The release of the latest Tear Film and Ocular Surface DEWS (Dry Eye Workshop) report has provided us with up-to-date relevant information regarding the current evidence based consensus when it comes to dry eye disease (DED) and its classification, diagnosis and management. The first two articles in this series concentrated on the classification and clinical work-up we as practitioners should aim to follow in practice. This, the final article of the series, reviews the best evidence based management of patients diagnosed with DED. A stepwise strategic approach is considered the best tactic with staged management at its core according to disease severity. Being able to differentiate between aqueous deficient and evaporative disease is crucial if we are to manage the conditions effectively.
THE ULTIMATE AIM
Management is ultimately aimed at restoring the homeostasis of the ocular surface by breaking the vicious cycle of the disease and offering long-term options to prevent a return to the vicious cycle and a resurgence of symptoms.
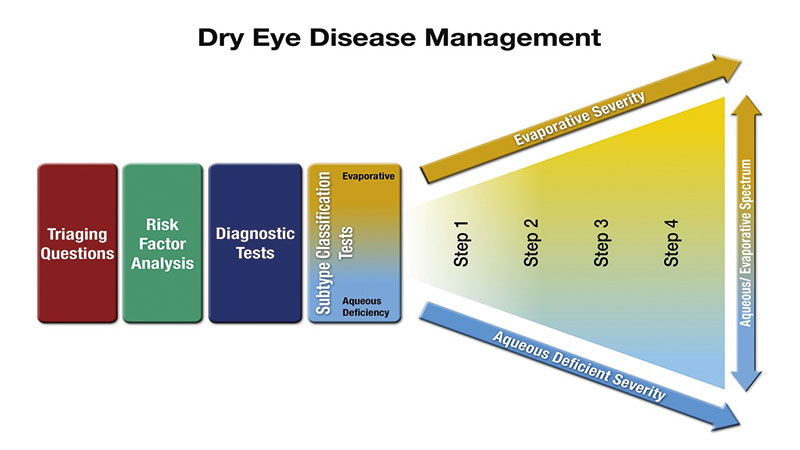
Figure 1: The DEWS II approach to managing dry eye disease (DED). Talking to the patient about risk factors and history and the diagnostic work up was covered in article 2 in this series. Once the working differential diagnosis and disease severity has been reached, the treatment plan can be developed
Which is which?
It is now generally believed that most people with symptoms relating to ocular surface disease (OSD) suffer from variable combinations of both abnormal meibomian gland function (evaporative eye disease – EDE) and tear underproduction (aqueous deficient eye disease – ADDE) rather than them being entirely separate conditions. Patients with DED have been shown to be over three times more likely to have evaporative dry eye than aqueous deficient dry eye and 30% of patients had both types.
Figure 2: Application of non-preserved lubricant
Staged management and treatment recommendations for dry eye disease
The basis of effective management begins with accurate diagnosis. It is important to categorise an individuals dry eye disease in terms of the degree to which EDE, ADDE and any other ocular surface conditions are contributing to the overall picture for that patient. The concept of determining the major causitive factor is key to appropriate and effective management. While there are treatments which are indicated for multiple aspects of DED, others are specific to one particular aspect of an individual’s ocular surface condition.
The steps outlined below show the most evidence-based staged management of DED depending on severity. The exact nature of an individual’s DED will dictate the range and number of management options selected from one or more steps. Options within a category are not ranked according to importance and may be equally valid.
Step 1:
- Education regarding the condition, its management, treatment and prognosis
- Modification of local environment
- Education regarding potential dietary modifications (including oral essential fatty acid supplementation)
- Identification and potential modification/elimination of offending systemic and topical medications
- Ocular lubricants of various types (if meibomian gland dysfunction is present, then consider lipid containing supplements)
- Lid hygiene and warm compresses of various types
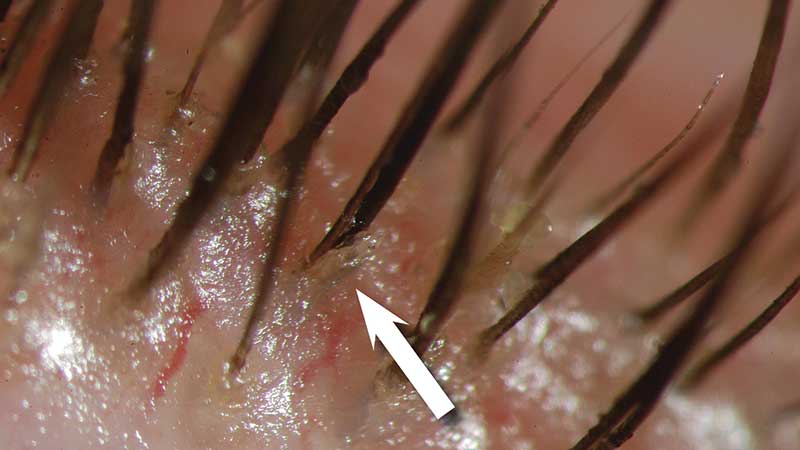
Figure 3: Evidence of Demodex (courtesy of Professor E Bitton)
Step 2:
If above options are inadequate consider:
- Non-preserved ocular lubricants to minimise preservative-induced toxicity (figure 2)
- Tea tree oil treatment for Demodex (if present – figure 3)
- Tear conservation
- Punctal occlusion (figure 4)
- Moisture chamber spectacles/goggles
- Overnight treatments (such as ointment or moisture chamber devices)
- In-office, physical heating and expression of the meibomian glands (including device-assisted therapies)
- In-office intense pulsed light therapy for MGD
- Prescription drugs to manage DED
- Topical antibiotic or antibiotic/steroid combination applied to the lid margins for anterior blepharitis (if present)
- Topical corticosteroid (limited duration)
- Topical secretagogues
- Topical non-glucocorticoid immunomodulatory drugs (such as cyclosporine)
- Topical LFA-1 antagonist drugs (such as lifitegrast)
- Oral macrolide or tetracycline antibiotics
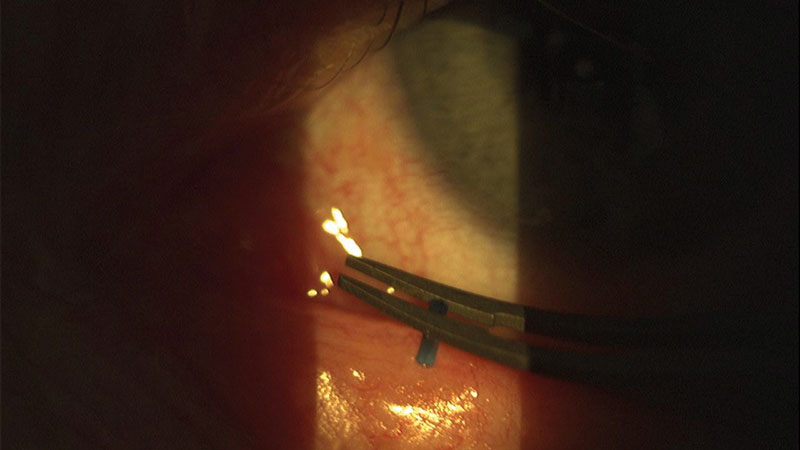
Figure 4: Punctal plug application
Step 3:
If above options are inadequate consider:
- Oral secretagogues
- Autologous/allogeneic serum eye drops
- Therapeutic contact lens options
- Soft bandage lenses
- Rigid scleral lenses
Step 4:
If above options are inadequate consider:
- Topical corticosteroid for longer duration
- Amniotic membrane grafts
- Surgical punctal occlusion
- Other surgical approaches (eg tarsorrhaphy, salivary gland transplantation)
It is now more understood that symptoms and signs do not always correlate, and this needs to also be considered when determining the severity of the condition. It is important to bear in mind that in cases where there are chronic symptoms but no signs that neuropathic pain rather than DED should be considered. Conversely, in situations where the individual has few symptoms but clear signs, reduced corneal sensitivity may be a factor and treatment is important to avoid ongoing damage.
POTENTIAL STEP TREATMENTS: A BRIEF SUMMARY OF THE MAIN IN-PRACTICE OPTIONS
Ocular lubricants
Lubricants remain the mainstay of early treatment for DED. There are numerous options available, but they do not specifically target the underlying pathophysiology of DED. Formulations often vary enormously in composition, osmolarity, viscosity and pH can have similar major components:
- Viscosity enhancing agents have a range of mechanisms including increasing tear film thickness, protecting the ocular surface, promoting tear retention and improving goblet cell density. Higher viscosity drops can increase retention time but also cause transient visual disturbance. Examples are carbomer and hyaluronic acid.
- Osmotic agents that are hypotonic may have an impact on improving mucus-producing goblet cell density.
- Osmoprotectants are a group of solutes that protect cells under osmotic stress by balancing the osmotic pressure without disturbing the cell metabolism. An example is trehalose (found in Thealoz).
- Antioxidants are considered potentially beneficial due to the presence of oxygen free radicals in the tears of patients with DED. Examples are vitamin A (in Hycosan Night).
- Preservatives are increasingly being removed from formulations, due to well founded evidence that chronic exposure to preservatives such as benzalkonium chloride result in toxicity and adverse changes to the ocular surface. Formulations are now more commonly being developed to be either preservative-free or using newer variants of preservatives designed to have a lower impact on the ocular surface.
- Inactive agents such as buffers to maintain pH and keep the drop comfortable on instillation or electrolytes to reflect the tear profile and increase goblet cell density (for example TheraTears).
- Lipid supplements incorporate oils in the formulation as an emulsion to help restore the lipid layer. Different types of emulsion and lipids have been explored to try to mimic the natural meibum. Phospholipids are thought to help thicken and stabilise the lipid layer and are found in formulations (such as Systane Balance and the liposomal sprays available).
Biological tear substitutes
This category describes serums derived from the human body and is typically reserved for management of more severe cases such as recurrent corneal erosions, chemical burns or Stevens-Johnson syndrome (figure 5).
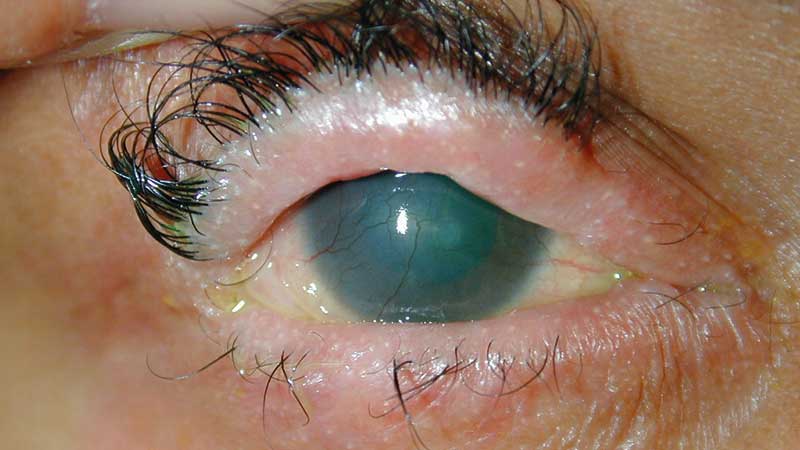
Figure 5: Stevens-Johnson syndrome
Autologous serum is the fluid component of a patient’s own blood that remains after clotting and has the advantage of being biochemically similar to tears. Studies show that serum can enhance corneal epithelial wound healing. It has also been found to inhibit the release of inflammatory cytokines and to increase the number of goblet cells and mucin expression in the conjunctiva. Production of the serum is usually done at blood banks and is a costly process. It is also difficult to store, and is only stable at minus 20°C for up to nine months. Allogenic serum and umbilical cord serum is derived from donors rather than the patient’s own blood.
Essential fatty acid (EFA) supplementation
Two key EFAs are omega 3 and 6. Omega 3 exist as short chain (alpha-linolenic acid) ALA and long-chain eicosapentaenoic acid EPA and docosahexaenoic acid DHA. ALA is found in foods like flaxseed and walnuts and EPA and DHA is present in oily fish. Short chains can be converted into long chains by the body.
Omega 6 is commonly derived from vegetable oils in the form of linolenic acid LA. In the body, omega 3 and 6 compete with each other for the enzymes that regulate their metabolism and modulate inflammation. Most omega 6 derivatives are pro-inflammatory, but some are anti-inflammatory. Omega 3 EFA derivatives are anti-inflammatory, and essential for limiting and resolving inflammation. The relative ratio of 3 to 6 influences the overall inflammatory status of the body. Supplementing to improve the ratio can yield systemic anti-inflammatory benefits that can help inflammatory conditions like DED. A number of studies exist to support the use of EFA supplementation in DED, although exact dosage and protocol is still yet to be established.
Lid hygiene
The value of lid hygiene is widely accepted, but evidence is limited in terms of the correct exact strategy and optimal combinations of treatment. If used appropriately it can reduce lipid by-products and lipolytic bacteria associated with these conditions. A recent level one study demonstrated the efficacy of lid scrubs for removal of crusts in blepharitis with both commercial lid cleanser and baby shampoo. However, the dedicated cleanser showed reduced surface inflammatory markers, improved lipid layer quality and was better tolerated than the baby shampoo. Baby shampoo was also found to potentially have an adverse effect on goblet cell function by reducing ocular surface mucin levels. Compliance with lid hygiene instruction is notoriously poor with a study showing only 55% of patients compliant after six weeks of use.
Demodex infestation and tea tree treatment
Demodex infestation is a causative factor in many cases of blepharitis and is associated with dry eye symptoms, but there is currently no evidence to link it directly with MGD. Appropriate management is with the use of topical products containing tea tree oil, which is toxic to Demodex, and has been shown to be more effective than baby shampoo at eradicating ocular Demodex. It can be toxic to the eye and causes stinging and irritation so needs to be used with caution. There is emerging evidence for the role of oral ivermectin, an anti-parasitic drug which is low cost and a single dose has been shown to reduce the number of Demodex on patients with blepharitis.
Punctal occlusion
Punctal occlusion mostly takes the form of plugs, which can be either absorbable (to test and determine the efficacy of occlusion) or non-absorbable. They are either at the level of the punctum or deeper within the canaliculus. Surgical occlusion can also be achieved, usually with cautery. Occlusion is a widely used tear conservation approach, and the rationale for ADDE can be understood, however, its use in EDE remains controversial and the results are equivocal as to the effectiveness in improving meibomian gland status or lipid layer.
Also, given the acceptance of the importance of inflammation to the continued cycle of DED, there is limited research on the potential for such intervention to impact on ocular surface inflammation by retaining inflammatory mediators on the ocular surface that needs to be considered. Although punctal plugs provide symptomatic improvement, few studies demonstrate a benefit of plugs over a comparison intervention, and no current level one large scale studies exist to support the contention that punctal occlusion of any form is effective in the management of DED.
MGD management
Warm compresses are proven to be effective, but compliance is often poor due to the time required and difficulty maintaining the temperature of the compress. The current suggestion is that heating the individual gland to at least 40°C with optimal contact between the compress and the eyelid is to be recommended. Some studies conclude that a wet surface is best to transmit heat through to the lid, but others find that this tends to allow for more rapid cooling after heating due to evaporation from the wet lid surface. Commercial devices like Blephasteam goggles and MGDRx Eyebag have been shown to be more effective than warm towel treatment.
Expression can be used both diagnostically to assess the glands and therapeutically to help remove obstructed material. Forceful expression to help remove material is limited by the pain the patient can tolerate. A study over a six-month period involving four in-office expressions with at-home warm compressing showed the number of expressible glands, quality of secretion and lipid layer thickness significantly improved and all patients reported improved comfort and decreased symptoms. Intraductal probing can also be used to open the obstructed orifice.
Debridement scaling
One of the primary drivers of MGD is hyperkeratinisation of the lid margin and duct orifices. As keratinised material builds up around and within the orifice the gland is obstructed and meibum cannot be delivered from the gland. Debridement of the lid edge along the mucocutaneous junction is believed to mechanically removing accumulated debris and keratinised cells from the lid margin, thus allowing increased flow of meibum.
In office devices: Lipiflow and IPL
Lipiflow is a heating and massaging device applied to the eyelid in-office. An intense pulsed light (IPL) device uses light to treat the area around MG to promote activity in the gland (figure 6). These have both been shown to offer potential relief when used in conjunction with at home warm compressing management.
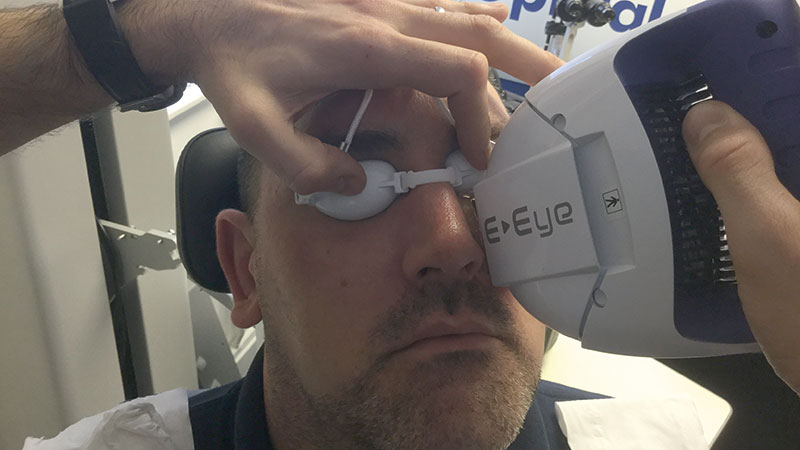
Figure 6: Use of an intense pulsed light device for MGD management
Prescription drugs
The role of systemic antibiotics is still poorly understood and optimal dosing remains uncertain. Short-term doses of topical antibiotics such as ofloxacin based ointment have been shown to help in patients with obstructive MGD. Azithromycin is believed to have anti-inflammatory action rather than simply reducing the bacterial lid flora.
Tear stimulation approaches: topical and oral secretagogues
These agents trigger tear stimulation, and can be categorised as agents that stimulate aqueous, mucin and/or lipids. They are not currently available in the UK.
Therapeutic contact lenses
Despite their association with dryness, contact lenses have a potential role in the management of DED. Their therapeutic role is thought to stem from the mechanical protection and reduction in corneal desiccation. CLs for DED are often used on an extended wear basis and the risks of microbial keratitis must be considered. Both soft and RGP lenses are useful; soft have a ‘bandage’ effect and scleral RGP lenses create a tear reservoir.
Anti-inflammatory therapy
Inflammation has a definite role in the vicious circle of dry eye, so it makes sense that anti-inflammatory treatment would have a role in its management. Studies have shown the clinical value of short term use of topical corticosteroid preparations, but their long-term use is not without risk of complications. These include ocular hypertension, cataract and opportunistic infection.
Cyclosporine is an immunomodulating medication with anti-inflammatory properties, and is used typically as anti-rejection treatment in organ transplant patients, and in auto-immune diseases. Its use for dry eye has been shown to reduce inflammation and improve elevated osmolarity, and lead to recovery of goblet cell density.
Tetracycline analogues
These are broad-spectrum antibiotics that inhibit protein synethesis. It is thought that they may act to decrease bacteria-producing lipolytic exoenzymes and inhibit lipase production, both of which result in meibomian lipid breakdown, thus improving clinical parameters in MGD and anterior blepharitis. These agents also have anti-inflammatory properties by decreasing the production or activity of various inflammatory mediators. Studies show that they seem to be most effective when blepharitis and rosacea are present. There are currently no robust studies that demonstrate the efficacy of these antibiotics over lid hygiene or other treatments.
Azithromycin
Reports exist to highlight the positive impact of systemic azithromycin macrolide to treat MGD. A short five-day course appears to actually stimulate function of meibomian glands. Its anti-inflammatory properties may also help to control bacterial flora and lid inflammation. Topical azithromycin has also been shown to be potentially effective in MGD management.
Environmental factors
Thought should be given to modifying environmental elements that could have a role in the DED. Influencing factors include chronic topical preserved medications (such as glaucoma management), systemic medications, blink rates, air conditioning and pollutants, contact lens wear.
The enormous range of evidence-based management options currently available means we are in the best position we have ever been to effectively manage DED in the primary care setting. The logical stepwise approach allows us to work through a devised management strategy with a long term plan of action as and when we need to move up to the next level of treatment options.
It all starts with an accurate diagnosis, which really is the key driver to developing the best treatment for an individual. TFOS DEWS II has provided us with a wonderful recipe book of treats to call upon to help each and every one of our dry eye patients. There really is no excuse for not treating each and every dry eye we see any more.
Sarah Farrant is a therapeutic optometrist with a specialist interest in dry eye disease practising in Somerset, UK. She is the UK ambassador for the Tear Film and Ocular Surface Society TFOS.
The relevant section of TFOS DEWSII can be downloaded from http://dx.doi.org/10.1016/j.jtos.2017.05.001 or accessed with the DEWSII app.
