According to the World Health Organisation, glaucoma is one of the principal causes of vision problems worldwide, causing 2% of visual impairment and 8% of blindness,1 while paediatric glaucoma causes 5% of childhood blindness.2
Paediatric glaucoma is a sight-threatening condition that needs early identification and treatment. It may also be the first manifestation of syndromes which require multi-disciplinary medical input, such as neurofibromatosis 1.3 This article will discuss primary congenital glaucoma, including its diagnosis and management. It will then discuss secondary paediatric glaucoma resulting from both ocular conditions such as aphakia, aniridia, trauma and juvenile idiopathic arthritis and also those associated with systemic conditions, such as neurofibromatosis 1 and Sturge-Weber syndrome.
Primary congenital glaucoma
Primary congenital glaucoma (PCG) is a rare condition. In the UK the incidence of PCG is one in 18,500 live births. The British Infantile and Childhood Glaucoma (BIG) Eye study found nearly nine times the incidence of glaucoma in participants of Pakistani origin compared to those of Caucasian origin.4 PCG is characterised by an idiopathic anterior chamber developmental abnormality with no associated ocular or systemic condition. Opinions differ as to why and how the angle is different, but theories suggest an abnormal refining or remodelling process of the embryological angle during development. This impairs aqueous outflow. Figure 1 shows the ill-defined angle structures with scalloped iris edge due to bridges of tissue across the trabecular meshwork.
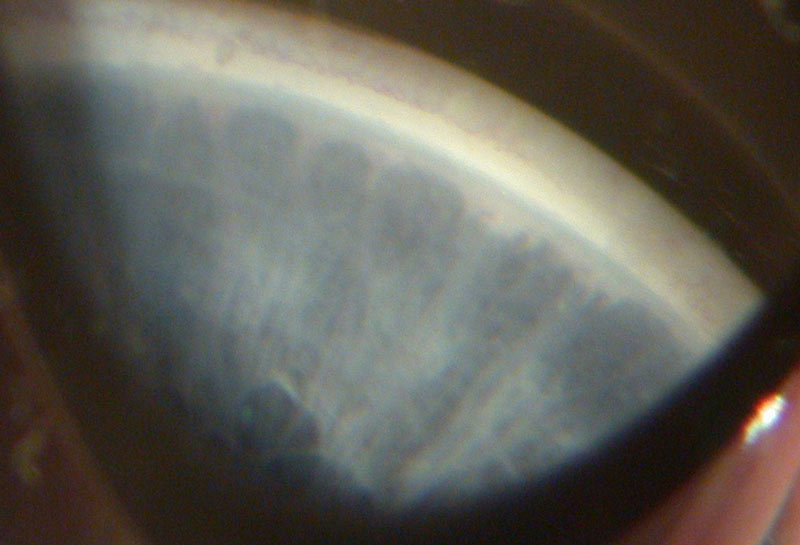
Figure 1: Gonioscopy view of the angle showing indistinct structures (courtesy of Chris Lloyd)
PCG can be classified by the age at which it presents as follows:
- Newborn – present at birth following raised intra-ocular pressure (IOP) intrauterine
- Infantile – occurs between four months and two years
- Juvenile – from two to 16 years
In 75% of cases, both eyes are affected but the condition is frequently asymmetrical.5 Although most cases are sporadic, gene loci associated with PCG have been identified in recent years.6
Clinical signs
Parents may be the first to notice the signs of glaucoma. These include:
- Cloudy or hazy corneas
- Watery eyes
- Photophobia
In more advanced cases, the parents may notice the eye becoming larger and bluer especially in infants with brown irides. On clinical assessment, children are found to have:
- Raised intraocular pressure (IOP).
- Corneal haze due to epithelial and stromal oedema, which dulls the red reflex and causes the blue appearance of the iris. Figure 2 shows a resolving central corneal haze.
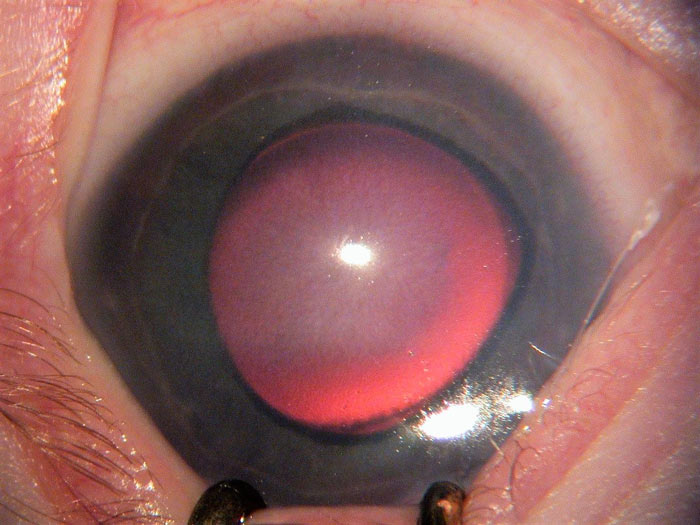
Figure 2: A high IOP causes a hazy appearance particularly noticeable centrally (courtesy of Chris Lloyd)
- Photophobia, epiphora and blepharospasm, which can make examination challenging.
- Buphthalmos, where the eye enlarges due to increased IOP. This happens in infants as the ocular tissues are more elastic than in adults, leading to an increase in the corneal diameter. A cornea measuring more than 11mm in a newborn, 12mm in an infant less than one year old and over 13mm in any child should raise suspicion of glaucoma.
- Scleral thinning, due to stretching of the sclera. This reveals the uvea which causes a blue hue of the sclera.
- Increasing myopic refractive error or myopic shift due to increasing axial length.
- Haab’s striae (figure 3). As the cornea is stretched by raised IOP, Descemet’s membrane breaks or splits and aqueous floods into the stroma. Once the pressure is reduced, the endothelial cells migrate across the split and the cornea can start to clear. Haab’s striae are the remains of the split as they appear once the cornea has cleared. They are tend to be horizontal.
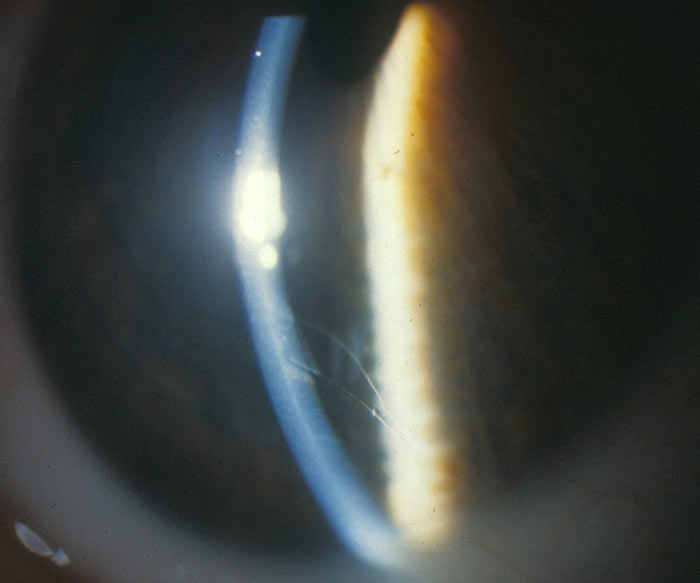
Figure 3: Haab’s striae (courtesy of Chris Lloyd)
- Optic disc cupping (figure 4) or asymmetry.
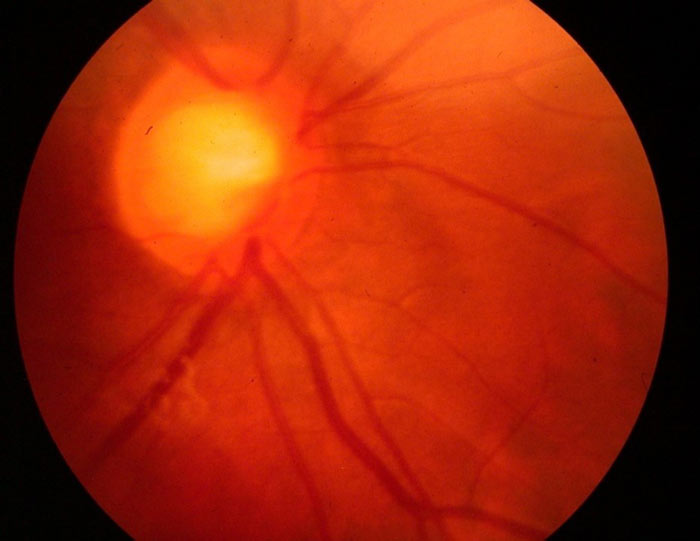
Figure 4: Cupped disc in a seven-year-old with glaucoma (courtesy of Chris Lloyd)
In some cases where glaucoma has spontaneously halted some of these signs may be present without a raised IOP.5
Tools for assessment
When assessing infants and children, it is important to be flexible with the order that tests are carried out and often there is a need to prioritise certain tests if patient’s attention is limited. Feeding or sleeping infants can be easier to assess.
Essential equipment includes:
- A direct ophthalmoscope for anterior eye examination and red reflex.
- Retinoscope for measuring refractive error and also useful for assessing the red reflex. A myopic shift may be an indicator of uncontrolled IOP.
- A hand-held slit lamp for assessing the corneal appearance, anterior chamber and lens.
- A variety of tonometers for measuring intraocular pressure. The rebound Icare tonometer or a non-contact tonometer (such as the Keeler Pulsair) allow a relatively easy measurement of an infant’s cornea, but where more accurate measurements are required, applanation techniques such as Perkins or Tonopen are preferable. Some children over three years old, who are able to sit at a slit lamp, can be remarkably cooperative when it comes to measuring pressure using Goldmann tonometry. However, for a child who is very difficult to examine, sometimes a digital palpation pressure reading is all that can be managed in the outpatient clinic.
- A corneal diameter rule. This can be a diameter gauge on a pen torch or a card (figure 5).

Figure 5: Card gauge to measure corneal or pupil diameters (courtesy of Lynne Speedwell)
- A head-set indirect biomicroscope for disc and fundus assessment.
Examination under anaesthetic (EUA)
An EUA allows for more detailed assessment and formal diagnosis. Some anaesthetics can affect IOP readings, for example sevoflurane has the effect of lowering IOP, so ketamine may be prefereable.4,7 Intubation also increases IOP. Measurements therefore need to be taken immediately following induction of anaesthesia and prior to the child being intubated.
As well as repeating tests from outpatient appointments, the EUA might include:
- Gonioscopy.
- Central corneal thickness – this is thinner in children with glaucoma correlating with increased axial length and corneal diameter.8
- Ultrasound – to measure axial length is helpful when the view through the cornea is limited.
In order to avoid too many extra anaesthetics, which may affect the developing brain structure and cognitive development,9 angle surgery may be performed at the initial EUA if the view of the angle is sufficient to negate the need for a further anaesthetic.
Continual monitoring of IOP, corneal diameter, optic disc cup-to-disc ratio and refractive error help indicate if treatment is successful. Optic disc cupping can be reversible in infants after treatment that successfully lowers the IOP.10 In older children, visual fields and OCT imaging can be useful tools for monitoring.
Differential diagnosis
The differential diagnosis of PCG includes the following:
- Epiphora and photophobia from corneal pathology or a nasal lacrimal duct obstruction.
- Corneal haze from:
- Birth trauma. Forceps delivery related ruptures of Descemet’s membrane may occur. These ruptures tend to be vertical compared to the horizontal Haab’s striae.
- Systemic conditions such as rubella and mucopolysaccharidoses can also cause corneal haze.
- Congenital hereditary endothelial dystrophy (CHED) causes corneal haze which can manifest from birth.
- A large corneal diameter can be found in:
- Megalocornea is a condition in which the corneas are large (>13mm) and clear with no other pathology. It can be hereditary.
- High myopia.
- Marfan’s syndrome.
Buphthalmos can also be caused by intraocular tumours, gliomas, connective tissue disorders and axial myopia.5,6
Surgical treatment
In PCG, treatment options are often specific to the subtype.
Newborn congenital glaucoma does not respond well to topical medication so surgery is usually the first line of treatment, although drops such as timolol, used off licence, may be given pre-operatively to try to reduce the corneal haze. Trabeculectomy and aqueous shunt insertion are the initial surgeries of choice. This is due to the anatomical developmental abnormalities of the drainage angle.11
Infantile paediatric glaucoma is more common, tends to be less severe and responds well to goniosurgery.12
Juvenile glaucoma treatment is dependent on age, severity and drainage angle anatomy and may require drops and or surgery.
In goniotomy, commonly used in infantile glaucoma, a surgical incision is made into the anterior trabecular meshwork in order to open up the aqueous drainage. This surgery requires visualisation of the angle using a gonioscope lens. Anterior chamber depth needs to be maintained throughout this procedure and this is done using a viscoelastic. This procedure can be repeated if necessary.
If the angle cannot be seen clearly due to corneal haze or if goniotomies have failed, a trabeculotomy procedure is indicated. A probe called a trabeculotome, is fed into Schlemm’s canal and then pulled into the anterior chamber to rupture through the trabecular meshwork. Angle surgery can be highly successful but does sometimes require repeat procedures for long-termsuccess.13
More invasive surgery, such as trabeculectomy, may be used especially if multiple angle surgeries have failed or when the cornea is over 14mm indicating advanced disease and poor prognosis. Trabeculectomy surgery involves creating an alternative drainage route for aqueous, via a passage in the sclera, which drains under the conjunctiva. These surgeries require antimetabolites to prevent the new drainage filtration flap or ‘bleb’ that is created during surgery from scarring.5 Aqueous shunt implants, such as Baerveldt or Ahmed valves, are rarely used as a primary surgery in PCG4 but may be indicated as a secondary procedure where other forms of surgery have not been successful. The surgery involves a plastic tube being place into the anterior chamber that drains up through a plate under the conjunctiva. This surgery may offer a long term IOP reduction but has risks such as hypotony and the need for repeat procedures such as tube repositioning if the tube touches the endothelium.14
Cyclodiode laser to ablate the ciliary body, and therefore reduce aqueous production, may be required in eyes that have not responded well to other interventions, particularly in eyes with poor visual prognosis. This intervention is short term and may need to be repeated, increasing the risk of complications.15
Multiple EUAs are required in infants and younger children and at the time of consent the medical team will discuss the options of potential surgical intervention to be done at the time of the EUA depending on the findings, as already discussed.9
Topical treatment
As mentioned previously, drops are used in PCG to reduce IOP and corneal haze prior to surgery; they are also used as an additional treatment post-operatively. They are prescribed as a first line of treatment in some juvenile and secondary glaucomas. Punctal occlusion when administering drops to infants is advisable to reduce systemic absorption. Most drops are not licensed for use in children and safety profiles are not fully known.
The following groups of drugs are used off licence:
- Beta blockers work by reducing aqueous production but must be avoided in asthmatic children. Parents and clinicians must be vigilant for any signs of respiratory side effects.
- Topical carbonic anhydrase inhibitors are a second line treatment but are contra-indicated if the corneal endothelium is compromised due to the risk of corneal decompensation.
- Oral acetazolamide is rarely used due to the systemic side effects.15
- Of the prostaglandin analogues, only latanoprost is licensed for use in children.16
- Pilocarpine may be used pre- and post-angle surgery as the miosis helps to open the drainage angle. It may also be used in aphakic glaucoma.
- Alpha agonists, such as such as brimonidine, can cross the blood-brain barrier and have been associated with severe systemic side effects.17 They are therefore contra-indicated in children under two years old and if prescribed, they are unlicensed.18
Effects of glaucoma
- Vision – all children require visual development monitoring. They are at risk of refractive error and strabismus and therefore amblyopia which may require occlusion therapy.
- Photophobia – children with glaucoma can suffer from photophobia so spectacles with tinted or photochromic lenses may be required to help with the glare, and peaked caps can be useful.
- Registration – sight impaired or severely sight impaired as appropriate. During early school years, visual development may be delayed and registration should be considered. If, when they are older, their visual acuity or visual field is better than expected registration can be withdrawn.
- Low vision aids – a low vision assessment can feedback information to the school and local teams. Modifications, such as classroom position, being able to wear a cap in school and writing in pen, can easily be made while awaiting visual impairment (VI) team input.
- Treatment – children may be on multiple drops and therefore may need to have the school nurse administer drops for them, depending on the drop regime prescribed. Drops used post-operatively often need to be used for several months and at frequent intervals.
- Side effects – all topical treatments have side effects and consideration should be given to the longevity of treatment. For example, a teenager started on a prostaglandin analogue drop may need to be on that drop for the rest of their life. The side effects of orbital fat loss, skin discolouration and lash growth may become very significant and they need to be counselled appropriately.
- School attendance – due to multiple hospital appointments and procedures, school attendance may be affected.
- The psychological effects of glaucoma in a young person should be considered. There can be a fear of losing sight or going blind. Groups that work with young people with visual impairment, such as LOOK-UK or the Royal Society for Blind Children (RSBC), can be useful contacts for both the child and their families.
Secondary glaucoma
Cataract surgery
Glaucoma following congenital cataract surgery is the most common cause of secondary glaucoma in children.4 The condition is either closed angle and occurs soon after surgery or more commonly, open angle which can happen at any time after surgery. Depending on the type of glaucoma, treatment will vary from drops to laser to surgery. The risk of developing glaucoma after cataract surgery is increased with microcornea, persistent foetal vasculature and post-operative inflammation. The risk also increases the earlier the cataract surgery is undertaken, so a balance between intervention to gain best visual prognosis and risk of glaucoma needs to be established.19
Intraocular lens implants were not found to be protective against glaucoma and required more surgical interventions such as capsulotomy.20 Rates as high as 59% of children having glaucoma or ocular hypertension 10 years after cataract surgery have been found. If young children present with a myopic shift in their refraction, raised IOP must be ruled out. Aphakic children are therefore monitored for life 21 although once they reach teenage years this can be done by a community optometrist. Annual sight tests need to include IOP measurements.
Anterior segment dysgenesis
This is a broad term that includes conditions such as:
- Axenfeld-Rieger syndrome
- Peter’s anomaly
- Aniridia
Treatment of glaucoma in anterior segment dysgenesis is challenging and may require surgical intervention such as trabeculectomy or aqueous shunt implant.22
Axenfeld-Rieger describes a spectrum of ocular and systemic manifestations with glaucoma occurring in 50% of patients.23 Anomalies occur in tissues derived from neural crest cells.24 It is a bilateral condition but may be asymmetrical and is associated with glaucoma due to iris hypoplasia. Axenfeld anomaly, shown in figure 6, manifests with posterior embyrotoxon and peripheral iris stands attached to the cornea. Rieger anomaly includes posterior embryotoxon, iris abnormalities such as correctopia and iris adhesions to the cornea in the angle.5 Dental, facial, cardiac and renal anomalies occur in Rieger syndrome.
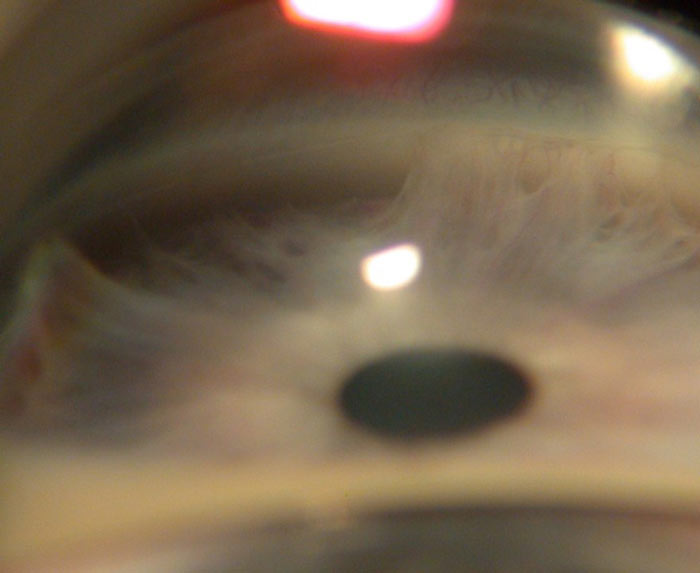
Figure 6: Axenfeld anomaly showing iris strands attached to the cornea (anterior synechiae) (courtesy of Chris Lloyd)
Peter’s anomaly is characterised by corneal opacity, irido-corneal adhesions and lens adherence to the cornea as shown in figure 7.
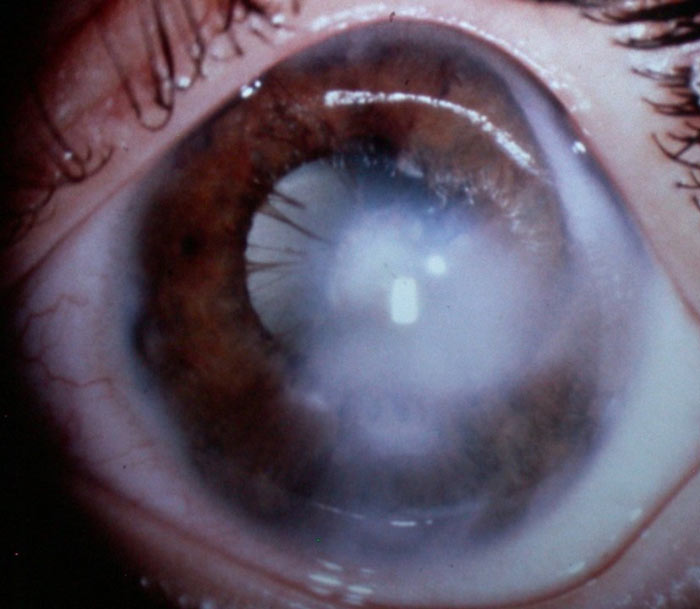
Figure 7: Peter’s anomaly (courtesy of Chris Lloyd)
Glaucoma develops in approximately 50% of patients.5 Peter’s anomaly is also due to neural crest cell developmental anomalies and can therefore also be associated with systemic manifestations including defects in the central nervous system, heart disease and craniofacial anomalies.25 Peter’s Plus syndrome has ocular manifestations alongside short stature, cleft lip and palate, and intellectual disability.26
Aniridia is a bilateral condition characterised by partial or total lack of iris (figure 8) and also affects other ocular structures:
- Corneal limbal stem cell deficiency leading to corneal problems including dryness, scarring and vascularisation.
- Corneal thickness is increased and must be considered when measuring IOP.
- Cataracts, lens subluxation and/or congenital aphakia.
- Fundal abnormalities include: foveal hypoplasia often resulting in nystagmus;5 choroidal coloboma and optic nerve hypoplasia.
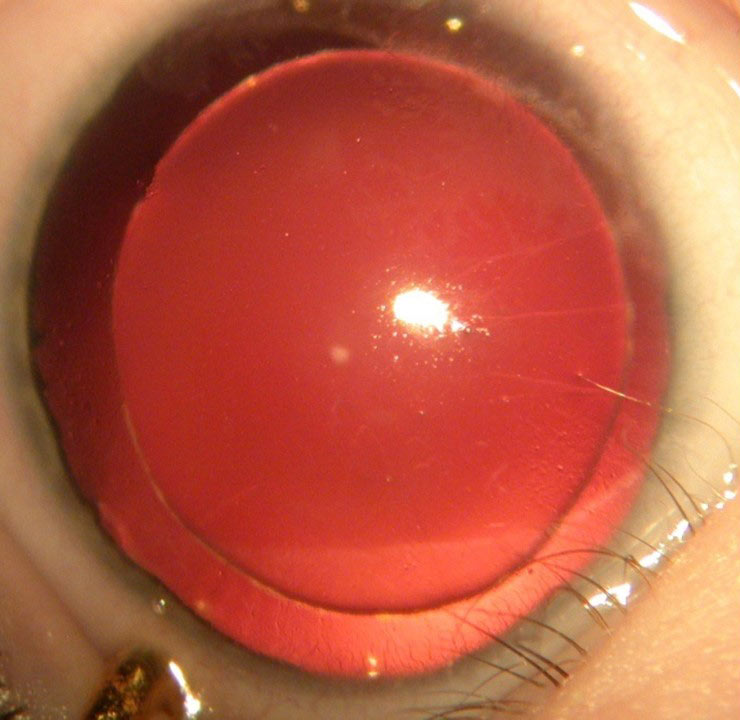
Figure 8: Aniridia showing total lack of iris (courtesy of Chris Lloyd)
Glaucoma in these children tends to develop later into childhood and is due to progressive obstruction of the drainage angle by rotation of iris remnant tissue.27 Up to 75% can develop glaucoma.
Two thirds of cases of aniridia have an autosomal dominant inheritance pattern and in these patients, usually only the eyes are affected. The gene that causes aniridia is the PAX6 gene.
Sporadic cases of aniridia should be screened for systemic associations such as WAGR syndrome. This syndrome manifests with Wilms’ tumour, aniridia, genitourinary abnormalities and intellectual disability. Wilms’ tumour is a nephroblastoma so abdominal ultrasound screening is advised.5
Retinoblastoma
Retinoblastoma can cause glaucoma, usually by iris neovascularisation leading to peripheral anterior synechiae and therefore closure of the angle. It can also cause pupil block via an exudative retinal detachment. Inflammation can occasionally be the cause of glaucoma in retinoblastoma.28
Retinopathy of prematurity (ROP)
Angle closure glaucoma can arise from anterior synechiae forming in eyes with total retinal detachment and the resultant fibrovascular ring of tissue causing shallowing of the angle.5 It can also occur in eyes treated with a surgical buckle for detachment and can occasionally be caused by neovascularisation of the angle.29
Ectopia lentis
This is a condition where the lens is dislocated. In ectopia lentis et pupillae, the pupil is also displaced, often in the opposite direction from the lens. If the lens dislocates into the anterior chamber, acute glaucoma can arise from pupil block. Ectopia lentis can be associated with systemic conditions, the most common of which is Marfan’s syndrome. These patients are thought to have irregular drainage angles that can give rise to glaucoma as well as the risk of lens dislocation induced glaucoma.5
Sturge-Weber syndrome
This is characterised by a facial cutaneous vascular malformation, a port-wine stain that follows the route of the trigeminal nerve and its branches. It is mostly unilateral. Systemic features involve venous angiomas of the leptomeninges, seizures, hemiparesis and hemianopia. The mechanism for glaucoma is thought to be due to dysgenesis of the drainage angle in infants and to raised episcleral venous pressure in older children. The episcleral venous pressure is raised by arteriovenous shunts in the haemangioma, thus the glaucoma is ipsilateral to the facial haemangioma.30,31 Medical and surgical options are considered depending on the age of onset of glaucoma. Other ocular manifestations include episcleral and choroidal haemangiomas.
Neurofibromatosis type 1 (NF1)
This is a multisystem condition where neurofibromas can occur throughout the body along the nervous system. Patients are at risk of intracranial tumours such as meningiomas or gliomas. Ocular signs may include Lisch nodules on the iris which appear from early childhood, eyelid neurofibromas and fundal lesions. Glaucoma is usually unilateral and is associated with ipsilateral eyelid neurofibromas.5 However, glaucoma can be present in new-borns so NF1 must be excluded as a cause in newborn glaucoma.3
Uveitis
There are a few possible mechanisms leading to secondary glaucoma (see Paediatric eye care – Part 2 C58735 January 19, 2018):
- The trabecular drainage can become blocked by protein and blood cell debris from the inflammation in an open angle.
- Angle closure from posterior synechiae and pupil block
- Anterior synechiae.
- Steroid use. Steroids are required to treat uveitis and can cause a raised IOP. Some studies show as high as a third of these children with uveitis get secondary glaucoma.32
Juvenile idiopathic arthritis is the most common systemic disease associated with uveitis in children. Treatment tends to result in the need for surgery including trabeculectomy or aqueous shunt implant.4 When glaucoma is caused by pupil block a peripheral iridotomy is required. The nature of this glaucoma can be difficult to manage due to its varied course, some may have transient episodes while others may have aggressive persistent raised IOP.
Juvenile xanthogranuloma
This is a rare and benign non-Langerhans cells histiocytosis, and normally presents with single or multiple skin lesions. Ocular involvement and systemic involvement is rare. Iris lesions may lead to spontaneous hyphaema, uveitis and glaucoma. In cases of infantile unilateral glaucoma this diagnosis should be considered.33
Lowe syndrome
Also known as oculocerebrorenal syndrome, Lowe syndrome is a rare, x-linked metabolic condition that causes hypotonia, renal problems, bilateral cataract and congenital glaucoma. The cataracts are present at birth and glaucoma develops in 50% of patients.5 Earlier onset glaucoma in these patients tends to require surgical management and later onset is more likely to be conservatively managed as in primary congenital glaucoma.34 The mechanism is thought to be similar to that of PCG with angle anomalies, however there may be an overlying aphakic glaucoma element.35
Conclusion
Paediatric glaucoma is a vast and varied condition that can have many different presentations. The mechanism for cause of glaucoma needs to be established to enable appropriate treatment choices to be made. Glaucoma may be the first sign of a systemic condition and therefore thorough investigation is essential. Children with glaucoma or at risk of secondary glaucoma require lifelong follow-up.
Steph Figg is a specialist optometrist at Great Ormond Street Hospital and Moorfields Eye Hospital.
References
- World Health Organisation Global Data on Visual impairments, 2010
- Gilbert CE, Rahi JS, Quinn GE. Visual impairment and blindness in children. In Johnson GJ Minassian DC Weale RA West SK eds. The Epidemiology of Eye Disease. 2003; 2nd ed. 260–286. Edward Arnold
- Satran L et al. Neurofibromatosis With Congenital Glaucoma and Buphthalmos in a Newborn. Am J Dis Child. 1980;134(2):182-183
- Papadopoulos M, Cable N, Rahi J, Khaw PT; The British Infantile and Childhood Glaucoma (BIG) Eye Study. Invest Ophthalmol Vis Sci. 2007 Sep;48(9):4100-6.
- Kanski J. (2007) Clinical Ophthalmology, A systemic approach. 6th Edition. Butterworth Heinemann Elsevier.
- Armstrong R, The genetics of primary congenital glaucoma Optometry Today December 2008
- Jones L, Sung V, Lascaratos G, et al Intraocular pressures after ketamine and sevoflurane in children with glaucoma undergoing examination under anaesthesia British Journal of Ophthalmology 2010;94:33-35
- Henriques et al. Corneal Thickness in Congenital Glaucoma Journal of Glaucoma: 2004 13 (3) 185-188
- Loepke, A, Soriano, S. (2008) An Assessment of the Effects of General Anesthetics on Developing Brain Structure and Neurocognitive Function. Anaesthesia and Analgesia 106:1681-1707
- Quigley HA Results with trabeculectomy and study of reversible cupping Ophthalmology 1982 89 (3) 219-226
- Perry LP et al. Newborn primary congenital glaucoma: Histopathologic features of the anterior chamber filtration angle. Journal of American association for pediatric ophthalmology and strabismus. 2012 16(6)565-568
- Walton DS, Katsavounidou G. Newborn primary congenital glaucoma: 2005 Update. Journal of Pediatric ophthalmology and strabismus. 2005, 42(6)333-41
- Anderson DR. Trabeculotomy compared to goniotomy for glaucoma in children. Ophthalmology. 1983;90:805–6.
- Beck AD et al. Aqueous shunt devices compared with trabeculectomy with Mitomycin-C for children in the first two years of life. American journal of ophthalmology 2003 136(6) 994-1000
- Papadopoulos M, Khaw PT. Advances in the management of paediatric glaucoma. Eye 2007. 21, 1319-1325
- NICE BNFC Glaucoma https://bnfc.nice.org.uk/treatment-summary/glaucoma.html (accessed March 2018
- Bowman et al. Ocular and systemic side effects of brimonidine 0.2% eye drops (Alphagan) in children. Eye 2004; 18: 24-26
- Coppens G et al. The safety and efficacy of glaucoma medication in the pediatric population. Journal of pediatric ophthalmology and strabismus. 2009; 46(1):12-18
- Grigg J, Fenerty C. (2017) Glaucoma Following Cataract Surgery in Aphakic or Pseudophakic Children. In: Lloyd I., Lambert S. (eds) Congenital Cataract. Springer, Cham
- Solebo et al. (2015) Risks and outcomes associated with primary intraocular lens implantation in children under 2 years of age: the IOLunder2 cohort study. 2015. British journal of ophthalmology; 99:1471-1476.
- Egbert J et al. The natural history of glaucoma and ocular hypertension after pediatric cataract surgery. Journal of American association for pediatric ophthalmology and strabismus. 2006; 10(1): 54-57
- Wallace D et al. Surgical results of secondary glaucomas in childhood. Ophthalmology 1998; 105(1): 101-111
- Alward W. Axenfeld-Rieger syndrome in the age of molecular genetics. American journal of ophthalmology 2000; 130(1): 107-115
- Ozeki H et al. Anomalies associated with Axenfeld-Rieger syndrome. Graefe’s archive for clinical and experimental ophthalmology. 1999. 237(9); 730-734
- Traboulsi E, Maumenee I. Peter’s anomaly and associated congenital malformations. Arch. Ophthalmol. 1992; 110 (12):1739-1742.
- Maillette de Buy Wenniger-Prick L. The Peters’ plus syndrome: a review Annales de Genetique. 2002; 45 (2) 97-103.
- Grant WM, Walton DS. Progressive changes in the angle in congenital aniridia, with devlopment of glaucoma. American journal of ophthalmogy 1974; 78(5) 842-847
- Yoshizumi M et al. Glaucoma-inducing mechanisms in eyes with retinoblastoma. Arch Ophthalmol. 1978;96(1):105-110.
- Michael A et al. Management of late-onset angle-closure glaucoma associated with retinopathy of prematurity. Ophthalmology. 1991;98(7):1093-1098
- Phelps CD. The pathogenesis of glaucoma in Sturge-Weber syndrome. Ophthalmology 1978; 85(3):276-286
- Cibis GW et al. Glaucoma in Sturge-Weber syndrome Ophthalmology 1984 91(9) 1061-1071
- Sijssens KM et al. Ocular hypertension and secondary glaucoma in children with uveitis. Ophthalmology 2006; 113(5):853-859
- Vendal Z, Walton D, Chen T. Glaucoma in juvenile xanthogranuloma. Seminars in ophthalmology. 2006; 21(3) 191-194
- Kruger S et al. Cataracts and glaucoma in patients with oculocerebrorenal syndrome. Arch Ophthalmol. 2003; 121(9):1234-1237
- Walton D et al. Glaucoma with the oculocerebrorenal sundrome of Lowe. Journal of glaucoma. 2005; 14(3):181-185
