This is the second of a series of two articles which have been developed to provide clinicians with an overview of myopia control studies and discuss key aspects of clinical trial design and how they relate to studies intended to investigate the efficacy of methods to control myopia progression, with an emphasis on contact lenses.
In part 1 (Optician 03.11.17), a number of topics relating to myopia control studies were discussed including the prevalence and progression of myopia, evaluation of clinical research evidence, methods of control and treatment of myopia, ethical issues and how progression of myopia can be assessed. Part 2 will discuss the design of clinical studies and how clinical research relating to myopia control is evaluated. With this knowledge, eye care practitioners should be better equipped to evaluate the clinical research literature for this important topic.
Study Design
The cornerstone of any robust clinical study is its design.1 Studies that are designed to investigate the progression of myopia should be prospective, with participants being followed going forward in time, and should allow evaluation of the intervention in comparison with one or more other treatments. While simple observational studies may be able to provide valuable information regarding myopia treatment, they are subject to potential bias, particularly with respect to the selection of which participants receive the treatment(s) that may limit their quality.
Cross-over design myopia control studies have been conducted;2 however, they are not appropriate for the longer term evaluation required for investigating the efficacy of these treatments, since in addition to unnecessarily extending the study duration, the child would be at a different stage of development for the treatment and control arms of the study. Contralateral eye comparisons are widely used in animal studies investigating progression of myopia3, and have also been undertaken with children4, but these carry the risk of inducing unwanted anisometropia and participants may accidentally reverse the treatment and control lenses. A parallel group design is the best option for investigating the progression of myopia in children. Brief descriptions, advantages and limitations of each of these study designs as they relate to myopia control studies are summarised in table 1.
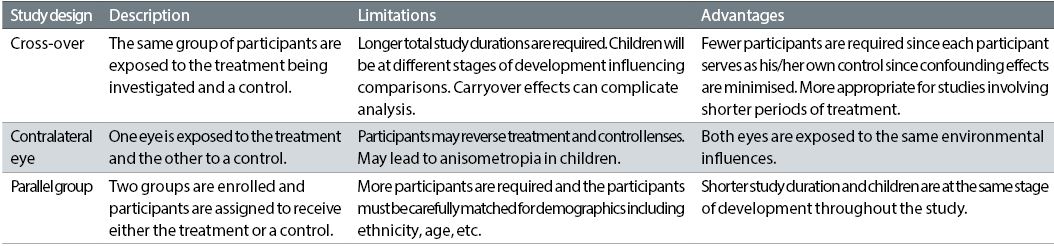
Table 1: Myopia control study designs
Since it can be challenging to distinguish between real treatment effects and the natural progression of myopia, one or more comparable groups of study participants is required in clinical trials evaluating myopia control; one group, termed the treatment group, receives the therapy under investigation and the other group(s), termed the control group(s) receive the conventional correction of their refractive error with either spectacles or single vision contact lenses.5 When soft contact lenses designed for myopia control are being investigated, it is preferable that a similar single vision soft design and material as the test lens is selected as the control, both to aid in masking (see later) and also to rule out possible confounding factors. Utilising two or more study groups allows the investigators to estimate both favourable and unfavourable treatment effects.
The allocation to study group should be determined by chance alone, with neither the participant nor the investigator being involved in the process. This process is referred to as ‘randomisation’.6 While randomisation does not guarantee that all groups are evenly matched at baseline for all known or unknown risk factors relating to myopia progression, if the number of participants is sufficiently high it is expected that the differences will be minimal; however, analyses should be conducted once the study has been completed to confirm that this was indeed the case. Stratified randomisation, incorporating a block design, is often used in myopia control studies to achieve balance between treatment and control groups in key characteristics such as age, ethnicity, entering level of myopia and clinical site.1
By the same token, the children participating and the investigators conducting the study should be masked with respect to which group they have been assigned to (double masked). While knowing which group they are in may not affect objective measurements, it may influence compliance with treatments, subjective assessments and the propensity to continue in the study to its conclusion. In order to achieve masking, all product identification on contact lens packaging should ideally be removed, or if this is not possible, covered.7 In cases where a treatment group is wearing orthokeratology contact lenses or spectacles it is not possible to mask the children; however, the investigators conducting the measurements of refractive error and axial length could be masked if necessary (single masking).8, 9 In studies evaluating treatment of myopia progression with topical drops (eg atropine), a placebo drop may need to be dispensed for use by the control group to maintain appropriate masking.10

The visual world for the growing child has changed
Objectives, Hypotheses, Endpoints and Methods of Measurement
A detailed protocol is required for all prospective, randomised, clinical trials. This protocol needs to be reviewed and approved by an independent ethics committee which ensures that the study objectives will be addressed and that the participants will not be exposed to undue harm compared with any expected benefit(s).11 Myopia control study publications should include information from the protocol clearly detailing the study objectives, hypotheses, endpoints and methods of measurement. The objectives are the research questions that the trial was designed to answer, and usually relate to the efficacy of the myopia control treatment(s) being investigated. Hypotheses are more specific and establish the basis for statistical evaluation. Clearly defined endpoints or outcome measures should also be stated, with the primary outcome measure being the endpoint of greatest importance, for example, progression of myopia. The method(s) of measurement of the primary outcome must also be defined, for example change in axial length and/or spherical equivalent refractive error relative to baseline. In a recent Food and Drug Administration workshop on controlling the progression of myopia with contact lenses that was conducted in the United States, it was recommended that the primary endpoint in myopia control studies should be axial length since this measurement has better repeatability; however, the measurement of cycloplegic autorefraction is still recommended as a secondary endpoint.12 Precision of measurements for all outcomes is extremely important, and best achieved by standardisation of measurement methods, training of observers, and the use of automation. Wherever possible, objective measurements and assessments should be made.
Length of Treatment and Number of Visits
Most of the myopia control studies using bifocal and peripheral add multifocal contact lenses that have been conducted to date have been two years or less in duration.13 It is important to investigate for how long, and to what degree any effect of the progression of myopia persists; therefore, studies in which treatment effects are measured for up to three years, but at least for two years are recommended to ensure that the treatment continues to accumulate over time.12 Figure 1 shows the change in both refractive error and axial length measured in a series of studies of varying duration.
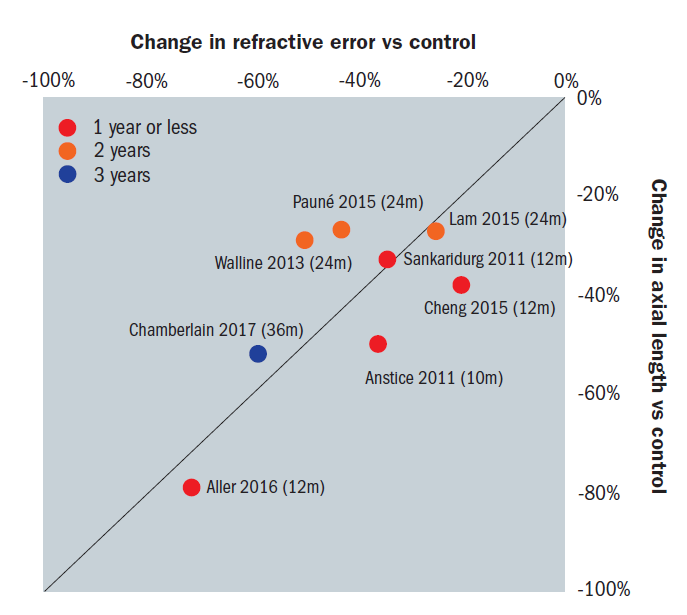
Figure 1: Change in refractive error and axial length measured in a series of studies of varying duration. Courtesy Philip Morgan
It should be recognised though that the longer a study continues, the greater the attrition rate will be and this can result in challenges relating to data analysis at the conclusion of the study. The interval between follow-up visits is also significant. Since the children participating in these studies are generally new to contact lenses, the visits during adaptation will likely be more frequent; however, it is important to balance the burden of attending follow-up visits with the requirement to conduct the primary outcome measurements. Intervals of six months are ideal once adaptation has occurred and allow modifications to lens power to be made to ensure optimal correction throughout the study period.
Study Population Demographics and Inclusion / Exclusion Criteria
It is particularly important that the children taking part are representative of the target populations for the treatment, although it must be recognised that this needs to be balanced with the population that is accessible for the study. For myopia control studies children with low degrees of myopia aged between seven or eight and 12 years should ideally be enrolled, to reflect the ages during which the fastest progression has been reported to occur.12,14,15 Children who are current or previous rigid gas permeable and/or orthokeratology lens wearers or who have previously been treated for myopia control with spectacles, contact lenses or pharmacological agents should be excluded from all myopia control studies. Since a number of genetic, environmental and lifestyle predisposing factors have been implicated in the development and progression of myopia,16-19 it is preferable to recruit children from as broad a demographic reach as possible. This can also be facilitated through multicentre studies (see below). Documentation of the demographics (including ethnicity) of and activities undertaken by the children participating, and where possible the refractive status of their biological parents, is also important for subsequent data analysis and interpretation.
Number and Location of Study Sites
When conducting clinical trials, it is desirable to enrol participants over as short a period as possible so that interim and final analyses can be conducted as expeditiously as possible after study milestones are reached by all participants. Multicentre studies facilitate this goal in addition to allowing recruitment of a larger number of participants from different geographic locations and a wider range of population groups. They also provide the ability to compare results among centres. All of these factors increase the generalisability of the results from the study, which is particularly important for research into myopia progression. Since it is also important that the study is conducted under the same protocol and in exactly the same way at all sites, limiting the sites to a number that can be carefully managed is recommended.
Number of Participants and Sample Size Calculations
The number of children participating in a study evaluating the progression of myopia is crucial to the interpretation of its results. The individual responses to myopia control treatments can vary considerably. If too few children are enrolled in a study a clinically important difference in progression between the treatment and the control groups may be reported, but it may not be determined to be statistically significant according to the a priori analysis that was conducted. In order to avoid this, sample size calculations are conducted during the study design process.20 These calculations take into consideration a minimum treatment effect (eg a mean reduction of 0.25D progression per year in the treatment compared to the control group) and the standard deviation of the expected treatment effect based on similar, previously conducted studies. The investigators should state how they have calculated the number of participants they required for the study, including the desired ‘power’, which is the probability that they will find a statistically significant difference when such a difference does actually exist; a power of between 80% and 90% is commonly chosen for clinical trials of this nature. They should also state the level of significance that they have chosen, that is the risk of concluding that a difference exists when there actually is no difference; a level of significance of p = 0.05 is generally chosen (ie 5% chance that a difference is found when there is none). When conducting these calculations an attrition, or drop-out rate, should also be taken into consideration, based on comparable studies in children.
To avoid conducting a study without sufficient power to determine whether an intervention can reduce the progression of myopia, investigators may be tempted to enrol large numbers of participants; however, this can not only be extremely costly and time consuming, it may also not be ethically sound, particularly if participating in the study is putting the children at increased risk. A balance between sufficient and excessive enrolment must therefore be achieved. Table 2 summarises the importance of achieving the right balance for enrolment of children in myopia control studies.
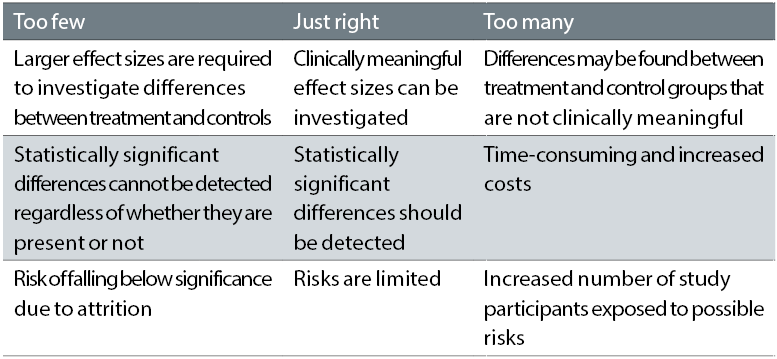
Table 2: Enrolment of study participants when evaluating myopia control
Safety and adverse events
Safety data may not always be reported in myopia control studies; however, when these data are presented, ECPs should carefully evaluate the rates and types of adverse events reported, to help them determine the relative risk to benefit ratio for the treatment being evaluated.21 This was discussed in further detail in Part 1.

It is particularly important that the children taking part are representative of the target populations for the treatment
Compliance Issues
The efficacy of any treatment is determined by the individual’s compliance with following instructions for the treatment. In the case of myopia control studies with contact lenses the wearing time, both hours per day and days per week is of great importance since presumably when the contact lenses are not being worn, the children either revert to spectacles or no correction and this practice may diminish or to some degree negate any therapeutic benefit of the treatment. Minimum wearing times should be required for continuation in the study and can be monitored by the use of questionnaires during the study, and wearing times for treatment and control groups should be carefully reported in the publications. A recent study has reported a greater reduction in myopia progression in wearers of contact lenses designed to slow myopia progression who were compliant with the instructions for wearing time.22 Compliance is also important in myopia control studies investigating efficacy of treatment with topical drops such as atropine.
Data Management and Reporting
In myopia control studies, statistical analysis for testing the hypotheses is generally only performed on the participants who successfully complete the study (‘per-protocol’) rather than on all the enrolled participants (‘intent to treat’) since the studies are being conducted to determine the effect, if any of the treatment. Notwithstanding this it is important to report the accountability of the participants, providing the number and reasons for discontinuation from the study. If there are marked differences in the dropout rates between the test and control groups, this should be addressed in both the analyses and reporting of the data to minimise any attrition or selective reporting biases.
When presenting the results for change in spherical equivalent refractive error and axial length, it is preferable for confidence intervals (CIs) for the estimated effect(s) to be reported, rather than just p values. Commonly 95% CIs are used which provide information about the upper and lower boundaries of observed treatment differences if tested 95 out of 100 times. The advantage of CIs is that they show the clinician the range of any true benefit that may occur as the result of treatment. This is particularly important for myopia control studies since the individual responses can vary considerably.
What Happens After Study Completion?
At the conclusion of myopia control studies, the children who have participated are generally returned to the care of their ECP for continued refractive and ocular health management. Consideration should be given to whether and how treatment for myopia control is continued and raises ethical and product availability issues, since the treatment under investigation may not be currently marketed. In one study investigating soft contact lenses with positive spherical aberration for myopia control, a withdrawal phase was incorporated in which all participants wore the control single vision soft lenses and during this phase no rebound effect was reported.23 Further studies are needed to determine the optimal length of time required for myopia control treatment and how the effects can be maintained.
Myopia Control Soft Contact Lens Research Studies
A comprehensive review of all studies investigating treatments to control the progression of myopia is beyond the scope of this article; however, a summary of the studies that have been conducted investigating the effect of soft contact lenses of differing designs on slowing the progression of myopia in school-aged children is provided in a recent publication by Li et al.13 The authors conducted a meta-analysis of eight studies, five randomised, controlled trials,2,24-27 and three cohort studies.28-30 While both concentric ring bifocal/dual focus soft contact lenses and peripheral ADD multifocal soft contact lenses were found to be effective in controlling myopia progression (up to 38% for myopia and up to 50% for axial elongation), the concentric ring / dual focus designs were reported to show a greater effect. It is important to recognise that the results from these studies were variable; while a consistent change in both myopia progression and axial elongation was found in three of the studies,2,24,29 the other studies reported inconsistent changes in these outcomes.25-28,30 Despite the results from these studies being promising, none were conducted using currently marketed lenses specifically designed for myopia control, and the maximum length of time evaluated was only two years.
A further clinical trial has been recently conducted to quantify the effectiveness of a CE marked daily disposable dual focus soft contact lens (MiSight, CooperVision Inc) in slowing the rate of progression of juvenile-onset myopia over a three-year period.31 One-hundred-and-forty-four myopic children aged eight to 12 years, with no prior contact lens experience, were enrolled at sites in the UK, Canada, Portugal and Singapore and randomly assigned to wear either the daily disposable dual focus soft contact lens or Proclear 1‑Day soft contact lenses. The three-year results were recently presented at the 2017 British Contact Lens Association Clinical Conference and the MiSight contact lenses were found to be effective in controlling myopia progression by 59% for spherical equivalent refractive error and 52% for axial length.
Translating Research from Clinical Studies to Clinical Practice
Myopia control is rapidly becoming a major component in day-to-day clinical practice for many ECPs. Parts 1 and 2 of this article have highlighted some of the key considerations with respect to clinical studies that are conducted to evaluate the efficacy of treatment methods. By integrating evidence from the literature in conjunction with their clinical expertise, ECPs will be better equipped to be able to determine the most appropriate methods of management for the young patients in their practices.
Dr Kathy Dumbleton is a clinical research scientist and consultant based in Berkeley, California where she is also a clinical Associate Professor at the School of Optometry, University of California, Berkeley.
This article was written with support from CooperVision
References
- Altman DGS, K. F.; Moher, D.; Egger, M.; Davidoff, F.; Elbourne, D.; Gotzsche, P. C.; Lang, T.; Consort, Group. The revised CONSORT statement for reporting randomized trials: explanation and elaboration. Ann Intern Med. 2001;134:663-94.
- Anstice NSP, J. R. Effect of dual-focus soft contact lens wear on axial myopia progression in children. Ophthalmology. 2011;118:1152-61.
- Woods JG, S. E.; Keir, N.; Dillehay, S.; Tyson, M.; Griffin, R.; Choh, V.; Fonn, D.; Jones, L.; Irving, E. Inhibition of defocus-induced myopia in chickens. Invest Ophthalmol Vis Sci. 2013;54:2662-8.
- Swarbrick HAA, A.; Watt, K.; Lum, E.; Kang, P. Myopia control during orthokeratology lens wear in children using a novel study design. Ophthalmology. 2015;122:620-30.
- Walline JJJ, L. A.; Sinnott, L.; Manny, R. E.; Gaume, A.; Rah, M. J.; Chitkara, M.; Lyons, S.; Achieve Study Group. A randomized trial of the effect of soft contact lenses on myopia progression in children. Invest Ophthalmol Vis Sci. 2008;49:4702-6.
- Altman DG. Randomisation. BMJ. 1991;302:1481-2.
- Keir NL, D.; Woods, C. A.; Bergenske, P.; Fahmy, M.; Fonn, D. Effect of Masking on Subjective Responses to Daily Disposable Contact Lenses. Optom Vis Sci. 2016;93:828-35.
- Charm JC, P. High myopia-partial reduction orthokeratology (HM-PRO): study design. Cont Lens Anterior Eye. 2013;36:164-70.
- Cho PC, S. W. Retardation of myopia in Orthokeratology (ROMIO) study: a 2-year randomized clinical trial. Invest Ophthalmol Vis Sci. 2012;53:7077-85.
- Chua WHB, V.; Chan, Y. H.; Tong, L.; Ling, Y.; Quah, B. L.; Tan, D. Atropine for the treatment of childhood myopia. Ophthalmology. 2006;113:2285-91.
- ISO 14155: 2011 Clinical investigation of medical devices for human subjects — Good clinical practice.
- FDA. Public Workshop - Controlling the Progression of Myopia: Contact Lenses and Future Medical Devices. FDA White Oak Campus, Silver Spring, MD2016.
- Li SMK, M. T.; Wu, S. S.; Meng, B.; Sun, Y. Y.; Wei, S. F.; Liu, L.; Peng, X.; Chen, Z.; Zhang, F.; Wang, N. Studies using concentric ring bifocal and peripheral add multifocal contact lenses to slow myopia progression in school-aged children: a meta-analysis. Ophthalmic Physiol Opt. 2017;37:51-9.
- Donovan LS, P.; Ho, A.; Naduvilath, T.; Smith, E. L., 3rd; Holden, B. A. Myopia progression rates in urban children wearing single-vision spectacles. Optom Vis Sci. 2012;89:27-32.
- McCullough SJOD, L.; Saunders, K. J. Six Year Refractive Change among White Children and Young Adults: Evidence for Significant Increase in Myopia among White UK Children. PLoS One. 2016;11:e0146332.
- Flitcroft DI. The complex interactions of retinal, optical and environmental factors in myopia aetiology. Prog Retin Eye Res. 2012;31:622-60.
- Lin ZV, B.; Jhanji, V.; Mao, G. Y.; Gao, T. Y.; Wang, F. H.; Rong, S. S.; Ciuffreda, K. J.; Liang, Y. B. Near work, outdoor activity, and their association with refractive error. Optom Vis Sci. 2014;91:376-82.
- O'Donoghue LK, V. V.; McClelland, J. F.; Logan, N. S.; Owen, C. G.; Saunders, K. J.; Rudnicka, A. R. Risk Factors for Childhood Myopia: Findings From the NICER Study. Invest Ophthalmol Vis Sci. 2015;56:1524-30.
- Pan CWR, D.; Saw, S. M. Worldwide prevalence and risk factors for myopia. Ophthalmic Physiol Opt. 2012;32:3-16.
- Fitzner KH, E. Sample size calculation and power analysis: a quick review. Diabetes Educ. 2010;36:701-7.
- Bullimore MA. The Safety of Soft Contact Lenses in Children. Optom Vis Sci. 2017;94:638-46.
- Sankaridurg PB, R. C.; Morgan, J.; Chen, X.; Tilia, D.; Ho, A.; Ehrmann, K.; Weng, R.; Conrad, F.; Smith, E.; Naduvilath, T.; Erickson, P. Novel contact lenses designed to slow progress of myopia: 12 month results. Association for Research in Vision and Ophthalmology. Baltimore, MD2017.
- Cheng KHL, S. L.; Hoekman, H. W.; Beekhuis, W. H.; Mulder, P. G.; Geerards, A. J.; Kijlstra, A. Incidence of contact-lens-associated microbial keratitis and its related morbidity. Lancet. 1999;354:181-5.
- Aller TAL, M.; Wildsoet, C. F. Myopia Control with Bifocal Contact Lenses: A Randomized Clinical Trial. Optom Vis Sci. 2016;93:344-52.
- Cheng XX, J.; Chehab, K.; Exford, J.; Brennan, N. Soft Contact Lenses with Positive Spherical Aberration for Myopia Control. Optom Vis Sci. 2016;93:353-66.
- Fujikado TN, Sayuri; Kobayashi, Takuma; Suzaki, Asaki; Nakada, Mitsuhiko; Nishida, Kohji. Effect of low-addition soft contact lenses with decentered optical design on myopia progression in children: a pilot study. Clinical ophthalmology (Auckland, NZ). 2014;8:1947.
- Lam CST, W. C.; Tse, D. Y.; Tang, Y. Y.; To, C. H. Defocus Incorporated Soft Contact (DISC) lens slows myopia progression in Hong Kong Chinese schoolchildren: a 2-year randomised clinical trial. Br J Ophthalmol. 2014;98:40-5.
- Paune JM, H.; Armengol, J.; Quevedo, L.; Faria-Ribeiro, M.; Gonzalez-Meijome, J. M. Myopia Control with a Novel Peripheral Gradient Soft Lens and Orthokeratology: A 2-Year Clinical Trial. Biomed Res Int. 2015;2015:507572.
- Sankaridurg PH, B.; Smith, E., 3rd; Naduvilath, T.; Chen, X.; de la Jara, P. L.; Martinez, A.; Kwan, J.; Ho, A.; Frick, K.; Ge, J. Decrease in rate of myopia progression with a contact lens designed to reduce relative peripheral hyperopia: one-year results. Invest Ophthalmol Vis Sci. 2011;52:9362-7.
- Walline JJG, K. L.; McVey, M. E.; Jones-Jordan, L. A. Multifocal contact lens myopia control. Optom Vis Sci. 2013;90:1207-14.
- Chamberlain P, Back A, Lazon De La Jara P. 3-year Effectiveness of a Dual-Focus 1 day Soft Contact Lens for Myopia Control. British Contact Lens Association Clinical Conference. Liverpool 2017.
