The tear film is composed of three integrated layers: a surface lipid layer, a thick muco-aqueous gel and base mucin layers that all play a role in protecting the ocular surface from damage.1-3 Disruption to any of these layers or their synergy can result in a poor quality tear film.4 Dry eye disease (DED) is a chronic condition impacting millions of people. The prevalence of DED is between 5% and 50%,5 a wide range as there is no gold standard for diagnosis of DED.6-12 DED has recently been redefined by the Dry Eye Workshop II (DEWS II) as ‘a multifactorial disease of the ocular surface characterised by a loss of homeostasis of the tear film, and accompanied by ocular symptoms, in which tear film instability and hyperosmolarity, ocular surface inflammation and damage, and neurosensory abnormalities play etiological roles’.4 DED is a complex condition for which a single etiology is not known. The complexity of both the disease and its diagnosis makes it difficult to establish a single test to diagnose DED.
Similar to the original DEWS report in 2007, the DEWS II report reaffirmed that tear film instability and increased tear osmolarity are key mechanisms in dry eye (DE), regardless of the underlying etiology.4,13 The inclusion of ‘homeostasis’ in the new definition emphasises that DED is not caused by any single factor but rather a fine balance of many different systems working in concert. Three homeostasis markers are highlighted in the 2017 DEWS II report: non-invasive tear break-up time, osmolarity and ocular surface staining.14 The diagnostic subcommittee of the 2017 DEWS II report recommends that, in the presence of symptoms, any one of these homeostasis markers be performed to establish a DED diagnosis.14 Given that tear film instability and hyperosmolarity are crucial factors within the disease, developing a standard procedure and criteria for DED diagnosis must involve these parameters.
Tear Osmolarity History
Osmolarity is a measure of the number of solute particles per litre of solution versus osmolality, which is the number of solute particles per kilogram. Tear osmolarity is a function of the balance between rate of tear production and tear loss from the eye.15,16 Tear volume is constantly decreasing as a result of evaporation and this needs to be balanced by adequate tear production in order to maintain isotonicity of the tears.16,17 In the setting of increased tear osmolarity, there is an increase in apoptosis rates of conjunctival, corneal and goblet cells which continues the cycle of tear film instability and ocular surface inflammation.18 Tear osmolarity is impacted by hydration levels, tear film lipid layer characteristics, palpebral aperture size, blink frequency, tear film stability and environmental factors, which makes increased tear osmolarity pertinent for both evaporative and aqueous-deficient types of DED.19 In the setting of evaporative DE, increased evaporation of the tear film increases tear osmolarity. In aqueous-deficient DE, decreased tear production increases tear osmolarity. As evaporative and aqueous-deficient DE are rarely mutually exclusive, the balance of tear production and evaporation can be inferred from the tear osmolarity, giving a broad overview of the equilibrium of the tear film.
Osmometers
There are several methods of measuring tear osmolarity including freezing point depression, vapour pressure and electrical impedance techniques.17,20,21 Water freezes at 0°C but the freezing point of a solution is lowered depending on the number of particles in that solution. The freezing point of a solution is directly proportional to the number of dissolved particles in that solution, so by determining the freezing point of a particular solution, the osmolarity can be directly calculated using known standards.17 Freezing point osmometers, such as the Clifton nanoliter osmometer, require a small volume of tears but also require significant expertise, laboratory access and substantial time to obtain results,20 making them less than ideal outside of a research setting. Vapor pressure osmometry relies on a similar relationship in that the vapour pressure drop of a solution, compared to a pure solvent of the same temperature and pressure, is proportional to its osmolarity.17 Vapour pressure osmometry requires a larger volume of tears and this longer collection time may increase reflex tearing, decreasing the osmolarity values that are measured.22,23
Newer electrical impedance osmometers, such as TearLab, measure osmolarity by measuring the number of charged particles within a sample (figure 1).
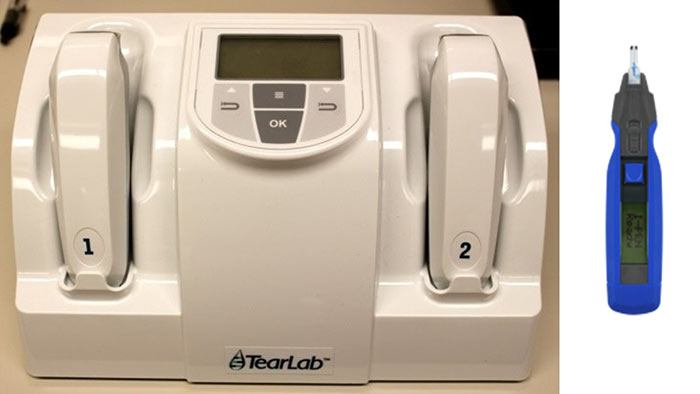
Figure 1: TearLab, left (Photo: Core) and i-Pen, right (Photo: osdcare.com/pages/i-pen)
The TearLab can obtain results from a tear sample size as small as 50nL in less than a minute.17,24 The electrical impedance osmometer collects the tear sample by touching a small testing chip into the tear meniscus without manipulation of the eyelids and without the need for anaesthetic (figure 2).

Figure 2: Illustration showing TearLab sampling from the lower tear meniscus (Photo copyright: Core)
The i-Pen is another point-of-care tear osmometer that uses electrical impedance technology (figure 1) but studies have not shown it to be as accurate in separating normal eyes from DEs as the TearLab.25,26 iPen has a flexible conductimetric sensor constructed using microelectronic techniques which is placed on the palpebral conjunctival membrane to measure the electrical conductivity of tear fluid.27 The ease of use and small tear sample required make newer in situ osmometers much more clinically accessible outside of a research facility and the shorter contact time required to obtain tear samples is more comfortable for the patient.
Tear osmolarity as a means to DED diagnosis
The ability to distinguish DE patients from non-DE patients via a single measurement is part of what makes tear osmolarity attractive as a DE tool. The trouble lies in establishing the threshold value for DE diagnosis. Most studies examining the threshold for DE diagnosis have produced values between 305-316 mOsm/L as a cut-off between normal and DE, with 308 mOsm/L being the most widely accepted value.24,28 Using a cut-off of 312 mOsm/L, tear osmolarity had a 72.8% sensitivity and 92.0% specificity in separating DE from normal eyes, whereas other DE testing, such as surface staining, TBUT and Schirmer testing all had less than 62% sensitivity or specificity.29 Another study found 73% sensitivity and 90% specificity when using a cut-off value of 316 mOsm/L.17 This gave a positive predictive value of 85%, which means that there is 85% probability that those eyes with osmolarity greater than 316 mOsm/L truly had DE.17
The difficulty in establishing a single threshold value comes from the fact that DE is, by definition, an instability of the tear film, so these values will fluctuate between measurements.4,30,31 Recognising that variability of tear osmolarity is part of the DED process, the difference in osmolarity values between eyes becomes important. With increasing severity of DED, there is more variability of osmolarity between eyes and between measurements of the same eye.20 In normal eyes, the osmolarity values are lower and show more consistency between eyes and between measurements.31 Normal eyes tend to vary by +/- 7 mOsm/L whereas DE may vary >11 mOsm/L between eyes or between tests but generally a difference of >8 mOsm/L between eyes indicates tear film instability.29 In a clinical setting, it is important to evaluate the measured osmolarity in each eye and the difference in osmolarity between eyes.
When considering other forms of DE testing, as tear production decreases as measured via the Schirmer test, tear osmolarity becomes elevated.32 While using tear osmolarity as a single metric to diagnose DED may not yet be appropriate, it may be most useful in grading severity of disease in DE patients. Tear osmolarity values show a relationship with the DEWS DE severity grade, wherein higher tear osmolarity corresponds to more severe grading of DED.32,33 While the utility of tear osmolarity does not preclude other forms of DE testing, understanding the strengths of each technique aids in the comprehensive evaluation of the DE patient.
Factors impacting tear osmolarity
There are several factors that can influence overall tear osmolarity. There are higher prevalence rates of DED in climates with lower humidity levels but this same relationship has not been shown with tear osmolarity and humidity levels.34,35 The literature is mixed with some studies demonstrating a relationship while others show no impact, though all of these studies lacked consistent control of other factors such as artificial tear use.20 Given the known association between DED prevalence rates and low humidity climates, there is a large opportunity for future research involving tear osmolarity and humidity levels.
Contact lens wear is a known risk factor for DE symptoms across several populations, with DED being four times more prevalent in contact lens wearers.36-38 In contrast to this finding, studies assessing tear osmolarity in contact lens wearers have shown equivocal results with some studies showing increase in osmolarity39-41 and some showing no impact on osmolarity.42-44 Whether this implies a different underlying etiology or mechanism for DE symptoms in contact lens wearers remains to be seen and certainly further research is warranted. The lack of correlation between elevated tear osmolarity and contact lens wear certainly reinforces the fact that tear osmolarity testing is not yet a standalone DE diagnostic tool for all patients but requires other testing to supplement its results.
There are several known systemic diseases associated with higher prevalence rates of DED and these same diseases show higher levels of tear osmolarity. Diabetes, thyroid dysfunction, fibromyalgia and traumatic brain injury are systemic conditions that are associated with increased tear osmolarity.45-48 The increase in osmolarity that occurs in diabetes may be due to decreased tear production resulting from small blood vessel damage at the level of the tear secretory glands.45 In thyroid disease, increased tear osmolarity is seen only if there are associated physical changes such as proptosis or increased palpebral aperture size that would increase tear evaporation rates.48 The underlying cause of increased osmolarity found in patients with pain disorders, like fibromyalgia, or patients with traumatic brain injury are not yet fully understood.
Certain ocular conditions can also impact tear osmolarity. Patients with pseudoexfoliation syndrome show higher tear osmolarity than controls, as do patients with pterygia.49,50 Interestingly, studies examining tear osmolarity in eyes with keratoconus have not found increased tear osmolarity.51,52
When examining the impact of DE therapies on osmolarity levels, the research is mixed. The use of topical cyclosporine (Restasis) has been shown to reduce tear osmolarity in some studies but have no impact in other studies,53,54 creating a large opening for future research about the relationship between osmolarity and cyclosporine. Artificial tear use has been found to reliably decrease tear osmolarity levels55,56 but this finding may be related to the osmolarity of the particular artificial tear used. The opportunity exists for future research examining whether treatment that results in a lower tear osmolarity improves patient symptoms, which might allow tear osmolarity to be a tool for tracking success of DE therapies.
Table 1 summarises the influences on tear osmolarity.
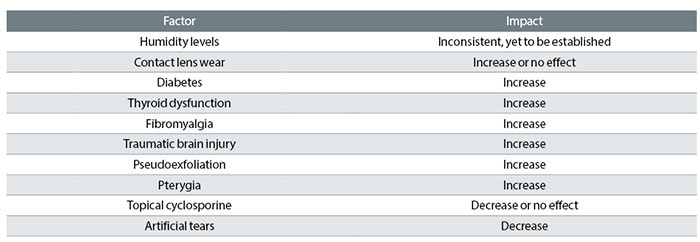
Table 1: Factors affecting tear osmolarity
Conclusion
DED continues to confound practitioners due to its varying aetiologies, varying signs and symptoms and lack of a single parameter that can diagnose DE from normal eyes. Tear osmolarity as a clinical technique has improved substantially in the last decade with osmometers becoming accessible outside of a research setting. Point-of-care osmometers are easy to use and comfortable for both the clinician and the patient. While it may not be sufficient as a single test for DE, osmolarity is an important part of the DE work-up. When performing tear osmolarity testing, the practitioner needs to be aware that while there may not be a single cut-off value to delineate DE from normal, any readings
>308 mOsm/L or >8 mOsm/L difference between eyes can indicate DED. These results need to be combined with that of other DE testing, such as staining with vital dyes, tear break-up times, tear production testing and assessment of the meibomian glands. This comprehensive view of the DE patient will allow for a better understanding of the mechanisms at play in each individual patient and aid in determining appropriate therapies targeted specifically for that patient.
Dr Marian Elder and Professor Sruthi Srinivasan are based at the Centre for Ocular Research & Education, School of Optometry & Vision Science, University of Waterloo
References
- Prydal, JI and FW Campbell, Study of precorneal tear film thickness and structure by interferometry and confocal microscopy. Invest Ophthalmol Vis Sci, 1992. 33(6): p. 1996-2005.
- Chen, HB, et al, Structure and composition of rat precorneal tear film. A study by an in vivo cryofixation. Invest Ophthalmol Vis Sci, 1997. 38(2): p. 381-7.
- Tiffany, JM, The normal tear film. Dev Ophthalmol, 2008. 41: p. 1-20.
- Craig, JP, et al, TFOS DEWS II Definition and Classification Report. Ocul Surf, 2017. 15(3): p. 276-283.
- Nelson, JD, et al, TFOS DEWS II Introduction. Ocul Surf, 2017. 15(3): p. 269-275.
- Chia, EM, et al., Prevalence and associations of dry eye syndrome in an older population: the Blue Mountains Eye Study. Clin Exp Ophthalmol, 2003. 31(3): p. 229-32.
- Lin, PY, et al, Prevalence of dry eye among an elderly Chinese population in Taiwan: the Shihpai Eye Study. Ophthalmology, 2003. 110(6): p. 1096-101.
- Schaumberg, DA, et al, Prevalence of dry eye syndrome among US women. Am J Ophthalmol, 2003. 136(2): p. 318-26.
- Galor, A, et al, Prevalence and risk factors of dry eye syndrome in a United States veterans affairs population. Am J Ophthalmol, 2011. 152(3): p. 377-384 e2.
- Paulsen, AJ, et al, Dry eye in the beaver dam offspring study: prevalence, risk factors, and health-related quality of life. Am J Ophthalmol, 2014. 157(4): p. 799-806.
- Tan, LL, et al, Prevalence of and risk factors for symptomatic dry eye disease in Singapore. Clin Exp Optom, 2015. 98(1): p. 45-53.
- Farrand, KF, et al, Prevalence of Diagnosed Dry Eye Disease in the United States Among Adults Aged 18 Years and Older. Am J Ophthalmol, 2017.
- Anonymous, The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf, 2007. 5(2): p. 75-92.
- Wolffsohn, JS, et al, TFOS DEWS II Diagnostic Methodology report. Ocul Surf, 2017. 15(3): p. 539-574.
- Mishima, S, Some Physiological Aspects of the Precorneal Tear Film. Arch Ophthalmol, 1965. 73: p. 233-41.
- Gilbard, JP and RL Farris, Tear osmolarity and ocular surface disease in keratoconjunctivitis sicca. Arch Ophthalmol, 1979. 97(9): p. 1642-6.
- Tomlinson, A, LC McCann, and EI Pearce, Comparison of human tear film osmolarity measured by electrical impedance and freezing point depression techniques. Cornea, 2010. 29(9): p. 1036-41.
- Baudouin, C, et al, Role of hyperosmolarity in the pathogenesis and management of dry eye disease: proceedings of the OCEAN group meeting. Ocul Surf, 2013. 11(4): p. 246-58.
- Bron, AJ, et al, TFOS DEWS II pathophysiology report. Ocul Surf, 2017. 15(3): p. 438-510.
- Potvin, R, S Makari, and CJ Rapuano, Tear film osmolarity and dry eye disease: a review of the literature. Clin Ophthalmol, 2015. 9: p. 2039-47.
- Tomlinson, A, et al, Tear film osmolarity: determination of a referent for dry eye diagnosis. Invest Ophthalmol Vis Sci, 2006. 47(10): p. 4309-15.
- Farris, RL, RN Stuchell, and ID Mandel, Tear osmolarity variation in the dry eye. Trans Am Ophthalmol Soc, 1986. 84: p. 250-68.
- Nelson, JD and JC Wright, Tear film osmolality determination: an evaluation of potential errors in measurement. Curr Eye Res, 1986. 5(9): p. 677-81.
- Jacobi, C, et al, Tear film osmolarity measurements in dry eye disease using electrical impedance technology. Cornea, 2011. 30(12): p. 1289-92.
- Nolfi, J and B Caffery, Randomized comparison of in vivo performance of two point-of-care tear film osmometers. Clin Ophthalmol, 2017. 11: p. 945-950.
- Rocha, G, et al, Randomized, masked, in vitro comparison of three commercially available tear film osmometers. Clin Ophthalmol, 2017. 11: p. 243-248.
- Maharaj, R, In Vivo Ocular Surface Osmolarity in a Dry Eye Population. Clinical and Refractive Optometry, 2017. 28(1): p. 3-6.
- Versura, P, V Profazio, and EC Campos, Performance of tear osmolarity compared to previous diagnostic tests for dry eye diseases. Curr Eye Res, 2010. 35(7): p. 553-64.
- Lemp, MA, et al, Tear osmolarity in the diagnosis and management of dry eye disease. Am J Ophthalmol, 2011. 151(5): p. 792-798 e1.
- Lemp, MA, et al, Distribution of aqueous-deficient and evaporative dry eye in a clinic-based patient cohort: a retrospective study. Cornea, 2012. 31(5): p. 472-8.
- Keech, A, M Senchyna, and L Jones, Impact of time between collection and collection method on human tear fluid osmolarity. Curr Eye Res, 2013. 38(4): p. 428-36.
- Suzuki, M, et al, Tear osmolarity as a biomarker for dry eye disease severity. Invest Ophthalmol Vis Sci, 2010. 51(9): p. 4557-61.
- Tukenmez-Dikmen, N, et al, Correlation of Dry Eye Workshop Dry Eye Severity Grading System With Tear Meniscus Measurement by Optical Coherence Tomography and Tear Osmolarity. Eye Contact Lens, 2016. 42(3): p. 153-7.
- Cho, HA, et al, Prevalence of dry eye syndrome after a three-year exposure to a clean room. Ann Occup Environ Med, 2014. 26: p. 26.
- Hwang, SH, et al, Potential Importance of Ozone in the Association Between Outdoor Air Pollution and Dry Eye Disease in South Korea. JAMA Ophthalmol, 2016.
- Bakkar, MM, et al, Epidemiology of symptoms of dry eye disease (DED) in Jordan: A cross-sectional non-clinical population-based study. Cont Lens Anterior Eye, 2016. 39(3): p. 197-202.
- Man, REK, et al, Incidence and risk factors of symptomatic dry eye disease in Asian Malays from the Singapore Malay Eye Study. Ocul Surf, 2017.
- Stapleton, F, et al, TFOS DEWS II Epidemiology Report. Ocul Surf, 2017. 15(3): p. 334-365.
- Herrero-Vanrell, R and A Peral, [International Dry Eye Workshop (DEWS). Update of the disease]. Arch Soc Esp Oftalmol, 2007. 82(12): p. 733-4.
- Miller, WL, et al, A comparison of tear volume (by tear meniscus height and phenol red thread test) and tear fluid osmolality measures in non-lens wearers and in contact lens wearers. Eye Contact Lens, 2004. 30(3): p. 132-7.
- Nichols, JJ and LT Sinnott, Tear film, contact lens, and patient-related factors associated with contact lens-related dry eye. Invest Ophthalmol Vis Sci, 2006. 47(4): p. 1319-28.
- Chen, SP, et al, Tear osmolarity and dry eye symptoms in women using oral contraception and contact lenses. Cornea, 2013. 32(4): p. 423-8.
- Muselier-Mathieu, A, et al, Ocular surface assessment in soft contact lens wearers; the contribution of tear osmolarity among other tests. Acta Ophthalmol, 2014. 92(4): p. 364-9.
- Sarac, O, et al, Comparison of tear osmolarity and ocular comfort between daily disposable contact lenses: hilafilcon B hydrogel versus narafilcon A silicone hydrogel. Int Ophthalmol, 2012. 32(3): p. 229-33.
- Sagdik, HM, et al, Tear film osmolarity in patients with diabetes mellitus. Ophthalmic Res, 2013. 50(1): p. 1-5.
- Turkyilmaz, K, et al, Dry eye in patients with fibromyalgia and its relevance to functional and emotional status. Cornea, 2013. 32(6): p. 862-6.
- Cockerham, GC, et al, Visual performance and the ocular surface in traumatic brain injury. Ocul Surf, 2013. 11(1): p. 25-34.
- Iskeleli, G, Y Karakoc, and A Abdula, Tear film osmolarity in patients with thyroid ophthalmopathy. Jpn J Ophthalmol, 2008. 52(4): p. 323-6.
- Julio, G, et al, Tear osmolarity and ocular changes in pterygium. Cornea, 2012. 31(12): p. 1417-21.
- Oncel, BA, E Pinarci, and YA Akova, Tear osmolarity in unilateral pseudoexfoliation syndrome. Clin Exp Optom, 2012. 95(5): p. 506-9.
- Mandathara, PS, et al, Pilot Study of Corneal Sensitivity and Its Association in Keratoconus. Cornea, 2017. 36(2): p. 163-168.
- Cho, KJ, et al, Changes in corneal sensation and ocular surface in patients with asymmetrical keratoconus. Cornea, 2013. 32(2): p. 205-10.
- Sullivan, BD, et al, Clinical utility of objective tests for dry eye disease: variability over time and implications for clinical trials and disease management. Cornea, 2012. 31(9): p. 1000-8.
- Bunya, VY, et al, Tear osmolarity in Sjogren syndrome. Cornea, 2013. 32(7): p. 922-7.
- Tomlinson, A, LC Madden, and PA Simmons, Effectiveness of dry eye therapy under conditions of environmental stress. Curr Eye Res, 2013. 38(2): p. 229-36.
- Iester, M, et al, Improvement of the ocular surface using hypotonic 0.4% hyaluronic acid drops in keratoconjunctivitis sicca. Eye (Lond), 2000. 14(Pt 6): p. 892-8.
