Fungal keratitis (or mycotic keratitis or keratomycosis) is a rare infection of the cornea, caused by several types of fungi. It is a difficult disease to diagnose and to treat due to the nature of the pathogen and the similarity of the clinical features across all types of microbial keratitis. In this review, we explore the known species that cause fungal keratitis as well as treatment and how to decrease risk of corneal infection.
What are fungi?
Fungi (single: fungus) constitute a large group of eukaryotic organisms that are quite diverse in nature as they range from edible mushrooms to microscopic yeasts and moulds. However, they all share certain characteristics including structural elements, like chitin and biochemical pathways. They are extremely abundant in the environment, but are normally inconspicuous because many of them are microscopic until they aggregate to colonise surfaces for long periods of time. We normally associate fungal growth with damp, temperate environments, but many have been isolated from unexpected extreme environments, such as deserts, areas with high salt concentrations and deep-sea sediments, while others have been documented as having survived space travel.1-3
Fungi as pathogens
Fungi have developed a number of symbiotic relationships with many different organisms, such as plants, algae and insects. Many of these relationships are mutually beneficial to both parties, with the host providing shelter and nutrients to the fungus and the fungus decomposing complex environments for the host. However, many fungi are known to cause serious disease in humans as pathogens or they can evoke dangerous allergic reactions.4 A major question arises from this: why is fungal disease rare when these organisms are ubiquitous in the environment?
The medical literature became enriched with studies and reports of fungal infection in humans causing serious and life-threatening infections in the mid 1980s, with the increased understanding and research into immunodeficiency (reduction in immune responses), including the description of the human immunodeficiency virus (HIV).5 Immunodeficient individuals are more susceptible to fungal pathogens, indicating that a normal immune response is sufficient to quickly suppress infection without progressing to severe disease.6 Phagocytes (dendritic cells, neutrophils, macrophages) are first response immune cells that have pattern recognition receptors (PRR) that can bind to fungal cell wall components. C-type lectin receptors, such as dectin-1 are important PRR as well as toll-like receptors (TLR), in particular TLR2.7-8
Both receptors bind to mannan, chitin and glucan in the cell wall and this ligation subsequently triggers signaling pathways in the phagocyte to initiate killing mechanisms that promote pathogen clearing. The inability to clear fungal pathogens due to immunodeficiency allows the fungus to colonise the site of infection, tissue area and/or organ. Examples of these are Candida albicans that causes vulvovaginal candidiasis (thrush) or oral candidiasis (mouth infection) and in some cases sepsis;9 Pneumocystis carinii that causes pneumonia10 and Aspergillus fumigatus that typically causes invasive pulmonary aspergillosis.11
Corneal susceptibility to fungal infection
Individuals affected by fungal keratitis are not typically immunodeficient, so why is fungal keratitis such as devastating disease? If we consider its anatomy, the cornea is separated from the rest of eye as it is not vascularised and only has certain elements of the immune response, such as dendritic cells, complement, cytokines produced by epithelial cells and the lacrimal immune response.12 As a consequence, this makes it particularly vulnerable to microbial invasion from contaminated sources. On the other hand, fungal spores are one of the most abundant particles in the environment so the cornea must come into contact with them regularly and not display signs of an active infection. What makes the cornea vulnerable to fungal infection is corneal trauma that include small abrasions to the corneal surface through contact lens wear. These can allow fungal pathogens to enter the epithelial layer and colonise the tissue early and rapidly.
Epidemiology of fungal keratitis
Cases of fungal keratitis have been reported worldwide, in countries as widespread as Brazil, Saudi Arabia, USA, India and African nations. In developing countries, such as India and Thailand, approximately 40% of all microbial keratitis cases are fungal in nature and mostly caused by Aspergillus species.13-15 Here, agricultural workers who have endured ocular trauma have been the predominant group affected. In contrast, in the USA, fungal keratitis is typically associated with contaminated contact lenses and incidence is increasing. Famously, in 2006 an outbreak of Fusarium keratitis was reported, linked with the contamination of ReNu with MoistureLoc contact lens solution from Bausch and Lomb. The proposed cause was the loss of fungicidal properties of the container due to elevated temperatures.16 This case strongly supports that fungi are everywhere in the environment, so they are difficult to avoid. Therefore, control and prevention are of high importance in reducing the risk of infection.
Causative agents
Filamentous fungi, such as Aspergillus and Fusarium, and yeast-like fungi such as Candida are commonly associated with fungal keratitis. Each type has some unique features that are worthwhile taking into consideration.
Fusarium
Fusarium is a large group of filamentous fungi, typically found in soil microbial communities. Despite their ubiquitous nature, they are relatively poorly known by the public, but surprisingly they are found under many guises. For example, Fusarium venatum is used as a popular meat substitute as it was found to contain a high level of protein content. It is marketed today under the commercial name of Quorn.17 Other Fusarium species are plant pathogens. For example, Fusarium oxysporum has caused a wide spread outbreak of disease in the American continent in banana plants (Panama) and palm trees (California). Interestingly, it was considered as a mycoherbicide to stop coca plantations in South America,18 but with its potential to also cause human disease, including fungal keratitis, this was not pursued as the environmental transmission risk was too great. As well as fungal keratitis, some Fusarium species can cause opportunistic infections in humans such onchomycosis (nail infections) and disseminated infection in immunocomprised individuals.18 F. oxysporum is among the most common causes of fungal keratitis.19 Others include F. solani, F. verticilloides, and F. proliferatum. Fusarium keratitis has predominantly been associated with contact lens solution contamination (figure 1).20

Figure 1: Fusarium keratitis has predominantly been associated with contact lens solution contamination
Aspergillus
There are many different species of Aspergillus, some of which have been exploited for commercial purposes, such as microbial fermentation of alcoholic beverages; but many are also pathogenic to humans. They are highly adaptable organisms and their success is due to their ability to survive in extreme conditions, such as high salt or sugar concentrations. They grow well on carbon-rich substrates and often contaminate and colonise starchy food like potatoes and bread. They are highly aerobic and colonise high oxygen tension surface, often appearing in moist environments such as showers, sink and damp walls as mildew in the case of A. niger. The most common Aspergillus pathogens are A. fumigatus and A. flavus. It not only causes infection but the toxins it produces can also cause allergic reactions. In cases of Aspergillus keratitis, most cases have occurred following ocular surgical procedures;21 but there is growing evidence to suggest environmental Aspergillus contamination of contact lenses is an issue22,23 and there has also been an increase in reports in people engaged in outdoor activities, including agricultural work.24
Candida
In contrast, Candida are usually harmless commensals of human hosts. However, when the mucosal barriers are disrupted or the immune system is compromised they can cause disease. Candida albicans, the most common species, causes candidiasis or thrush in individuals and in particular those who have compromised immune systems.25 In some cases, Candida infection can occur after a course of antibiotics,26 which supports the fact that our microbiome is crucial in supporting our defences against this organism. In the case of Candida keratitis, disease is associated with use of corticosteroids.27 These are immunosuppressants used to reduce inflammation for other conditions and prior to corneal surgery (eg LASIK, chronic keratopathy or corneal graft). With a suppressed immune system, Candida becomes opportunistic to invade the unprotected tissue.25
Other species
Scedosporium apiospermum a ubiquitous environmental mould, is increasingly reported as causing invasive fungal disease in immunocompromised hosts. It is typically found in stagnant, polluted water and is classified as an emerging pathogen that is resistant to most anti-fungal treatments. From a biological point of view, it is the anamorphic (asexual) life cycle stage of Pseudallescheria boydii. Although rare, there are some case reports in clinical microbiology, mycology and ophthalmology journals that report successful treatment with voriconazole 28 and itranazole.29
Paecilomyces lilacinus (or Pupureocillium lilacinum) is a common filamentous fungus, isolated from a wide range of environments, including soils, estuarine sediments, sewage sludge and deserts.30 Its pathogenesis in humans is rare but there is an increasing trend of keratitis cases caused by this fungus in both contact lens wearers and non-wearers,30 where no history of any trauma was observed. In all cases there appears to be fungal colonisation (spread) of the cornea. Paecilomyces is resistant to many anti-fungals (see treatment and management section) and this poses a significant problem for its management.
Clinical features
Fungal keratitis presents in a similar manner to other types of microbial keratitis. Symptoms include:
- Severe pain
- Redness
- Blurred vision
- Photophobia
- Excessive tearing or discharge
In the later stages, it is actually possible to see the fungal growth across the cornea and corneal ulcer may form with a hypopyon, which is an aggregate of inflammatory cells forming a fluffy exudate near the ulcer (figure 2).31
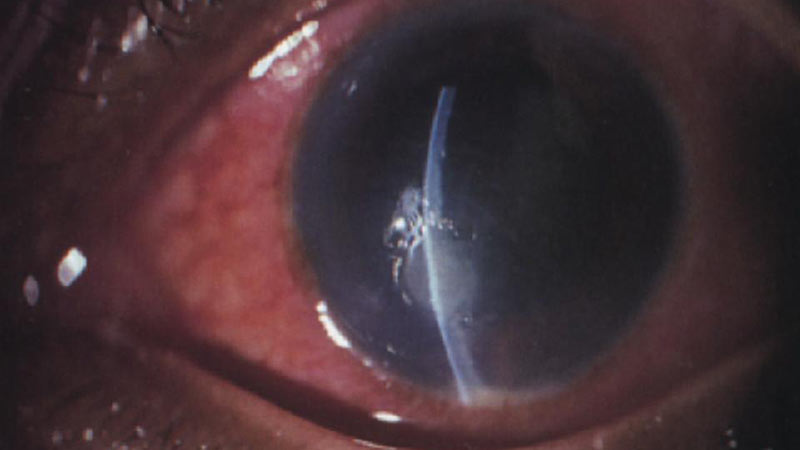
Figure 2: Later stage fungal keratitis showing ulceration and hypopyon.
Diagnosis
Fungal keratitis is often misdiagnosed as a bacterial infection and this may add to its persistence and the severity of infection as appropriate anti-fungal treatments are delayed in favour of broad-spectrum antibiotics (that cannot kill fungus) and in some cases anti-inflammatory corticosteroids (that can have an adverse effect and actually promote fungal growth).
A definitive diagnosis is provided by a positive culture from a corneal scraping and by amplification of fungal DNA. Collecting sample material for these diagnostics test can be a challenge and provide discomfort for the patient. While it can take up to a week to see any fungal growth, amplification of fungal DNA by polymerase chain reaction (PCR) is a preferred route to diagnosis. This speeds up identification in a precise manner, establishing the causative agent and its genotype, which is important for the appropriate anti-fungal treatment.15
Treatment and Management
Antifungal medicines
Antifungal compounds are a group of chemical agents that target fungal structure and biochemical components. Many anti-fungals target specifically the sterol biosynthesis pathway, which synthesises ergosterol. Ergosterol is a sterol compound found in the cell membrane of fungi. It is similar to cholesterol in humans, but differences between them in their structure and in their synthesis, make ergosterol an excellent candidate for an effective treatment with the aim to minimise adverse effects in the host. However, some antifungal medicines may cause allergic reactions in some individuals.32
Common treatments used in fungal keratitis include polyenes that can bind directly to ergosterol, thus disrupting the fungal cell membrane structure.33 Examples of Polyenes are natamycin, which is the drug of choice for treating Fusarium keratitis and amphotericin B, which is used in cases caused by Candida species. Polyenes do not penetrate the ocular surface well and compounds from the azole family are usually considered for deep fungal infections as they are absorbed systemically. These include ketonazole, miconazole, fluconazole, and clotrimidazole, which is one of the few anti-fungal medications that can be bought over the counter, sold under the brand name Canesten. This group inhibits 14-alpha demethylase, which is an enzyme that participates in ergosterol biosynthesis.33 The use of voriconazole and itranazole in the treatment of fungal keratitis is increasing.28,29
Unfortunately, antifungal resistance is emerging and other types of treatment have to be considered. Disinfectants such as iodine and silver nitrate have been used successfully as an alternative.34
Corticosteroids
Corticosteroids are routinely used to reduce the level of inflammation in the eye as they downregulate immunological features that cause inflammation (immune cell recruitment and activation). The use of corticosteroids should be avoided during a fungal infection as their presence promotes fungal growth because the immune pressure that ‘fight’ the fungal pathogen is not present if corticosteroids are used.15 Their application is only recommended in case where inflammation is causing severe damage to the eye.35
Surgical intervention
Patients who do not respond to medical treatment usually require surgical intervention. This includes procedures such as corneal debridement (physical removal of the fungus and direct application of anti-fungal medication to the site of infection) or a cornea transplant (keratoplasty). Antifungal treatment should be continued following surgical intervention.36
Risk factors
The ubiquitous nature of fungi and their ability to become facultative or opportunistic pathogens provides two points to consider:
- Fungi surround us. They are in our homes, gardens, cities and rural areas.
- Mammals have effective immune systems in place to manage potential infection and protect us from disease.
However, incidence of fungal keratitis is high and therefore if we take the first point, we can assume the fungi that surround us are colonising and invading the cornea. It is likely that cases and solutions can become contaminated, especially if we think that we normally store them on the sink or bathroom shelves, where mildew and aspergillus can linger even if it is not evident to the human eye (figure 3).

Figure 3: Using contact lens cases in bathrooms increases the risk of fungal infection
Outdoor trauma is often reported with incidence of fungal keratitis, such as gardening and sport activities. If grit gets into our eyes it is a normal reaction to wipe it away with our hands or fingers; thus, transferring fungus to the cornea and even causing small lesions by which the fungus can enter the epithelial layers.
In terms of our immune system, fungal keratitis patients are not typically immunocompromised. However, we must remember that the cornea does not possess the same immune system features as the rest of our bodies. In addition, it is normal practice in the treatment of microbial keratitis to reduce inflammation with corticosteroids. This reduces any immune response that is upregulated, allowing the fungal pathogen to grow quickly without any challenge.
Prevention
There are several key factors that should be considered in the prevention of fungal keratitis from patient, practitioner and commercial points of view.
Patient:
1 Individuals should wear safety glasses with a side shield when performing outdoor activities. Protective eye wear will protect the cornea from grit, dust and soil particles that may enter the eye through hand to eye contact (eg rubbing the eye); wind and actions that promote particles to become airborne (eg shaking and digging). This will limit potential abrasions, which allow fungal spores in the grit, dust and soil to enter the cornea. Wearing glasses while doing outdoor sport activities, such as running and cycling will also prevent fungal pathogens carried in the wind and air to enter the eye.
2 Contact lens care regimen should be respected at all times. The lenses and cases should not come into contact with tap water, nor should they be left for prolonged periods of time. If any black or mildew deposits appear on the cases (even the outside), the case should be immediately discarded (although if manufacturer’s instructions are followed this should not happen in the recommended lifetime of the cases. If lenses are contained within these contaminated cases, immediate consultation with a contact lens specialist should take place. All eye care solutions should also be kept with closed caps and discarded after the recommended use by date.
3 General hygiene should be maintained on both personal and environmental levels. Hands should be washed prior to handling contact lenses. The towel used to dry hands after washing should be clean and dry. In addition, the area where eye care solutions are kept should be damp-free.
Practitioner:
1 Non-essential steroid medication should be avoided. Although steroids are useful anti-inflammatory agents, which downregulate the immune response, and it is important to reduce inflammation in the eye; the immune response is required to clear fungal pathogens. The use of corticosteroids has been associated with the promotion of fungal growth.37
2 Prevention of fungal keratitis has also been approached through prophylactic use of anti-fungal medication particularly in geographical areas with a notable persistence of fungal keratitis in the local populations. This has resulted in a decrease of corneal ulceration cases.38 This preventative intervention has to be tightly controlled at all times due to high risk of the emergence of antimicrobial resistance.39
Commercial
1 The notable case of the association with Bausch and Lomb’s ReNu with MoistureLoc contact lens solution with the outbreak of Fusarium keratitis in the USA 16 reminds us of the importance of sterility during manufacture despite this case being linked to the container type and its loss of antifungal properties under certain conditions. Industry is encouraged to secure a microbial screening programme to ensure all lenses and solutions are sterile before use.
More prevention recommendations can be found at https://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/HomeHealthandConsumer/ConsumerProducts/ContactLenses/ucm062584.htm.
Conclusions
As with other types of microbial keratitis, patients displaying symptoms should be immediately referred to an ophthalmology unit. Cases of fungal keratitis are rare in western countries, but they are on the increase in developing countries, especially in South-East Asia. Here in the UK, if a patient presents with symptoms a travel history could be taken to support diagnosis. Awareness and education of the risks, causes and consequences of fungal keratitis should be given particularly to contact lens wearers, especially those undertaking agricultural work and outdoor activities. Importantly, contact lens care and hygiene is critical to cornea health and vision well-being and eye care solution and contact lens guidelines should be respected to achieve this.
Fiona L Henriquez is Professor of Parasitology, Infection and Microbiology Group Leader, Institute of Biomedical and Environmental Health Research, University of the West of Scotland.
References
- Sterflinger K, Tesei D, Zakharova K. Fungi in hot and cold deserts with particular reference to microcolonial fungi, Fungal Ecology, Volume 5, Issue 4,2012, Pages 453-462,
- Plemenitaš A, Lenassi M, Konte T, et al. Adaptation to high salt concentrations in halotolerant/halophilic fungi: a molecular perspective. Frontiers in Microbiology. 2014;5:199.
- Gomoiu I, Chatzitheodoridis E, Vadrucci S, Walther I, Cojoc R. Fungal spores viability on the International Space Station. Origins of life and evolution of the biosphere: The Journal of the International Society for the Study of the Origin of Life. 2016 April 22
- https://www.cdc.gov/fungal/diseases/index.html
- Limper AH, Adenis A, Le T, Harrison TS. Fungal infections in HIV/AIDS. Lancet Infect Dis. 2017 Nov;17(11):e334-e343.
- Low C-Y, Rotstein C. Emerging fungal infections in immunocompromised patients. F1000 Medicine Reports. 2011;3:14.
- Netea MG, Van der Graaf C, Van der Meer JW, Kullberg BJ. Recognition of fungal pathogens by Toll-like receptors. Eur J Clin Microbiol Infect Dis. 2004 Sep;23(9):672-6. Epub 2004 Aug 18.
- Jose L Blanco, Marta E Garcia,Immune response to fungal infections, Veterinary Immunology and Immunopathology, Volume 125, Issues 1–2,2008,Pages 47-70.
- Delaloye J, Calandra T. Invasive candidiasis as a cause of sepsis in the critically ill patient. Virulence. 2014;5(1):161-169.
- Minielly JA, Mills SD, Holley KE. Pneumocystis carinii pneumonia. Canadian Medical Association Journal. 1969;100(18):846-854.
- M Kousha, R Tadi, AO Soubani. Pulmonary aspergillosis: a clinical review European Respiratory Review Sep 2011, 20 (121) 156-174.
- DelMonte DW, Kim T. Anatomy and physiology of the cornea. J Cataract Refract Surg. 2011 Mar;37(3):588-98. doi: 10.1016/j.jcrs.2010.12.037.
- Saha S, Banerjee D, Khetan A, Sengupta J. Epidemiological profile of fungal keratitis in urban population of West Bengal, India. Oman Journal of Ophthalmology. 2009;2(3):114-118.
- Thomas PA, Kaliamurthy J. Mycotic keratitis: epidemiology, diagnosis and management. Clin Microbiol Infect. 2013 Mar;19(3):210-20.
- Ansari Z, Miller D, Galor A. Current Thoughts in Fungal Keratitis: Diagnosis and Treatment. Current fungal infection reports. 2013;7(3):209-218.
- Bullock JD, Elder BL, Khamis HJ, Warwar RE. Effects of time, temperature, and storage container on the growth of Fusarium species: implications for the worldwide Fusarium keratitis epidemic of 2004–2006. Arch Ophthalmol. 2011;129:133–136.
- T Finnigan, L Needham and C Abbott, Chapter 19 - Mycoprotein: A Healthy New Protein With a Low Environmental Impact, In Sustainable Protein Sources, edited by Sudarshan R. Nadathur, Janitha P.D. Wanasundara and Laurie Scanlin, Academic Press, San Diego, 2017, Pages 305-325, ISBN 9780128027783
- Kuruvilla TS, Dias M. Fusarium solani: A Causative Agent of Skin and Nail Infections. Indian Journal of Dermatology. 2012;57(4):308-309.
- Gracia-Garza JA, Fravel DR, Bailey BA, Hebbar PK. Dispersal of Formulations of Fusarium oxysporum f. sp. erythroxyli and F. oxysporum f. sp. melonis by Ants. Phytopathology. 1998 Mar;88(3):185-9.
- Epstein AB. In the aftermath of the Fusarium keratitis outbreak: What have we learned? Clinical ophthalmology (Auckland, NZ). 2007;1(4):355-366.
- Thomas PA. (2009) Aspergillus Keratitis. In: Comarú Pasqualotto A. (eds) Aspergillosis: From Diagnosis to Prevention. Springer, Dordrecht
- Sionov, E, Sandovsky-Losica, H, Gov, Y and Segal, E (2001), Adherence of Aspergillus species to soft contact lenses and attempts to inhibit the adherence. Mycoses, 44: 464–471.
- Cuadros J, Gros-Otero J, Gallego-Angui P, Scheu AK, Montes-Mollón Á,Pérez-Rico C, Moreno JP, Gómez-Herruz P, Soliveri J, Teus M. Aspergillus tamarii keratitis in a contact lens wearer. Med Mycol Case Rep. 2017 Nov 26;19:21-24.
- Thomas PA, Kaliamurthy J. Mycotic keratitis: epidemiology, diagnosis and management. Clin Microbiol Infect. 2013 Mar;19(3):210-20.
- Lopes JP, Stylianou M, Nilsson G, Urban CF. Opportunistic pathogen Candida albicans elicits a temporal response in primary human mast cells. Scientific Reports. 2015;5:12287.
- Kashkin PN, Krassilnicov NA, Nekachalov VY. Mycopathologia et Mycologia Applicata 1961; 14: 173.
- Berson EL, Kobayashi GS, Becker B, Rosenbaum L; Topical Corticosteroids and Fungal Keratitis. Invest Ophthalmol Vis Sci. 1967;6(5):512-517.
- Hernández Prats C, Llinares Tello F, Burgos San José A, Selva Otaolaurruchi J, Ordovás Baines JP. Voriconazole in fungal keratitis caused by Scedosporium apiospermum. Ann Pharmacother. 2004 Mar;38(3):414-7.
- Saracli MA, Erdem U, Gonlum A, Yildiran ST. Scedosporium apiospermum keratitis treated with itraconazole. Med Mycol. 2003 Apr;41(2):111-4.
- Jennifer Luangsa-ard, Jos Houbraken, Tineke van Doorn, Seung-Beom Hong, Andrew M. Borman, Nigel L. Hywel-Jones, Robert A. Samson; Purpureocillium, a new genus for the medically important Paecilomyces lilacinus, FEMS Microbiology Letters, Volume 321, Issue 2, 1 August 2011, Pages 141–149
- Dalmon C, Porco TC, Lietman TM, et al. The Clinical Differentiation of Bacterial and Fungal Keratitis: A Photographic Survey. Investigative Ophthalmology & Visual Science. 2012;53(4):1787-1791.
- Tang MM, Corti MAM, Stirnimann R, Pelivani N, Yawalkar N, Borradori L. and Simon D. Severe cutaneous allergic reactions following topical antifungal therapy. Contact Dermatitis, 2013; 68: 56–57.
- Ghannoum MA, Rice LB. Antifungal Agents: Mode of Action, Mechanisms of Resistance, and Correlation of These Mechanisms with Bacterial Resistance. Clinical Microbiology Reviews. 1999;12(4):501-517.
- Fili S, Schilde T, Perdikakis G, Kohlhaas M, (2016) Two Cases of Keratomycosis Caused by Fusarium solani: Therapeutic Management. J Eye Cataract Surg 2:9.
- Peponis V, Herz JB, Kaufman HE. The role of corticosteroids in fungal keratitis: a different view. The British Journal of Ophthalmology. 2004;88(9):1227.
- Xie L, Dong X, Shi W. Treatment of fungal keratitis by penetrating keratoplasty. Br J Ophthalmol. 2001 Sep;85(9):1070-4.
- http://bmec.swbh.nhs.uk/wp-content/uploads/2013/03/FUNGAL-KERATITIS.pdf
- Srinivasan M, Upadhyay MP, Priyadarsini B, Mahalakshmi R, Whitcher JP. Corneal ulceration in southeast Asia III: prevention of fungal keratitis at the village level in south India using topical antibiotics. The British Journal of Ophthalmology. 2006;90(12):1472-1475.
- Sanglard D. Emerging Threats in Antifungal-Resistant Fungal Pathogens. Frontiers in Medicine. 2016;3:11.
