas should be particularly emphasised to new contact lens wearers and reiterated at contact lens follow-up visits – these can be considered as our three key targeted behaviours. This is not to suggest that various other areas are unimportant – and clearly each patient will have their individual areas of poor compliance – but in a busy practice with usually fixed times for contact lens discussions, these three areas warrant particular focus and indeed, deserve mention to all patients as relevant to their type of contact lenses.
Targeted behaviour 1: Hand washing and drying
A lack of hand washing has been repeatedly shown to be associated with an increase in CIEs17,22 – including a 13x increase in risk according to recent information from Singapore;23 further to this, hand-washing with soap is associated with reduced contact lens case contamination.24 Studies of the effectiveness of hand washing can be counterintuitive25 and some studies indicate that there is little difference between the performance of anti-microbial soap brands and conventional soap and indeed, the ability of hand washing at reducing microbial contamination of the hands (including the fingertips which interact with a contact lens prior to application) seems limited for a single hand wash. However, there appears to be a significant positive impact of sustained and consistent hand hygiene practices over time.21 Clearly, this issue should be addressed at each contact lens aftercare examination irrespective of the lens types worn (daily disposables and reusable lenses). Open questions should be put to the patient to reveal the extent and methods of the hand washing (table 1).
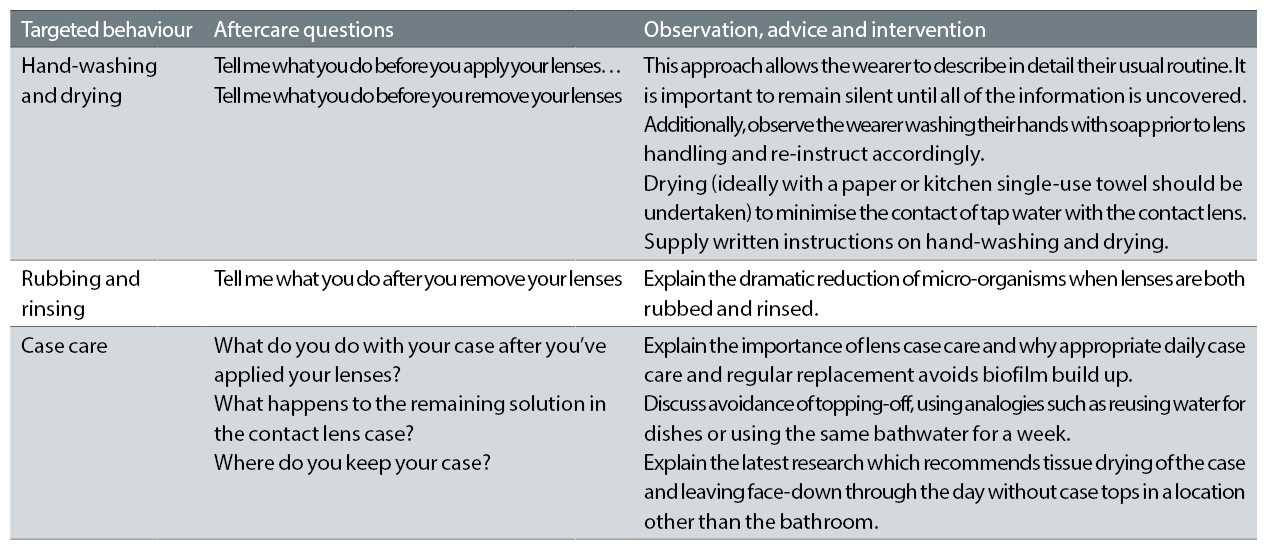 Table 1: Targeted behaviours and communication
Table 1: Targeted behaviours and communication
A related consideration is the relationship between Acanthamoeba infection and contact with water in contact lens wearers.26 Radford and co-workers have reported a great regional variation in Acanthamoeba keratitis across the United Kingdom, associated with differences in local water hardness, and concluded that the contamination of lenses and lens cases with tap water is an important risk factor for this form of ocular infection.27
This suggests that it is important to specifically direct contact lens wearers to avoid obvious steps such as storing their lenses in tap water (or indeed any other solution except approved regimens) or rinsing their lenses in water prior to application. However, there is something of a clash between recommending ongoing and consistent hand washing and avoiding tap water. It is therefore key that patients dry their hands before handling their contact lenses; indeed the action of drying with a single use paper towel or cloth towel can further reduce contamination (including Acanthamoeba) of the skin.28
Advising patients about hand washing prior to contact lens application requires a carefully considered set of patient instructions;29 clearly, though, this is one important area where wearers are able to take simple steps to reduce the likelihood of a CIE.
Targeted behaviour 2: Rubbing and rinsing
When reusable lenses are prescribed, the simple steps of rubbing and rinsing a contact lens with solution has a dramatic impact on bacterial contamination; this phenomenon was carefully studied by Zhu and colleagues in 2011.30 Using the five micro-organisms – three bacteria, a fungus and a yeast – required by the relevant standard for contact lens solutions (ISO14729), in addition to Acanthamoeba polyphaga, this group explored the effect of rubbing and rinsing prior to storage in multipurpose solutions. For all types of micro-organisms, a regimen of rinsing and storage approximately halved the numbers remaining on the lens
compared to storage alone. Adding the rubbing step delivered a more potent effect, almost totally removing bacterial contamination. Again, here is a quick practical step which wearers are able to take to reduce their chances of a contact lens related infection. This part of lens care should be discussed at each aftercare visit and remedial action implemented as required.
Targeted behaviour 3: Case care
A much-overlooked area for reusable lenses is the appropriate use of the lens storage case despite the link between poor case hygiene practices and CIEs. This situation has probably been exacerbated by conflicting, unclear and inconsistent information from professional bodies, regulators and practitioners.31 One important consideration is the development of biofilm (a persistent microbial coating) in the storage case with ongoing use. A recent study of the use of current generation solutions found that over 80% of patients were using contaminated cases, with a greater proportion of contamination when using a one-step peroxide than multipurpose products.32 Such contamination has the propensity to increase the numbers of micro-organisms reaching the eye at lens application.
Research on case care can be utilised to inform practitioners and wearers about the best practices to minimise biofilm formation. Wearers of reusable lenses should be aware of the recommended case hygiene procedure:
- After lenses have been applied, rinse the lens case and lids with disinfecting solution33 not tap water
- Immediately tissue dry the lens case33
- Leave to air dry face down on a clean tissue Ω preferably not in a bathroom34 during the day
- Replace the lens case according to manufacturer instructions
The location of case storage is important and bathrooms should be avoided. The aerosol effect from the toilet flushing in bathrooms mobilises micro-organisms through the room. For this reason, in addition to the potential for contamination with water, contact lens cases are best kept in a bedroom, and if stored in the bathroom, this should be inside a closed cabinet to minimise contamination.
Best practice, then, for the use of a contact lens case is for it to be stored dry through the day. In addition to this approach being associated with reduced contamination in its own right, this advice also prevents the practice of ‘topping up’ or ‘topping off’ solution. These interchangeable terms describe the situation when a wearer fails to discard their solution after lenses are applied in the morning, such that when lenses are returned to the case in the evening, it is already partially filled and the case can be ‘topped up’ with a small volume of fresh solution.
Such an approach may be adopted by the patient as a cost-saving measure or perhaps in some cases because this appears to be quite reasonable. This method of solution/case misuse is associated with increased levels of CIEs35-37 presumably because of the increase in resulting concentration of microbes in the lens case38 as a progressively less potent solution sees reducing performance against increasing levels of microbes.
Information about the best use of the lens case needs to be delivered at aftercare consultations. Daily care for the case should be discussed along with its replacement cycle and the avoidance of topping up.
Delivering the key messages
Knowledge about the best way to care for contact lenses needs to be understood by the staff members in practice dealing with lens wearers. This includes, of course, optometrists and opticians, but it is vital that other staff members, including front-line colleagues who might take on the role of instructing new contact lens wearers and who deal with incoming contact lens queries in person or over the telephone, are updated about the latest information. Front-line staff may be reliant on their eye care professional for ongoing training and updates. Periodic reviews of the instructions given to new and existing wearers by support staff is vital if wearers are to receive the most current information. In turn, this means that the eye care professional has a responsibility to keep updated in the latest research and advice, and to disseminate this to their practice staff and importantly their patients Ω some of whom they may see only once a year or less frequently.
Importantly, our understanding of best practice with contact lenses changes over time. For example, we now have a better understanding of the contributory factors of inflammation and infection in contact lens wear than a decade or two ago. As such, all patients – even experienced wearers – need to be questioned and advised about their individual wear and care behaviours. For example, it is entirely possible they are compliant with the advice provided on their initial lens care instruction some years ago but with improvements in understanding over time, new information needs to be conveyed.
Summary and conclusions
Spending time at contact lens consultations focusing on the three targeted behaviours reported here Ω hand-washing and drying, rubbing and rinsing, and case care Ω can realistically be expected to reduce the risk of adverse events in individual contact lens wearers; contact lens wearers are able to reduce their own risk of infection and CIEs if these messages are communicated to them effectively. Contact lens wear can quickly become an everyday essential of life, and taking these three simple steps to reduce the potential for inflammation and infection helps to maintain a happy and successful wearing experience.
Philip Morgan is a Professor of Optometry at the University of Manchester, and optometrist Sarah Morgan is a Vision Sciences Fellow at the same institution and a renowned expert in communication.
This article was written with support from an educational grant from CooperVision
References
- Dryden R, Williams B, McCowan C & Themessl-Huber M. What do we know about who does and does not attend general health checks? Findings from a narrative scoping review. BMC Public Health 2012 12:1 12, 723 (2012).
- Dalton A, Bottle R, Okoro C, Majeed F & Millett C. Uptake of the NHS Health Checks programme in a deprived, culturally diverse setting: Cross sectional study. Journal of Epidemiology & Community Health 65, A21-A21 (2011).
- Miller NH. Compliance with treatment regimens in chronic asymptomatic diseases. The American Journal of Medicine 102, 43-49 (1997).
- Thrall G, Lip GYH & Lane D. Compliance with pharmacological therapy in hypertension: Can we do better, and how? J Hum Hypertens 18, 595-597 (2004).
- Barron TI, Connolly RM, Bennett K, Feely J & Kennedy MJ. Early discontinuation of tamoxifen: A lesson for oncologists. Cancer 109, 832-839 (2007).
- Okeke CO et al. Adherence with Topical Glaucoma Medication Monitored Electronically. Ophthalmology 116, 191-199 (2009).
- Efron N & Morgan PB. Rethinking contact lens associated keratitis. Clinical and Experimental Optometry 89, 280-298 (2006)
- Wu P, Stapleton F & Willcox MDP. The Causes of and Cures for Contact Lens-Induced Peripheral Ulcer. Eye & Contact Lens: Science & Clinical Practice S63-S66 (2003). doi:10.1097/00140068-200301001-00018
- Fleiszig SMJ & Evans DJ. Pathogenesis of contact lens-associated microbial keratitis. Optometry and Vision Science 87, 225–232 (2010).
- Shin H et al. Changes in the Eye Microbiota Associated with Contact Lens Wearing. MBio 7, e00198 (2016).
- Szczotka-Flynn LB, Pearlman E & Ghannoum M. Microbial contamination of contact lenses, lens care solutions, and their accessories: a literature review. 36, 116-129 (2010).
- Boost MV & Cho P. Microbial Flora of Tears of Orthokeratology Patients, and Microbial Contamination of Contact Lenses and Contact Lens Accessories. Optometry and Vision Science 82, 451-458 (2005).
- Radford CF, Bacon AS, Dart JKG & Minassian DC. Risk factors for acanthamoeba keratitis in contact lens users: A case-control study. British Medical Journal 310, 1567-1570 (1995).
- Stapleton F et al. The Incidence of Contact Lens-Related Microbial Keratitis in Australia. Ophthalmology 115, 1655-1662 (2008).
- Young G. Disinfection in 49 Steps. Contact Lens Spectrum 53-54 (2012).
- Morgan PB, Efron N, Toshida H & Nichols JJ. An international analysis of contact lens compliance. Contact Lens and Anterior Eye 34, 223-228 (2011).
- Dart JKG, Radford CF, Minassian D, Verma S & Stapleton F. Risk Factors for Microbial Keratitis with Contemporary Contact Lenses. Ophthalmology 115, 1647-1654.e3 (2008).
- Chalmers RL, Keay L, McNally J & Kern J. Multicenter case-control study of the role of lens materials and care products on the development of corneal infiltrates. Optometry and Vision Science 89, 316-325 (2012).
- Claydon BE, Efron N & Woods C. A prospective study of the effect of education on non-compliant behaviour in contact lens wear. Ophthalmic & physiological optics : the journal of the British College of Ophthalmic Opticians (Optometrists) 17, 137-146 (1997).
- Shih KL, Hu J & Sibley MJ. The microbiological benefit of cleaning and rinsing contact lenses. Int Contact Lens Clin 12, 235-242 (1985).
- Larson E et al. Short- and long-term effects of handwashing with antimicrobial or plain soap in the community. J Community Health 28, 139-150 (2003).
- Stapleton F, Keay L, Jalbert I & Cole N. The Epidemiology of Contact Lens Related Infiltrates. Optometry and Vision Science 84, 257–272 (2007).
- Lim CHL et al. Risk factors for contact lens-related microbial keratitis in Singapore. Eye (London, England) 30, 447-455 (2015).
- Wu YT, Willcox MDP & Stapleton F. The Effect of Contact Lens Hygiene Behavior on Lens Case Contamination. Optometry and Vision Science 92, 167-174 (2015).
- LY VT, Edrington TB, Wechsler S, De Land PN & Simmons PA. Efficacy of Hand Washing Procedures on Bacterial Contamination of Hydrogel Contact Lenses. Optometry and Vision Science 74, 288-292 (1997).
- Kilvington S. Acanthamoeba Keratitis: The Role of Domestic Tap Water Contamination in the United Kingdom. Invest Ophthalmol Vis Sci 45, 165-169 (2004).
- Radford CF, Minassian DC & Dart JKG. Acanthamoeba keratitis in England and Wales: Incidence, outcome, and risk factors. British Journal of Ophthalmology 86, 536-542 (2002).
- Patrick DR, Findon G & Miller TE. Residual moisture determines the level of touch-contact-associated bacterial transfer following hand washing. Epidemiology & Infection 119, 319-325 (1997).
- McMonnies CW. Hand hygiene prior to contact lens handling is problematical. Cont Lens Anterior Eye 35, 65-70 (2012).
- Zhu H et al. Importance of rub and rinse in use of multipurpose contact lens solution. Optometry and Vision Science 88, 967-972 (2011).
- Wu Y, Carnt N, Willcox M & Stapleton F. Contact lens and lens storage case cleaning instructions: whose advice should we follow? 36, 68-72 (2010).
- Dantam J et al. Microbial Contamination of Contact Lens Storage Cases During Daily Wear Use. Optometry and Vision Science 93, 925-932 (2016).
- Wu YT, Zhu H, Willcox M & Stapleton F. Removal of biofilm from contact lens storage cases. Invest Ophthalmol Vis Sci 51, 6329-6333 (2010).
- Wu YT, Zhu H, Willcox M & Stapleton F. Impact of air-drying lens cases in various locations and positions. Optometry and Vision Science 87, 1-468 (2010).
- Saw S-M et al. Risk factors for contact lens-related fusarium keratitis: a case-control study in Singapore. Archives of Ophthalmology 125, 611-617 (2007).
- Joslin CE et al. The association of contact lens solution use and Acanthamoeba keratitis. Am J Ophthalmol 144, 169-180 (2007).
- Zimmerman AB, Emch AJ, Geldis J, Nixon GJ & Mitchell GL. Contact Lens Corneal Inflammatory Events in a University Population. Optometry and Vision Science 93, 42-49 (2016).
- Zhang S et al. Growth and Survival of Fusarium solani-F. oxysporum Complex on Stressed Multipurpose Contact Lens Care Solution Films on Plastic Surfaces In Situ and In Vitro. Cornea 25, 1210-1216 (2006).
