Assessing the anterior chamber (AC) is an essential part of evaluating the risk of glaucoma.1,7 Glaucoma is an eye disease characterised by a progressive optic neuropathy and associated visual field loss.4 In the UK it is the second leading cause of vision loss after AMD. Early detection is more likely to lead to a better prognosis 14 yet it is thought that 50% living with the disease are yet to be diagnosed.2
Glaucoma may be classified according to the appearance of the aqueous drainage pathway at the trabecular meshwork (figure 1).
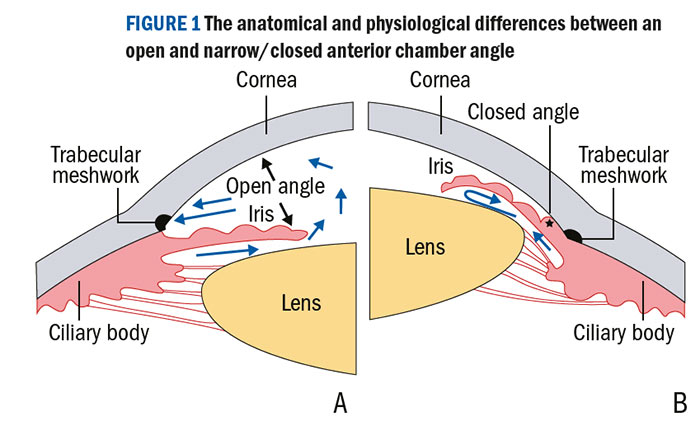
Figure 1: The anatomical and physiological differences between an open and narrow/closed anterior chamber angle
In open-angle glaucoma, despite a normal clinical appearance, aqueous outflow may be restricted. Contrastingly, in angle closure glaucoma, access to the trabecular meshwork is physically obstructed by mechanisms that either push the iris forward from behind or pull it forward.16 This is thought to account for around 10% of all glaucomas 19 but almost 50% of all glaucoma-related blind registrations, particularly in East Asia.13
The main risk factors for angle closure are:7
- Gender (female to male ratio 3:1)
- Ethnicity (greater prevalence among, for example, Chinese, Vietnamese, Inuit)
- Family history
- Smaller eyes (short axial length, hypermetropia, small corneal diameter)
- Shallow central AC depth
- Increasing age (AC becomes shallower as lens thickness increases)
- Small corneal diameter
This article will focus on the anterior chamber angle, primarily in the context of anterior segment OCT (AS-OCT) examination and provide some referral guidance.
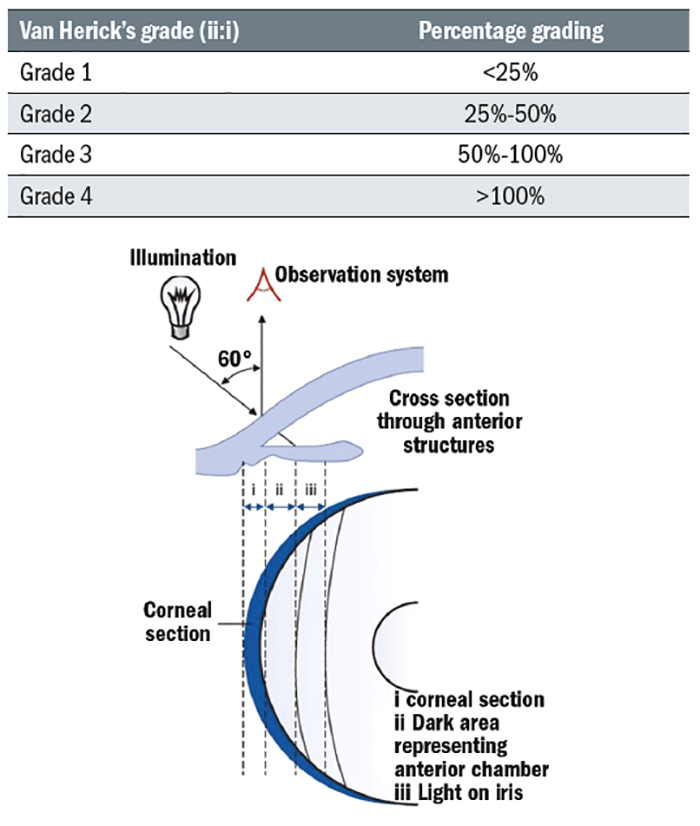
Assessing the central and peripheral anterior chamber depth
Gonioscopy allows a 360° colour view and is the gold standard for AC angle assessment.16,20 It is taught to those undertaking IP and higher glaucoma qualifications and is now being used more frequently in primary eye care and optometry-led community ophthalmology clinics. Gonioscopy is the only technique which allows direct en face visualisation of the trabecular meshwork. Disposable gonioscopy lenses are now available which offer greater convenience and sterility assurance without compromising image quality.26
The Van Herick technique has shown high sensitivity and specificity for the identification of narrow angles when compared to gonioscopy.21 It is a quick and simple test which should be done on every patient who can reach the slit-lamp and undertaken as follows:
- The illumination is set at 60° to the eyepiece.
- Use 16x magnification with a bright narrow beam focused at the limbus.
- The limbal anterior chamber depth (LACD) measurement is taken as soon as an optical section of the cornea is visualised.
- The nasal angle should also be measured (avoid touching the patient’s nose with the illumination system or rotate the coupled observation and illumination system temporally, slightly) although studies have shown that the superior angle is the most likely to close.3
The LACD grading system proposed by Van Herick 10 ranged from zero (closed) to four (open) and has more recently been further divided into a decimal (or percentage equivalent) system for more precise and linear grading.11,17 The systems are summarised in table 1.
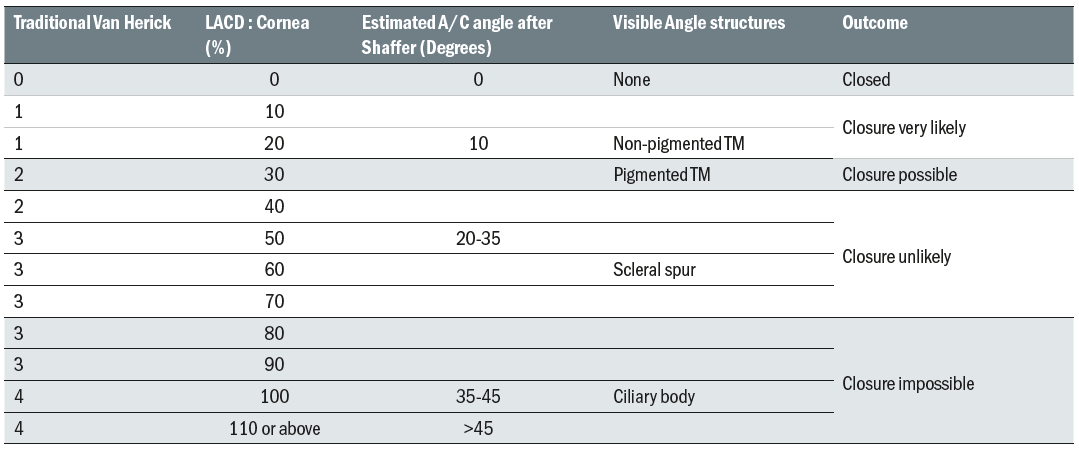
Table 1: Traditional Van Herick system for classifying LACD subdivided using the more sensitive percentage system. The approximate angle in degrees and angle structures visible on gonioscopy are shown along with possible outcomes
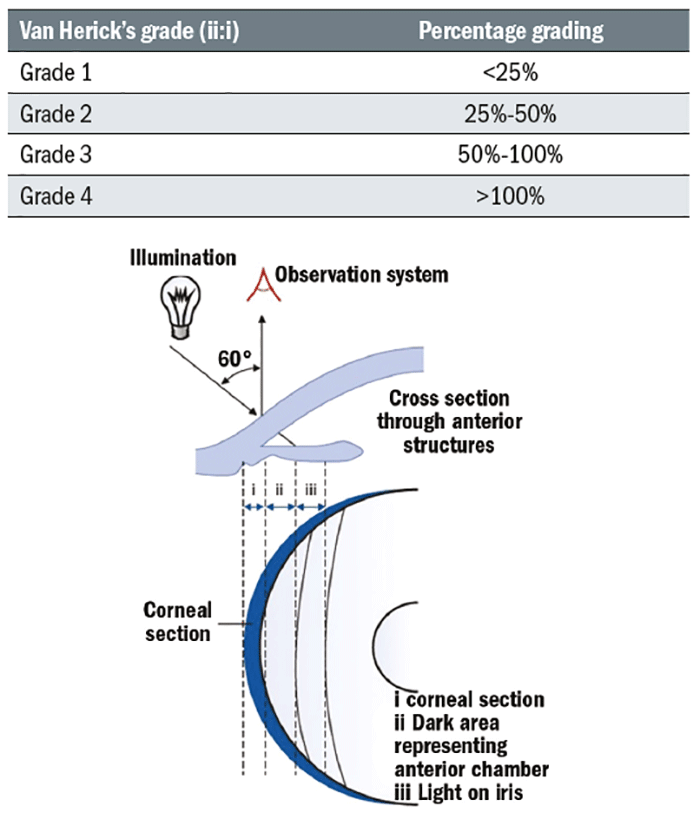
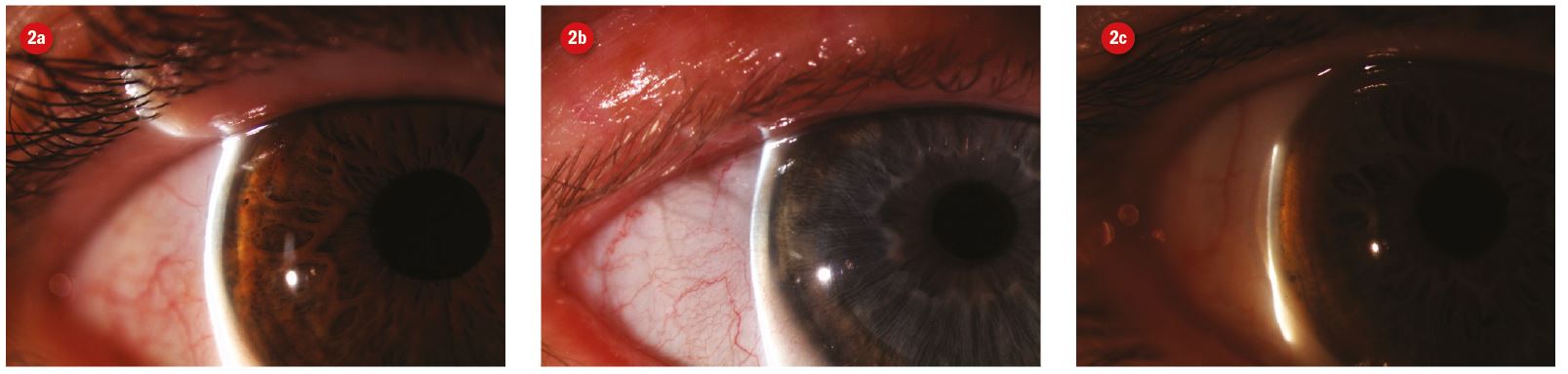
Figure 2: Slit lamp image and anterior segment cross section illustrating van Herisk technique based on the ratio of corneal thickness (i) and cornea to iris gap (ii). 2a shows LACD of grade 4 (1.2), 2b of grade 4 (0.7) and 2c narrower angle of grade 2 (0.3)
Central anterior chamber depth (CACD) is the perpendicular distance in mm from the corneal endothelium to the anterior surface of the lens measured at the corneal apex.18 The Smith technique12,14,17 is the commonest way to measure CACD without digital technology. A CACD of less than 2mm is considered to be at risk of occlusion.22
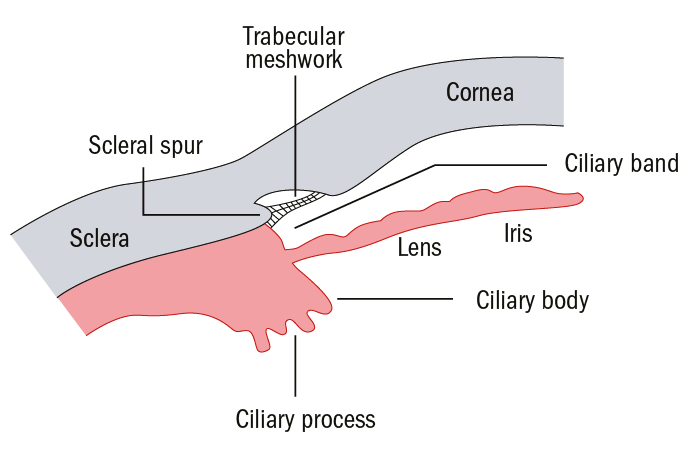
Figure 3: Schematic representation of an open anterior chamber angle showing the scleral spur and trabecular meshwork
Anterior segment optical coherence tomography (AS-OCT) is one of the most current methods for assessing LACD and CACD. It uses low-coherence interferometry to produce cross sectional images of the anterior segment. This offers practitioners a quick, easy 8 and non-invasive way to visualise the central and peripheral A/C qualitatively as well as providing quantitative data. Image capture requires an additional or integral capture lens for the anterior segment.
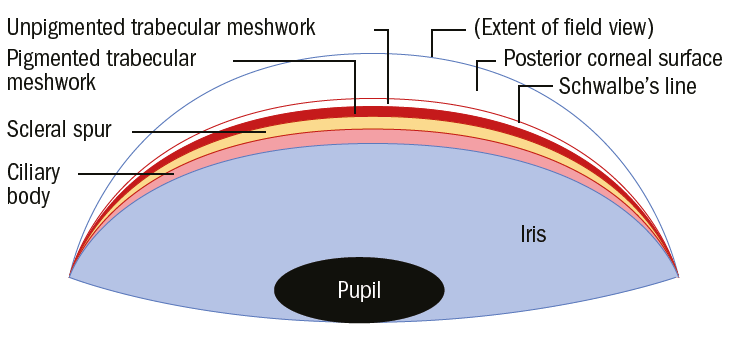
Figure 4: Schematic gonioscopic view of a wide open A/C angle – note that even the ciliary body (ciliary band) is visible
Unlike gonioscopy, no external pressure is applied to the eye (which can influence angle configuration) and with less incipient light there is less induced miosis thereby providing a truer and more representative appraisal of the angle.6 Furthermore, it is more objective than gonioscopy, forms a permanent record for comparison, may be delegated to trained staff and may identify eyes at risk of angle closure earlier than gonioscopy.23
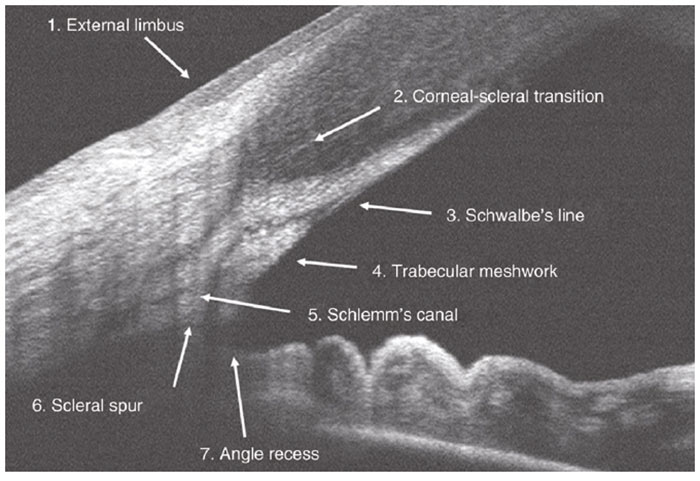
Figure 5: An AS-OCT scan of a wide open A/C angle showing key anatomical landmarks - the cornea, scleral spur, trabecular meshwork and iris. After Fernández-Vigo et al 24
Accurate interpretation of AS-OCT images requires the correct recognition of anatomical landmarks, particularly the scleral spur.8 This represents the junction between the inner wall of the trabecular meshwork and the sclera; it can be identified as the widest part of the sclera, seen as an inward protrusion at the inner surface;8 in some cases this can be difficult to locate.
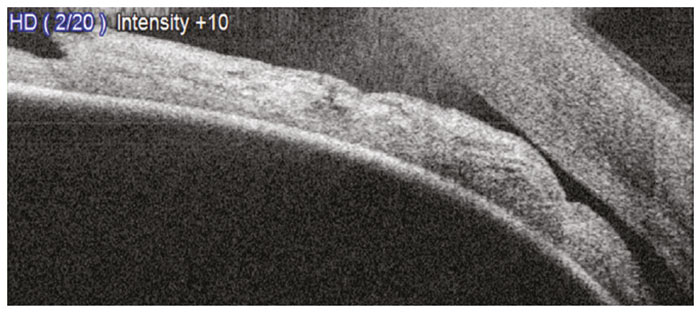
Figure 6: An AS-OCT scan showing an occludable angle (VH1). Note the iridocorneal touch anterior to the scleral spur despite the angle behind this area being open (courtesy of Birmingham Optical)
Iridocorneal contact anterior to the scleral spur is a sufficient indication of an occludable angle18 (figure 6). This should be differentiated from a closed angle (figure 7).
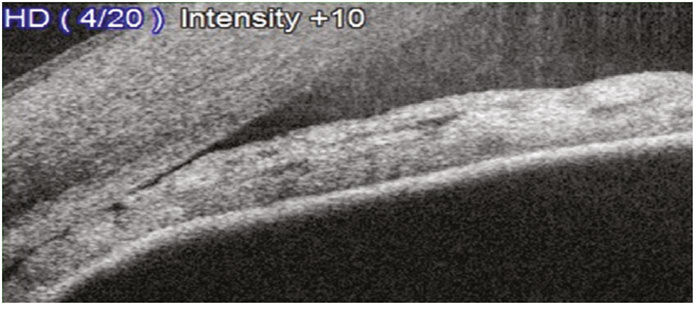
Figure 7: An AS-OCT scan showing a closed A/C angle (VH0) (courtesy of Birmingham Optical)
In cases of uncertainty, digital calipers may be used to measure the angle (in degrees), the angle opening distance (AOD) and the CACD. The operator is required to highlight certain landmarks on the acquired images for processing. Such data allows for more precise grading, risk assessment and safe monitoring.
The length of the trabecular meshwork from the scleral spur is approximately 500um. The angle opening distance is determined by a perpendicular line from the corneal endothelium to the anterior iris surface at a point 500-750um from the scleral spur; this is described in detail by Dabasia et al.18 An AOD of less than 190um is occludable.15
To measure the AC angle in degrees, the triangle is completed from the AOD lines. An angle of 160 or less is occludable.15
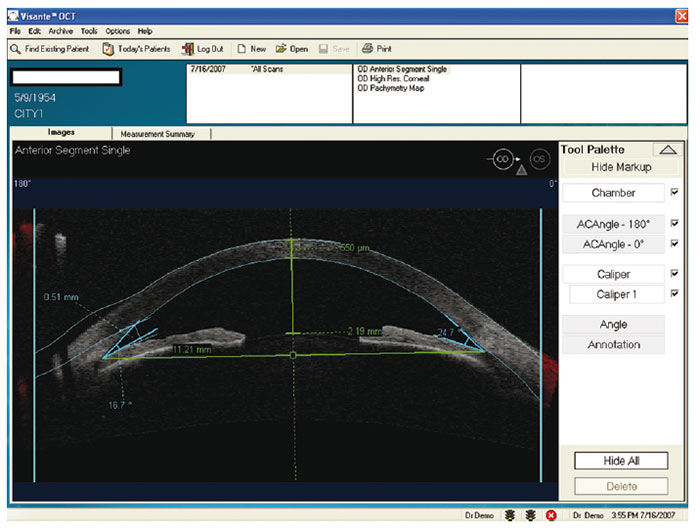
Figure 8: An AS-OCT scan showing the CACD measurement using digital calipers. CACD is measured between the corneal endothelium and a line joining the two opposite iris recesses. This example was taken using the Zeiss Visante (courtesy of W Harvey)
Figure 9 shows the process for using the digital calipers. The scleral spur is identified, a 750um line is drawn on the posterior cornea and a perpendicular line gives a distance of 153um to the iris (AOD) which is occludable. The completed triangle gives an angle of 10.3° (occludable). Based on table 1, we would expect this to correspond to a V/H grade 1 (20%). More importantly, we can judge that the outcome is an increased risk of angle closure, particularly if the temporal angle looks the same.

Figure 9: An occludable angle with a shallow AOD and A/C angle (courtesy of Birmingham Optical)
While AS-OCT is appropriate to delegate, there is value in the practitioner assessing the anterior chamber in real time and not relying solely on the static image.
AS-OCT has several limitations. Since the image is cross sectional, it is not possible to obtain an en face view of the trabecular meshwork. Also, most machines only allow a horizontal cross section therefore only the nasal and temporal angles are viewed (and a single cross-section risks missing angle features). Imaging of the superior angle quadrant is difficult and requires manipulation of the upper eyelid.8 Since OCT cannot penetrate the sclera or iris, the mechanism causing the angle closure cannot be shown (this would require Scheimpflug imaging) and peripheral anterior synechiae (PAS) cannot be detected.
Managing narrow A/C angles
Early detection of eyes at risk of angle closure allows prophylactic therapy to improve aqueous drainage and prevent PAS formation thereby halting progression of the angle closure process. Treatment options include laser peripheral iridotomy (LPI), peripheral iridoplasty and lens extraction.16
Acute angle closure occurs in approximately one in 50,000 patients in a Scottish population;9 20% of cases were induced by mydriatic drops in susceptible patients. There are various clinical signs such as pain, nausea, vomiting, history of intermittent blurred vision, raised (usually very high) IOP, conjunctival injection, corneal oedema and a mid-dilated non-reactive pupil. It is an acute event requiring emergency referral.7
For all other narrow angles, your first instinct may be to refer, but at what central/peripheral depth should you refer, to whom and how quickly? An unnecessary referral can cause needless anxiety for the patient as well as squandering NHS resources.
Referrers should consider:
- Is the limbal A/C depth so shallow that an Acute Angle Closure attack is likely to occur?
- Is the limbal A/C depth shallow enough/configured such that aqueous outflow might be chronically impaired resulting in, either now or in the future, damage to the optic nerve and subsequent field loss?
- Will dilating this patient add value or risk to the examination?
The first step is to establish a working diagnosis.16 It is important to consider the patient holistically including risk factors (described in the beginning of the article). Gonioscopy is required for a definitive differential diagnosis as follows:
- Primary angle closure suspect (PACS) – contact between the peripheral iris and the posterior trabecular meshwork is possible (ie shallow limbal A/C depth). IOP, discs and visual fields are normal.
- Primary Angle Closure (PAC) – obstruction of the trabecular meshwork by the peripheral iris has occurred. Disc and fields are normal. There is either raised IOP (appositional PAC) and/or peripheral anterior synechiae (synaechial PAC).
- Primary Angle Closure Glaucoma (PACG) - PAC with damage to the optic nerve and visual field change. Damage occurs through episodes of severe IOP elevation (so IOP might be normal or raised on presentation) or long-term elevated IOP.
A Van Herick grade of two or less (25%) compares very well with gonioscopy for highlighting patients at risk of angle closure,16 with College and SIGN guidance stating that this is the recommended referral threshold.7,4
Using the revised method of grading shown in table 1, a LACD of 20% should be referred whereas 30% could be monitored in practice if the patient has normal discs, fields and IOP. Inter and intra-observer variability is common6 so it is quite acceptable to ask a more experienced colleague for their opinion to help minimise false positives.
When monitoring shallow A/Cs, the following should be discussed with the patient and recorded in their notes:
- Overview of the condition being monitored, with patient consent.
- Issue booklet on angle closure (eg IGA).
- Signs and symptoms indicative of acute angle closure with advice to go to A&E.
- Drugs which can cause mydriasis (including medications eg anticholinergics and adrenergics).16
- Recall date.
Narrow angles in symptomatic patients require ‘urgent’ (typically two to four weeks) HES review even with normal discs, fields and IOP. Half of patients with an acute angle closure attack give a history of previous attacks therefore even intermittent symptoms must be taken seriously. Acute angle closure is an emergency (same day), as is IOP over 44 mmHg.
Narrow angles in asymptomatic patients (5% VH1) can be seen ‘soon’ (within six to eight weeks) although this might be escalated to ‘urgent’ if the IOP is raised (in the absence of other signs of acute angle closure); even with open angles, an IOP over 29 is usually considered ‘urgent’. Most other cases are ‘routine’.
Suspected PACG (ie glaucomatous optic neuropathy and/or field loss) should be referred since there is active disease which will require treatment. Urgency will be based on symptoms & IOP (as described above) and how advanced the disease is.
NB There may be local variations so check with your glaucoma consultant and always use your clinical judgment.
Tips for writing a referral for a patient with narrow A/C angles
When referring it is important to include sufficient information so the patient is seen in the most appropriate environment and in the most appropriate timescale.
Where community ophthalmology clinics exist (or experienced and well equipped clinical optometrists), many borderline narrow angles may be triaged there initially leaving only the high risk cases which are likely to require treatment for the HES.
The following information should be included:
- Refraction and VA
- F/H glaucoma and other risk factors
- Symptoms (especially indicative of previous acute attacks)
- Discs (C:D ratio, size, depth, notches, haemorrhages)
- IOP (time, equipment used, GAT/PAT)
- Fields (attach plus brief description, repeated)
- A/C (V/H grading including % and central depth)
- OCT – AS, disc and macula (attached)
- Proposed urgency
Case studies from a community ophthalmology clinic
The majority of narrow angle referrals seen in community ophthalmology are asymptomatic with normal IOP/discs/fields and being able to use an AS-OCT is incredibly useful.
Consider the two separate referrals below; both asymptomatic and referred in as V/H grade 0-1 with concern expressed about the risk of angle closure.
It was agreed that the first patient (figure 10) had narrow angles on Van Herick (grade 1) and this correlates with the AS-OCT where there is very nearly iridocorneal touch. The nasal angle was just as narrow, and the fellow eye was only slightly more open. This patient was referred ‘soon’ to the HES with information provided on laser iridotomies.
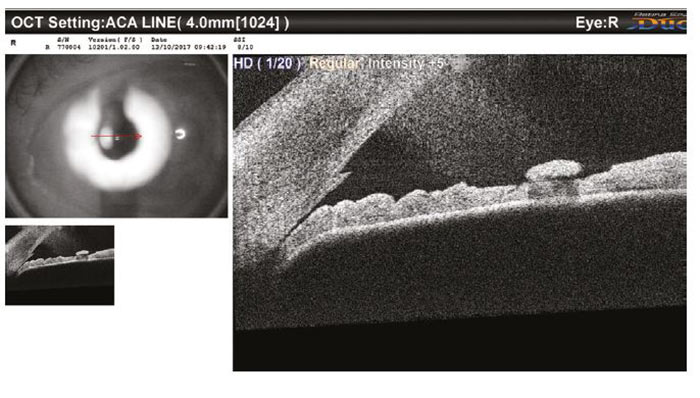
Figure 10: An AS-OCT image of a shallow A/C angle seen in community
Contrastingly, the second case (figure 11) was judged to have more open (VH 3) angles and this once again correlated with the AS-OCT, with the nasal angle and the other eye also being just as open. The patient was reassured and discharged.
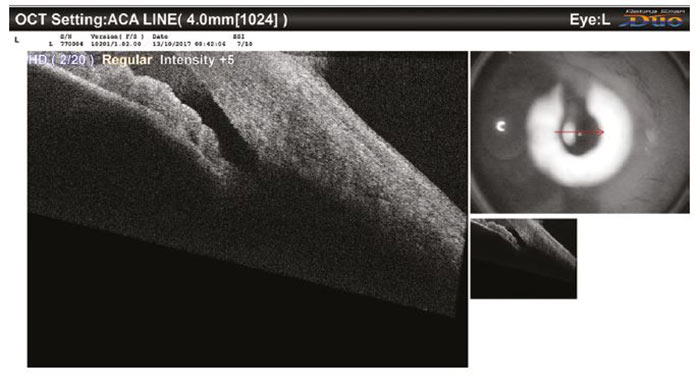
Figure 11: An AS-OCT image of an open A/C angle seen in community
It is interesting to note that there are occasions when the LACD looks shallow (eg V/H 1-2), but the AS-OCT scan is sufficiently open, which highlights the fact that Van Herick does not allow direct visualisation of the AC/A.
Conclusion
OCT is becoming increasingly common in primary eye care, including rollouts by small and national chains.
AS-OCT is a non-invasive, quick and straightforward way of identifying patients at risk of angle closure (chronic glaucoma and acute event) and for informing referral decisions in patients where the Van Herick technique has identified a risk factor. It is most useful when combined with gonioscopy.
Anterior chamber angle assessment should be performed on all patients who can reach a slit lamp (particularly adults and those at risk) and the results should be included in all glaucoma referrals.
Through appropriate use and interpretation of AS-OCT, a greater proportion of patients may be safely monitored in practice (with ophthalmologist oversight where possible), and, where required, referrals are more likely to be made in the most appropriate timescale and with fewer false positives.
Peter McElduff is community optometrist, eCare Medical Eye Clinic Ipswich. Christian Dutton is clinical & professional services optometrist. Lyn Price is clinical lead (optometry), Evolutio Care Innovations Ltd. Mr Simon Hardman-Lea is lead consultant ophthalmologist, Evolutio Care Innovations Ltd.
eCare is the clinical division of Evolutio Care Innovations Limited. eCare was developed to allow patient access to consultant level eye care, delivered utilising the very latest telemedicine technology, from suitably equipped optometry practices at an accessible price.
Affiliate optometrists wishing to join the eCare network are working under the clinical governance of an eCare ophthalmologist and can therefore work within core skills. However, eCare affiliates are encouraged to have or attain through the eCare network support, additional training such as glaucoma management and Independent Prescribing.
If you are interested in becoming an eCare affiliate then you can register your interest by emailing affiliate@myecare.co.uk or calling 0203 780 7860 and talking to an evolutio relationship manager.
Acknowledgments
Thanks to Jason Higginbotham and Birmingham Optical for their assistance. We use the Nidek RS 330 OCT Retina Scan Duo in our clinics.
References
- The College of Optometrists (2016) ‘Clinical management guidelines Ω Primary Open Angle Glaucoma’, [Online] Available at: https://www.college-optometrists.org/guidance/clinical-management-guidelines/glaucoma-primary-open-angle-poag-.html (Accessed 23/11/17).
- O’Connell R, and Nguyen D. (2015) ‘Glaucoma – Part 1 definitions and epidemiology’, Optician, 24/4/15, pp. 16-22.
- Foster P, He M and Liebmann J. (2006) ‘Epidemiology, Classification and Mechanism’. In: Weinreb, RN Friedman, DS (eds) Angle Closure and Angle Closure Glaucoma: Reports and Consensus Statements of the 3rd Global AIGS Consensus Meeting on Angle Closure Glaucoma, Kugler Publications, pp. 1–20.
- SIGN (2015) ‘Glaucoma referral and safe discharge’ [Online] Available at: http://www.sign.ac.uk/assets/sign144.pdf (Accessed 23/11/17).
- Terry L. (2017) ‘OCT: Past, present and future’, Optometry Today, 11/09/17, [Online] Available at: https://www.aop.org.uk/ot/CET/2017/09/11/oct-past-present-and-future/article (Accessed 23/11/17).
- Hiscox R. (2016) ‘Clinical applications of optical coherence tomography: what should I know?’, Optometry in Practice, 17(2), pp. 59-70 [Online] Available at: https://www.college-optometrists.org/oip-resource/clinical-applications-of-optical-coherence-tomography--what-should-i-know.html (Accessed 23/11/17).
- The College of Optometrists (2016) ‘Clinical management guidelines – Primary Angle Closure Glaucoma’, [Online] Available at: https://www.college-optometrists.org/guidance/clinical-management-guidelines/glaucoma-primary-angle-closure-pacg-.html (Accessed 23/11/17).
- Campbell P. (2016) ‘Assessing the anterior chamber angle’, Optometry Today, 14/03/16 [Online] Available at: https://www.aop.org.uk/ot/CET/2016/03/14/assessing-the-anterior-chamber-angle/article (Accessed 23/11/17).
- Chua P, Day A, Lai K, Hall N, Tan L, Khan K, Lim L, Foot B, Foster P, Azuara-Blanco A. (2017) ‘The incidence of acute angle closure in Scotland: a prospective surveillance study’ British Journal of Ophthalmology [Online] Available at: http://bjo.bmj.com/content/early/2017/08/09/bjophthalmol-2017-310725 (Accessed 23/11/17).
- Van Herick W, Shaffer R and Schwartz A. (1969) ‘Estimation of width of angle of anterior chamber. Incidence and significance of the narrow angle’, American Journal of Ophthalmology, 68, pp. 62-69.
- Cockburn D. (1982) ‘Slit lamp estimate of anterior chamber depth as a predictor of the gonioscopic visibility of angle structures’, American Journal of Optometry and Physiological Optics, 59, pp. 904-908.
- Smith R. (1979) ‘A new method of estimating the depth of the anterior chamber’, British Journal of Ophthalmology, 63, pp. 215-220.
- Quigley H and Broman A. (2006) ‘The number of people with glaucoma worldwide in 2010 and 2020’, British Journal of Ophthalmology, 90, pp. 262-267.
- Campbell M. (2016) ‘Assessing the anterior chamber in optometric practice ’, Optician [Online] Available at https://www.opticianonline.net/cet-archive/129 (Accessed 23/11/17).
- Hiscox R. (2014) ‘What you should know about OCT assessment Part 3 – Anterior eye’, Optician, 19/12/14, [Online] Available at: https://www.opticianonline.net/cet-archive/56 (Accessed 23/11/17).
- Dabasia P. (2013) ‘Methods of measurement of the anterior chamber angle Part 1: Angle closure glaucoma and gonioscopy‘, Optometry in Practice, 14(3), pp. 107 – 114.
- Dabasia, P. (2013) ‘Methods of measurement of the anterior chamber angle Part 2: Screening for angle closure and angle closure glaucoma’, Optometry in Practice, 14(4), pp. 147 – 154.
- Dabasia, P. (2014) ‘Methods of measurement of the anterior chamber angle Part 3: Screening for angle closure and angle closure glaucoma using advanced technologies’, Optometry in Practice, 15(1), pp. 11 – 18.
- Goyall S, Moutsou M, Arora A. (2014) ‘Is Primary Angle Closure (PAC) Glaucoma (G) Rare in the UK? Ω Prevalence Rates from a Glaucoma Clinic in the UK’, ARVO meeting abstracts, 419, p. 429.
- NICE Guidance (2017) ‘Glaucoma Diagnosis and management’, [Online] Available at: https://www.nice.org.uk/guidance/ng81/chapter/Recommendations#diagnosis (Accessed 23/11/17).
- Foster P, Devereux G, Alsbirk H, Lee P, Uranchimeg D, Machin D, Johnson G, Baasanhu J. (2000) ‘Detection of gonioscopically occludable angles and primary angle closure glaucoma by estimation of limbal chamber depth in Asians: modified grading scheme’, British Journal of Ophthalmology, 84, pp. 186–192.
- Wishart P, Batterbury M. (1992) ‘Ocular hypertension: correlation of anterior chamber angle width and risk of progression to glaucoma’, Eye, 6(3), pp. 248–256.
- Baskaran M, Iyer J, Narayanaswamy A, He Y, Sakata L, Wu R, Liu D, Nongpiur M, Friedman D, Aung T. (2015) ‘Anterior Segment Imaging Predicts Incident Gonioscopic Angle Closure’, Ophthalmology, 122(12), p.2380.
- Fernández-Vigo J, Fernández-Vigo C, Martínez de la Casa J, Sáenz-Francés F, Santos-Bueso E, García Feijóo J. (2016) Identificación de estructuras del ángulo iridocorneal mediante tomografía de coherencia óptica de dominio Fourier. Arch Soc Esp Oftalmol, 91, pp. 74–80.
- Lee DG, Choi SH. (2009)’Measurement of Anterior Segment Using Visante OCT in Koreans’ Journal of the Korean Ophthalmological Society, 50(4), pp. 542-550. [Online] Available at: https://synapse.koreamed.org/DOIx.php?id=10.3341/jkos.2009.50.4.542 (Accessed 27/11/17)
- Lee B, Szirth BC, Fechtner RD, Khouri AS. (2017) ‘Are Disposable and Standard Gonioscopy Lenses Comparable?’ Journal of Glaucoma, 26(4), pp.157-159.
