Primary care optometrists do not often come up against active inflammatory diseases of the posterior chamber, however, these conditions may be sight-threatening, and may have life-threatening systemic aetiologies, therefore it is paramount that optometrists have an understanding of their signs, symptoms and management, as well as of the underlying conditions. More common conditions encountered routinely in practice may have overlapping symptoms, therefore care should be taken when differentially diagnosing conditions by careful history taking and examination. This article aims to cover the more common and more serious inflammatory conditions of the posterior chamber.
Classification and terminology
The terminology used to classify inflammatory diseases usually relates to the primary location of inflammation, and was formalised by the International Uveitis Study Group in the late 1980s. Anterior uveitis relates to inflammation of the anterior chamber structures, intermediate uveitis the vitreous, and posterior uveitis the choroid and retina. Panuveitis refers to those cases where there is involvement of the entire uveal tract with no predominant site.1,2 Within this classification, there are more specific terms which relate to inflammation of particular anatomical structures:
- Choroiditis is inflammation of the choroid and may be focal, multifocal or diffuse/geographic.
- Chorioretinitis is inflammation involving the choroid and retina.
- Retinitis is inflammation of the retina and may be focal or multifocal.
- Neuroretinitis is inflammation of the neural layers and optic nerve.
Other terms encountered when discussing posterior chamber inflammatory disease include:
- Vitritis, which is a sign of intermediate uveitis and is characterised by vitreous cells.
- Pars planitis is an idiopathic subdivision of intermediate uveitis, which appears as white exudation at the ora serrata commonly termed ‘snow banking.’
- Retinal vasculitis is inflammation of the retinal vessels, characterised by various features including perivascular sheathing, haemorrhages and cotton wool spots. Periphlebitis is perivascular inflammation of the veins and is more common than periarteritis (arteries). Vasculitides may be the primary condition or secondary to adjacent retinitis.
Chorioretinitis: causes, symptoms, signs and management
The aetiology of chorioretinitis may be exogenous (arising from outside the body) or endogenous (arising from within the body). The following sections will look at the many and varied causes. It is by no means exhaustive.
Infectious causes of chorioretinitis
Syphilis uveitis
Syphilis is caused by the spirochete bacterium Traponema pallidum, which is usually transmitted by close sexual contact, but may also be transmitted transplacentally. Its prevalence is increasing, with a recent report describing a 97% increase in diagnoses from 2012 to 2016 in England, the largest amount of new diagnoses since 1949 (5,920 patients), especially in men who have sex with men.3
Acquired syphilis is a three-stage disease, and ocular involvement may occur in the secondary or more commonly tertiary stage. Primary syphilis is characterised by chancre (painless ulcers) at the site of inoculation, secondary syphilis by fever, malaise and mucocutaneous lesions, and tertiary syphilis by gummas (soft tumour-like lesions), aortitis and neurosyphilis, which can cause, among others, meningitis, stroke, central nervous system abnormalities and dementia.4
Syphilis may cause anterior uveitis, which may be granulomatous or non-granulomatous, or posterior uveitis, whose signs are diverse. Lesions may be unilateral or bilateral, focal or multifocal, chorioretinitis or purely choroiditis.5 A typical presentation of syphilis uveitis is of punctate inner retinitis which appear as white spots and arteriolitis. More commonly the uveitis appears as so called acute syphilitic posterior placoid chorioretinitis – large, yellowish sub-retinal plaque-like lesions with overlying vitritis. Patients with syphilis may also develop neuroretinitis.4
Investigations may include venereal disease research laboratory testing (for disease activity), fluorescent treponemal antibody absorption (to detect current or previous Traponema infection) and sometimes a lumbar puncture.5 Patients are commonly tested for HIV as the two diseases often concomitantly exist.4
Treatment is in conjunction with genitourinary physicians and is usually with high-dose penicillin, especially where neurosyphilis is present. Inflammation may transiently worsen during treatment because of spirochete death. Systemic corticosteroid may be also be useful.6
Tuberculosis uveitis
The facultative intracellular bacterium Mycobacterium tuberculosis is the cause of tuberculosis (TB), and is usually contracted by airborne droplets. It is estimated that almost 1.9 billion people are infected with Mycobacterium tuberculosis worldwide,7 of which 10% develop active TB, and 1% develop ocular disease.5 It primarily affects the lungs in the form of pneumonia, pleural effusion and fibrosis, but may also affect other parts of the body.
Ocular signs include anterior uveitis (which is usually granulomatous), scleritis, phlyctenulosis and keratitis. Posterior chamber inflammation includes vitritis and pars planitis, choroidal tubercles (small, indistinct greyish-yellow nodules found at the posterior pole), tuberculoma (larger solitary tubercle, located anywhere in the choroid), serpiginous-like choroiditis (multifocal plaque-like choroiditis), sub-retinal abscesses, periphlebitis, Eale’s disease, cystoid macular oedema, vitreous haemorrhage and retinal detachment 4,8 (figure 1).
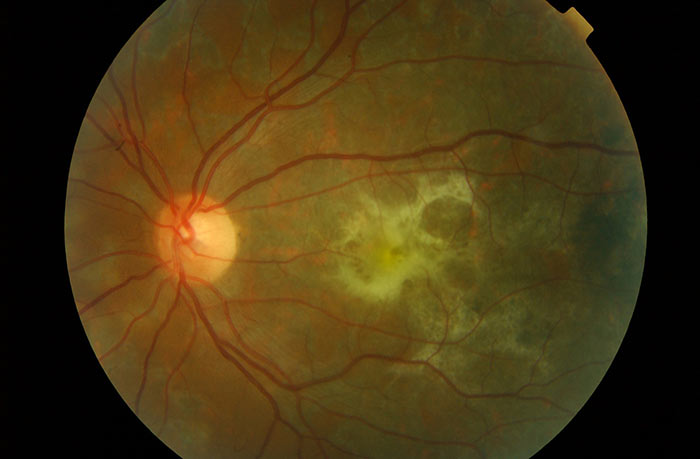
Figure 1: Scarring from previous TB-related chorioretinitis
Because the majority of cases in the UK are in individuals born outside of the country (about 73%),9 individuals from endemic areas, such as South Asia and Sub-Saharan Africa, showing posterior uveitis should cause a higher degree of suspicion. Polymerase chain reaction (PCR) of intraocular fluids are conducted to confirm a diagnosis, and sometimes interferon-gamma release assays are used to measure the ability of the patient to release interferon-gamma to specific TB antigens. Often though, presumed TB uveitis can be made from a combination of ocular and systemic clinical findings (eg chest x-ray, microbiological assessment of sputum and urine, or tuberculin skin test).
These patients are routinely treated systemically with four antibiotics – isoniazid, rifampicin, pyrazinamide and ethambutol for two months, then isoniazid and rifampicin for four months. Ocular inflammation may be managed with corticosteroids.5,8 As an aside, ethambutol itself has known possible ocular side effects including optic neuritis, dyschromatopsia and central scotomata.10
Endogenous and exogenous bacterial endophthalmitis
Cases of bacterial endophthalmitis are rare, and usually caused by Staphylococcus aureus and Streptococcus spp. Exogenous endophthalmitis may occur in healthy patients following, for example, trauma, surgery or, particularly over the last decade, intravitreal injection. Endogenous endophthalmitis usually results from bacteraemia, and occurs in patients who are immunocompromised for example HIV-positive patients, post-transplantation patients or those on immunosuppressant medication. In the USA, 40% of endogenous endophthalmitis is caused by endocarditis.11
Patients often present with pain, photophobia, floaters and vision loss and onset is usually more acute than fungal endophthalmitis, which is discussed later. Endogenous causes also display systemic symptoms, such as fever. Signs include anterior uveitis with hypopyon, vitritis, chorioretinal infiltrates, septic emboli and white-centred haemorrhages. Treatment includes systemic treatment of any underlying condition, and intravitreal antibiotics for the endophthalmitis. Vitrectomy is often indicated, although enucleation or evisceration are sometimes required.12
Herpetic uveitis
Herpes simplex virus (HSV) 1 and 2, known to the layperson as the ‘cold sore virus’ and ‘genital herpes’ respectively, are common viruses that infect humans. Varicella zoster virus (VZV) is another of the herpesvirus family, and causes chickenpox in younger patients and shingles in older adults. While the anterior segment complications of HSV (most typically HSV-1) and VZV are well known and outside the scope of this article, less commonly they may cause posterior chamber inflammation. HSV may rarely cause acute retinal necrosis (ARN, figure 2), while those with VZV may develop ARN or progressive outer retinal necrosis (PORN).4
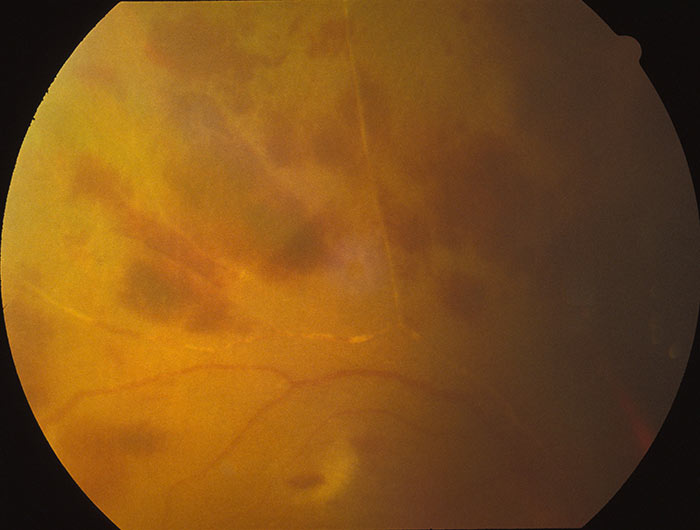
Figure 2: Acute retinal necrosis subsequent to herpetic retinal inflammatory response
ARN may occur in immunocompetent or immunocompromised hosts. It is rare, with an incidence of 1 in 1.6-2 million per year in the UK, with the majority being caused by VZV.13,14 Patients often present with unilateral reduced vision, floaters and an uncomfortable eye. Signs include one or more areas of retinal necrosis in the peripheral retina with discrete borders and vitritis. There is usually occlusive arterial vasculopathy and concurrent anterior chamber inflammation. Seventy-five percent develop rhegmatogenous or tractional retinal detachment.5 Aqueous tap and PCR can be used to identify viral DNA. Treatment involves intravenous aciclovir for up to a fortnight, then oral aciclovir for up to 12 weeks. Intravitreal antivirals have gained popularity recently, and some centres use prophylactic laser to localise retinal detachments.14
PORN is very rare, and usually caused by VZV in an immunocompromised host (most often patients with HIV). Patients usually present with unilateral or bilateral painless reduced vision. Ophthalmoscopically, PORN appears as multifocal retinal opacification, often at the macula, with minimal or absent vitritis, vasculitis and anterior uveitis. Progression is usually very rapid.15 Patients are often co-managed between an ophthalmologist with experience in HIV-related disease and a HIV physician, and treatment is usually IV and intravitreal antivirals (eg ganciclovir). Despite treatment, prognosis is poor5 and the risk of developing a rhegmatogenous retinal detachment is as high as 75%.4 Systemic antivirals are particularly important in bilateral PORN due to the risk of VZV encephalitis.4
HIV-associated posterior uveitis
HIV is a retrovirus that infects human CD4+ helper T cells, and can lead to AIDS, a condition which causes progressive failure of the immune system. CMV retinitis is the most important posterior chamber inflammatory disease in this patient group, however, they are more susceptible to other infectious causes such as Toxoplasmosis, and they may be more severe.16
Cytomegalovirus (CMV) retinitis
While CMV is very common among the general population, CMV uveitis is very uncommon and occurs in immunocompromised patients, typically patients with HIV, and usually when CD4+ count drops below 50 cells/mm3 (the reference value for a non-HIV-infected population is 500-1,500 cells/mm3). Patients are asymptomatic in half of cases but can present with floaters, scotomata, vision loss and discomfort.17 Anterior segment is usually quiet but may show mild cells with stellate keratic precipitates.5 Vitritis is usually mild, if present at all, and retinitis is varied in its appearance. There may be ‘frosted branch’ angiitis – perivascular sheathing, indolent granular retinitis – peripheral lesions with minimal oedema, exudate or haemorrhage, or ‘pizza pie’ fulminant retinitis – large areas of haemorrhage and retinal necrosis. Diagnosis is usually ophthalmoscopically, sometimes with the aid of PCR of CMV DNA.4,17
Management of CMV retinitis involves improving the immunological state of the patient. In HIV/AIDS, prophylaxis includes maintaining a CD4+ count of greater than 50 cells/mm3. Treatments for CMV specifically include systemic antivirals (ganciclovir, valganciclovir, foscarnet or cidofovir), and intravitreal ganciclovir implant or injection, or intravitreal foscarnet injection.18 With treatment, the majority of patients achieve stabilisation,17 however, many develop complications, including a third who progress to retinal detachment.19
Other viral causes of posterior uveitis
There are several other viruses which may cause posterior chamber inflammation. Measles, mumps, rubella,5 West Nile virus,20 Chikungunya,21 Ebola22 and, on a topical note, Zika virus23 among others have been known to cause chorioretinal inflammatory disease.
Nemotodal uveitis
Nematodes are a diverse phylum of animals, also known as roundworms, of which Toxocara canis is an important cause of uveitis. This parasite causes toxocariasis, which usually occurs in humans by accidental ingestion of their ova, often through soil contaminated by dog faeces. After ingestion, the ova hatch in the small intestine and pass through the intestinal wall, then spread via the bloodstream to various parts of the body causing, among others, hepatitis, pneumonitis and encephalitis.24,25 Prevalence of Toxocara in the blood of donors in the 1970s was 3% but in children it is often higher. In some countries and age groups prevalence may be as high as 84%. Ocular involvement is more prevalent in young children,26 particularly five to 10-year-olds.25
Diagnosis is usually clinical, but enzyme-linked immunosorbent assay (ELISA) may be used to check for antibodies.26 Patients may present with unilateral vision loss, and parents may have noticed leukocoria and/or strabismus.
Signs of ocular toxocariasis are varied, but may include:
- Endophthalmitis – appears as a white eye with chronic anterior uveitis (granulomatous keratic precipitates and posterior synechiae), vitritis and snowbanking, and eventually an exudative or tractional retinal detachment. A retrolental cyclitic membrane may also appear.
- Posterior pole chorioretinitis – a 1-4DD yellow-white lesion at the macula, sometimes with a greyish centre, with vitreous cells and retinal traction.
- Peripheral chorioretinitis – a yellow-white lesion in the peripheral fundus, with traction and sometimes a so-called falciform (sickle-shaped) fold in the mass, leading to a hetereotopic (abnormally located) macula or retinal detachment.
- Optic papillitis – an inflammatory reaction within the optic disc, which may lead to an artery occlusion.
- Occasionally a motile nematode, or its tracks, may be noted ophthalmoscopically. It may show no inflammatory response while alive, but may cause a severe reaction if the nematode dies within the eye.5,24
Treatment is usually by systemic or periocular steroids to control the inflammation along with antihelminthic drugs such as albendazole 25 or thiabendazole, although these are usually of limited use.5 Patients often require a vitrectomy.25
Protozoan uveitis
Toxoplasmosis is perhaps the most important protozoan uveitis. It is the result of an infection of the retina by Toxoplasma gondii, an obligate intracellular parasite which is present in endothermic animals, including humans, the world over. Estimates of the prevalence of infection varies between studies but is more common in Latin America and tropical Africa,27 and risk factors for contracting the parasite are living in crowded environments, having a low education level and working in an occupation that comes into contact with soil. The latter is linked to the presence of oocysts, which are only produced in cats and expelled in their faeces. Drinking contaminated water and eating raw or undercooked meat are also considered major sources of infection, in developing and developed countries respectively.5 Transplacental transmission is also an important cause, and active or inactive chorioretinitis may be present in up to 95% of neonates with toxoplasmosis,27 and is often bilateral and macular.5 It is important to note that neonatal toxoplasmosis can also cause severe systemic complications including hydrocephalus and intracranial calcifications.28 Transmission rates increase from the first to third trimester, however, complications are more severe if contracted earlier in the pregnancy.28
In the United Kingdom the incidence of ocular toxoplasmosis specifically has been reported at 0.8/100,000 per year, with a lifetime risk is 18/100,000.29 It is often cited as the commonest cause of posterior uveitis.27 Clinically the patient may be asymptomatic, but may complain of floaters or reduced vision. Like toxocariasis, in congenital toxoplasmosis, the parents may have noticed leukocoria and/or strabismus.28
Diagnosis of toxoplasmosis uveitis is usually a clinical one. It appears as a fluffy white chorioretinal lesion adjacent to a variably pigmented scar. Vitritis is often present and may appear as precipitates on the surface of the posterior hyaloid face. Vitritis may be severe enough to give the classic ‘headlight in the fog’ appearance of the white chorioretinal lesion behind the cloudy vitreous. Periphlebitis may be present.5,28 Less commonly seen presentations include punctate outer retinal lesions which have minimal vitritis.4 OCT findings in toxoplasmosis include retinal thickening and even macular schisis. Old inactive lesions appear as significant thinning of the retinal layers, even down to the choriocapillaris.4
Toxoplasmosis, in an immunocompetent patient usually resolves within two months, and therefore such patients are not routinely treated, primarily because of drug toxicity.28 Treatment is usually commenced when lesions involve the disc, macula or papillomacular bundle, if they are threatening a major vessel, if there is significant vitritis, or if the patient is immunocompromised.5 Treatment has traditionally been pyrimethamine, sulfadiazine and a corticosteroid, however, due to adverse drug reactions this is less common nowadays. Trimethoprim with sulfamethoxazole, atovaquone or azithromycin are sometimes used in place of the classic triple therapy.28 Of course, care should be taken on maternal cases due to teratogenicity of drugs. The macrolide antibiotic, spiramycin may be used to prevent transplacental spread in pregnant patients. Patients are routinely advised on measures to prevent exposure to Toxoplasma during pregnancy including cooking meat and washing fruit and vegetables thoroughly, avoiding unpasteurised cheeses and avoiding handing cat litter.5
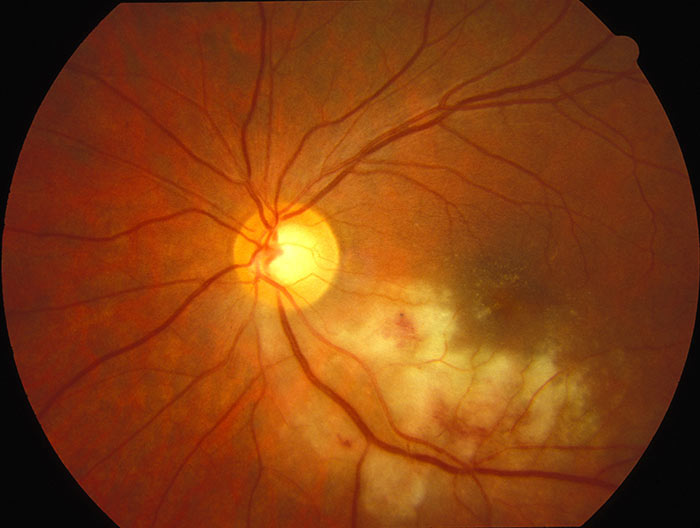
Figure 3: Cytomegalovirus retinitis
Fungal uveitis
Mycosis (fungal infection) is rare in healthy individuals, with exogenous causes being rarer than endogenous ones. Exogenous infection may occur after trauma or surgery or contagious spread of an external infection, while endogenous infection may be caused by intravenous drug use, indwelling venous catheters (ie a catheter left in a vein temporarily or permanently to aid, for example, drug administration) or immunosuppression.4 Intravenous drug abuse should be presumed as the cause until proven otherwise.5
The most common ocular mycosis is that caused by Candida albicans, a ubiquitous dimorphic fungus which is commensal but opportunistic. Other causes of fungal infections include Aspergillus sp. and Histoplasma capsultum.5
Fungal endophthalmitis appears ophthalmoscopically as multifocal chorioretinal lesions which are yellow-white and fluffy, often termed ‘cotton balls’. They start in the choroid and eventually work through the retinal layers and erupt into the vitreous, where they appear as a ‘string of pearls.’4
Treatment is challenging and usually includes a vitrectomy, intravitreal antifungal drugs (amphotericin B), intravitreal corticosteroid30 and systemic antifungals (eg fluconazole).5
Autoimmune and other non-infectious causes of chorioretinitis
Sarcoidosis
Sarcoidosis is a systemic multisystem granulomatous disease of unknown aetiology which has ocular involvement in up to 60% of cases. Primary presentation as said ocular involvement occurs in up to 30%32 and posterior uveitis makes up about a quarter of cases.5
The International Workshop on Ocular Sarcoidosis (IWOS)33 classifies seven signs suggestive of ocular sarcoidosis:
- Mutton-fat keratic precipitates and/or iris nodules (Koeppe or Busacca)
- Trabecular meshwork nodules and/or tent-shaped peripheral anterior synechiae
- Snowballs/string of pearls vitreous opacities
- Multiple chorioretinal peripheral lesions
- Nodular and/or segmental periphlebitis and/or macroaneursym (figure 4)
- Optic disc nodule/granuloma and/or solitary choroidal nodule
- Bilaterality
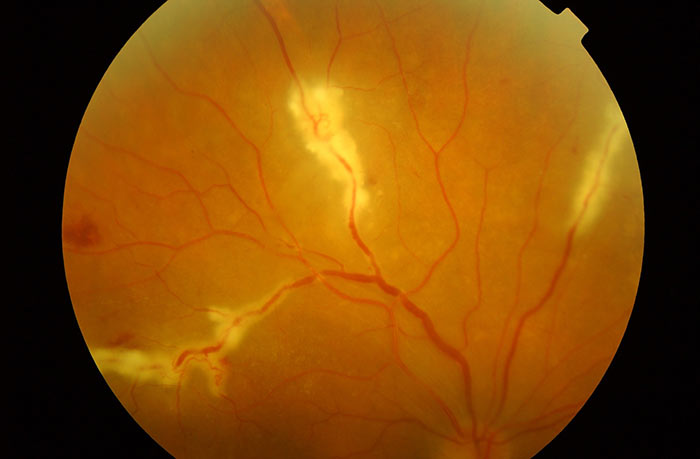
Figure 4: Periphlebitic exudation in sarcoidosis
Diagnosis of sarcoidosis is one of exclusion and is usually based on symptoms and tissue biopsy, the latter being the gold standard for diagnosis.32 Classification of intraocular sarcoid according to IWOS requires biopsy for a definite diagnosis.33 Because of the invasive nature of biopsy, IWOS also classifies cases where biopsies are not done, and a diagnosis of presumed ocular sarcoidosis occurs when there is a bihilar lymphadenopathy (enlargement of the lymph nodes of the pulmonary hila) on chest x-ray (CXR) along with uveitis. Sometimes CXR is inconclusive, and other tests are required such as serum angiotensin converting enzyme (which is elevated in sarcoid) or high resolution CT scan of the thorax.5
Systemically, these patients are monitored for cardiac, respiratory and neurological complications, and the mortality rate can be as high as 6%.34 Management of posterior segment inflammation may be purely observation if the visual acuity is good and there is no evidence of cystoid macular oedema. If treatment is required then topical, intravitreal or periorbital corticosteroids are required. Patients with posterior segment disease often require systemic steroid use. Chronic inflammation should mean a move away from steroid use due to their complications (eg obesity, diabetes, osteoporosis) and immunosuppressant therapies, such as methotrexate, are used.34
Systemic lupus erythematosus (SLE)
SLE is a life-threatening multisystem autoimmune condition. It affects 28 in 100,000 individuals, is more common in women (studies suggest somewhere between 78-96% of patients are female) and non-Caucasian populations.35,36 SLE may affect the kidney, heart, lung, brain, joints, skin, mucous membranes and sometimes the gastrointestinal tract. SLE nephritis is one of its most common and serious complications.37
Around a third of patients with SLE develop ocular disease, the commonest being ocular surface disease such as secondary Sjögren’s. They may also develop episcleritis, scleritis, ischaemic optic neuropathy and nerve palsies (commonly CN VI palsy).37 These patients are also 3.5 times more likely to experience a retinal vein occlusion.38 Relevant to this article, around 10% of patients with SLE develop posterior segment disease, which includes retinopathy which is more accurately described as a vasculopathy, much like hypertensive or diabetic retinopathy, rather than inflammatory vasculitis.39 While rare, the immunosuppressed state of these patients makes them more susceptible to infectious retinitis including CMV, ARN or PORN.39
Systemically, SLE is managed using immunosuppressant therapy including hydroxychloroquine, azathiaprine, mycophenolate, cyclophosphamide and occasionally corticosteroids. Ophthalmic signs of SLE are often a sign of poor systemic management and systemic treatment is often heightened. Evidence on the treatment of ophthalmic complications of SLE are limited at present, but oral or intravenous corticosteroid, or biologic therapies such as rituximab are sometimes used. Ocular surface disease is managed as one would otherwise manage dry eye.37
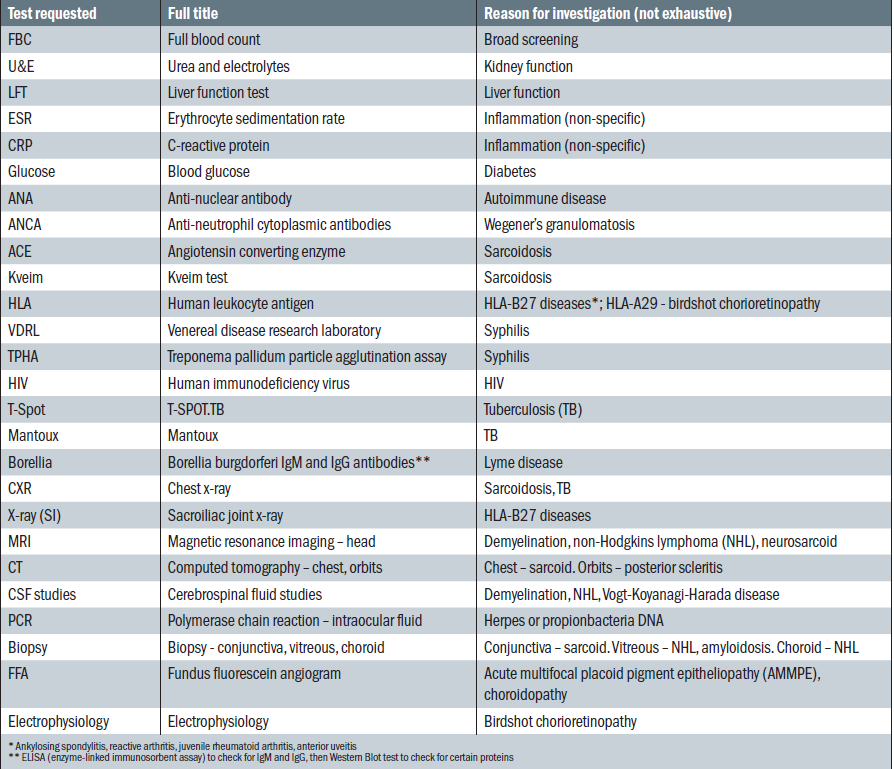
Table 1: An example of investigations conducted on patients with uveitis in secondary care
Behçet’s disease (BD)
BD is a chronic multisystem inflammatory disease of unknown aetiology. It generally presents in the third to fifth decade of life, and is more common in those along the ancient ‘Silk Route’ which extends from Eastern Asia to the Mediterranean. While women are more at risk in Eastern Asia, in the Middle East men are more at risk in a ratio of 10:1.40 While it is rare in the United Kingdom, with a prevalence of 6.4x10-4%, it is commonest in Turkey where the prevalence is 0.37%.41 This condition seems to have genetic and environmental elements, as those in Western Europe with Turkish ancestry have a higher risk than the general population, but still less than those living in Turkey. The HLA-B51 allele has been implicated in the genetic aspect of this disease, but the environmental risks are still unknown.41
In order for a confirmed diagnosis, the following signs are required:42
- They must have had three episodes of oral ulcers in a year
- They must have two of the following:
• Genital ulcers
• Skin lesions
• Positive pathergy test (skin pustule development after pricking the skin with a needle)
• Ocular involvement
Ocular involvement occurs in 70% of patients and is the primary presenting feature in 20%.40 BD may cause anterior uveitis, which is acute and non-granulomatous, often with a hypopyon, and posterior uveitis. Posterior uveitis may include vitritis, periphlebitis, retinal haemorrhage and oedema, and neovascularisation, which may lead to vitreous haemorrhage and tractional retinal detachment.5 Ocular BD has a particularly poor prognosis with a 13% chance of severe vision loss at 10 years, most commonly through ischaemic maculopathy secondary to branch retinal vein occlusion. The risk of severe vision loss can be reduced with adequate immunosupression.43
Treatment of BD involves a corticosteroid (eg oral prednisolone or intravenous methylprednisolone) and a steroid-sparing agent (eg azathioprine). For refractory cases the immunosuppressant ciclosporin, the anti-tumour necrosis factor agent infliximab, or others may be used.44
Chorioretinitis as an adverse drug reaction
Although rare, various systemic drugs have been implicated in chorioretinitis, including tumour necrosis factor antagonist agents such as etanercept (Enbrel) which are used in the treatment of autoimmune disorders such as rheumatoid arthritis, as well as with Bacille Calmette-Guerin (BCG) vaccine used in tuberculosis prophylaxis.31
Ceri Probert is an optometrist practising in Wales, a tutor at Cardiff University Department of Optometry and is College of Optometrists council member, Wales.
References
- Blochmichel E, Nussenblatt RB. International-uveitis-study-group recommendations for the evaluation of intraocular inflammatory disease. American Journal of Ophthalmology 1987;103(2):234-235.
- Jabs DA, Nussenblatt RB, Rosenbaum JT, Standardization Uveitis N. Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. American Journal of Ophthalmology 2005;140(3):509-516.
- Public Health England. Sexually Transmitted Infections and Chlamydia Screening in England, 2016. Public Health England. London 2017.
- Mandelcorn ED. Infectious causes of posterior uveitis. Canadian Journal of Ophthalmology-Journal Canadien D Ophtalmologie 2013;48(1):31-39.
- Denniston AKO, Murray PI. Oxford handbook of ophthalmology. 2014.
- Clement ME, Okeke NL, Hicks CB. Treatment of Syphilis A Systematic Review. Jama-Journal of the American Medical Association 2014;312(18):1905-1917.
- Dye C, Scheele S, Dolin P, Pathania V, Raviglione RC. Global burden of tuberculosis - Estimated incidence, prevalence, and mortality by country. Jama-Journal of the American Medical Association 1999;282(7):677-686.
- Gupta V, Gupta A, Rao NA. Intraocular tuberculosis - An update. Survey of Ophthalmology 2007;52(6):561-587.
- Ormerod LP. Further evidence supporting programmatic screening for, and treatment of latent TB Infection (LTBI) in new entrants to the UK from high TB prevalence countries. Thorax 2013;68(3):I-I.
- British National Formulary. British National Formulary 2017. Available at <https://www.medicinescomplete.com/mc/bnf/current/>. Accessed 10 November 2017.
- Durand ML. Endophthalmitis. Clinical Microbiology and Infection 2013;19(3):227-234.
- Durand ML. Bacterial and Fungal Endophthalmitis. Clinical Microbiology Reviews 2017;30(3):597-613.
- Muthiah MN, Michaelides M, Child CS, Mitchell SM. Acute retinal necrosis: a national population-based study to assess the incidence, methods of diagnosis, treatment strategies and outcomes in the UK. British Journal of Ophthalmology 2007;91(11):1452-1455.
- Cochrane TF, Silvestri G, McDowell C, Foot B, McAvoy CE. Acute retinal necrosis in the United Kingdom: results of a prospective surveillance study. Eye 2012;26(3):371-377.
- Engstrom RE, Holland GN, Margolis TP, Muccioli C, Lindley JI, Belfort R, Holland SP, Johnston WH, Wolitz RA, Kreiger AE. The progressive outer retinal necrosis syndrome - a variant of necrotizing herpetic retinopathy in patients with AIDS. Ophthalmology 1994;101(9):1488-1502.
- Rodgers CA, Harris JRW. Ocular toxoplasmosis in HIV infection. International Journal of Std & Aids 1996;7(5):307-309.
- Lee JH, Agarwal A, Mahendradas P, Lee CS, Gupta V, Pavesio CE, Agrawal R. Viral posterior uveitis. Survey of Ophthalmology 2017;62(4):404-445.
- Jabs DA, Ahuja A, Van Natta M, Dunn JP, Yeh S, Studies Ocular Complications A. Comparison of Treatment Regimens for Cytomegalovirus Retinitis in Patients with AIDS in the Era of Highly Active Antiretroviral Therapy. Ophthalmology 2013;120(6):1262-1270.
- Sandy CJ, Bloom PA, Graham EM, Ferris JD, Shah SM, Schulenburg WE, Migdal CS. Retinal-detachment in AIDS-related cytomegalovirus retinitis. Eye 1995;9:277-281.
- Kuchtey RW, Kosmorsky GS, Martin D, Lee MS, Collins F. Uveitis associated with West Nile virus infection. Archives of Ophthalmology 2003;121(11):1648-1649.
- Mittal A, Mittal S, Bharathi JM, Ramakrishnan R, Sathe PS. Uveitis during outbreak of Chikungunya fever. Ophthalmology 2007;114(9):1798-1799.
- Shantha JG, Yeh S, Nguyen QD. Ebola virus disease and the eye. Current Opinion in Ophthalmology 2016;27(6):538-544.
- Kodati S, Palmore TN, Spellman FA, Cunningham D, Weistrop B, Sen HN. Bilateral posterior uveitis associated with Zika virus infection. Lancet 2017;389(10064):125-126.
- Shields JA. Ocular toxocariasis - a review. Survey of Ophthalmology 1984;28(5):361-381.
- Despommier D. Toxocariasis: Clinical aspects, epidemiology, medical ecology, and molecular aspects. Clinical Microbiology Reviews 2003;16(2):265-+.
- Gillespie SH, Dinning WJ, Voller A, Crowcroft NS. The spectrum of ocular toxocariasis. Eye 1993;7:415-418.
- Furtado JM, Winthrop KL, Butler NJ, Smith JR. Ocular toxoplasmosis I: parasitology, epidemiology and public health. Clinical and Experimental Ophthalmology 2013;41(1):82-94.
- Butler NJ, Furtado JM, Winthrop KL, Smith JR. Ocular toxoplasmosis II: clinical features, pathology and management. Clinical and Experimental Ophthalmology 2013;41(1):95-108.
- Gilbert RE, Dunn DDT, Lightman S, Murray PI, Pavesio CE, Gormley PD, Masters J, Parker SP, Stanford MR. Incidence of symptomatic toxoplasma eye disease: aetiology and public health implications. Epidemiology and Infection 1999;123(2):283-289.
- Chakrabarti A, Shivappakash MR, Singh R, Tarai B, George VK, Fomda BA, Gupta A. Fungal Endophthalmitis Fourteen Years' Experience From a Center in India. Retina-the Journal of Retinal and Vitreous Diseases 2008;28(10):1400-1407.
- Moorthy RS, London NJS, Garg SJ, Cunningham ET. Drug-induced uveitis. Current Opinion in Ophthalmology 2013;24(6):589-597.
- Agrawal R, Gonzalez-Lopez JJ, Meier F, Gupta B, Pavesio C. Ocular and systemic features of sarcoidosis and correlation with the international workshop for ocular sarcoidosis diagnostic criteria. Sarcoidosis Vasculitis and Diffuse Lung Diseases 2015;32(3):237-245.
- Herbort CP, Rao NA, Mochizuki M, Sci Comm First Int Workshop O. International Criteria for the Diagnosis of Ocular Sarcoidosis: Results of the First International Workshop on Ocular Sarcoidosis (IWOS). Ocular Immunology and Inflammation 2009;17(3):160-169.
- Liu D, Birnbaum AD. Update on sarcoidosis. Current Opinion in Ophthalmology 2015;26(6):512-516.
- Smith PP, Gordon C. Systemic lupus erythematosus: Clinical presentations. Autoimmunity Reviews 2010;10(1):43-45.
- Alarcón GS, Friedman AW, Straaton KV, Moulds JM, Lisse J, Bastian HM, G McGwin J, Bartolucci AA, Roseman JM, Reveille JD. Systemic lupus erythematosus in three ethnic groups: III A comparison of characteristics early in the natural history of the LUMINA cohort. Lupus 1999;8(3):197-209.
- Papagiannuli E, Rhodes B, Wallace GR, Gordon C, Murray PI, Denniston AK. Systemic lupus erythematosus: An update for ophthalmologists. Survey of Ophthalmology 2016;61(1):65-82.
- Yen YC, Weng SF, Chen HA, Lin YS. Risk of retinal vein occlusion in patients with systemic lupus erythematosus: a population-based cohort study. British Journal of Ophthalmology 2013;97(9):1192-1196.
- Sivaraj RR, Durrani OM, Denniston AK, Murray PI, Gordon C. Ocular manifestations of systemic lupus erythematosus. Rheumatology 2007;46(12):1757-1762.
- Kacmaz RO, Kempen JH, Newcomb C, Gangaputra S, Daniel E, Levy-Clarke GA, Nussenblatt RB, Rosenbaum JT, Suhler EB, Thorne JE and others. Ocular Inflammation in Behcet Disease: Incidence of Ocular Complications and of Loss of Visual Acuity. American Journal of Ophthalmology 2008;146(6):828-836.
- Sakane T, Takeno M, Suzuki N, Inaba G. Current concepts - Behcet’s disease. New England Journal of Medicine 1999;341(17):1284-1291.
- Silman AJ. Criteria for diagnosis of behcets-disease. Lancet 1990;335(8697):1078-1080.
- Taylor SRJ, Singh J, Menezo V, Wakefield D, McCluskey P, Lightman S. Behcet Disease: Visual Prognosis and Factors Influencing the Development of Visual Loss. American Journal of Ophthalmology 2011;152(6):1059-1066.
- Hatemi G, Silman A, Bang D, Bodaghi B, Chamberlain AM, Gul A, Houman MH, Kotter I, Olivieri I, Salvarani C and others. EULAR recommendations for the management of Behcet disease. Annals of the Rheumatic Diseases 2008;67(12):1656-1662.
