Remember 1999? The end of the last millennium. The first time silicone hydrogel contact lenses became available in the UK. Although to some it may only seem to be the blink of an eye, in fact we are nearing the 20th anniversary of that date. In the last two decades, technology – such as the phone in your pocket – has changed almost beyond recognition. The same period of time has also seen significant change in silicone hydrogel contact lenses. Material technology has evolved, and choice of available modalities and prescriptions has grown considerably. This article examines some of the ocular physiological responses resulting from silicone hydrogel wear, and reviews how our understanding of those responses has helped to improve silicone hydrogel materials and their designs over time.
Prior to 1999, we already had access to a wide choice of frequently replaced hydrogel lenses, including daily disposable (DD) options. Arguably, one of the biggest issues remained outstanding: oxygen transmission (Dk/t). There is a limit to the amount of oxygen that can be dissolved in water, and therefore there is a limit to the amount of oxygen a hydrogel CL can transmit to the cornea. With particular focus on extended wear, there was a desire to significantly increase oxygen transmissibility. The properties of silicone as a conduit for oxygen were well understood. The challenge that lay ahead for material chemists and manufacturers was how best to incorporate hydrophobic silicone into a hydrophilic hydrogel material.
The commercialisation of silicone hydrogels was a landmark moment in the development of soft CLs. The problems of hypoxia, especially in relation to daily wear of CLs, were solved. Eyes that once exhibited active neovascular changes in the peripheral cornea showed an emptying of those vessels, and limbal hyperaemia reduced or disappeared.1 Not all the effects seen with wear of those early silicone hydrogel materials were quite so positive however. In a number of situations, the sequelae of wearing silicone hydrogels led eye care professionals and patients to believe they were seeing an eye which exhibited an allergic reaction to silicone. In fact, an allergic reaction directly to silicone is now known not to be biologically possible.2 The immune system is primed to recognise carbon-based molecules such as proteins,3 but cannot react directly to the firmly bound silicone components found within a silicone hydrogel lens. Despite this, it is understandable that some of the complications seen were thought to mimic allergic responses. Those signs and symptoms are discussed below, along with the changes to material, CL design and patient management that have occurred over the last few years. As both our knowledge and technology have improved, these changes have eliminated many of the problems observed by the technology incorporated into early silicone hydrogel materials.
Complications associated with early reusable silicone hydrogel materials
Many eye care professionals have had the experience of seeing a patient respond less than ideally to the initial introduction of silicone hydrogel contact lens materials. Examples of undesirable signs or symptoms associated with these earlier materials are shown in table 1. The CL may not be comfortable for the patient, or specific signs such as marked contact lens induced papillary conjunctivitis (CLIPC) or corneal inflammatory events (CIEs) may be noted. The root cause for these complications do not lie in a direct reaction to silicone and each complication has its own etiology.
CLIPC is known to occur as an inflammatory response to denatured protein on hydrogel lenses that are replaced less frequently than that typically seen with contemporary materials.4,5 A similar lid reaction can be seen in patients wearing silicone hydrogel contact lenses, although typically the papillae may be larger and concentrated in the central area of the tarsal conjunctiva.6,7 A key difference compared to hydrogel materials was the increased ‘stiffness’ or modulus of early generation silicone hydrogel materials. This was due to the incorporation of siloxane groups and reduced water content.8-10 The increase in modulus was thought to be responsible for CLIPC seen with early silicone hydrogel contact lenses, being produced by a mechanical stimulus, rather than a reaction of the immune system.11
Superior epithelial arcuate lesions (SEALs) are another example of a mechanical interaction seen with early silicone hydrogel contact lens designs.12 These were thought to occur as a result of the high modulus of early silicone hydrogel materials being unable to conform to the limbus,11 and from the combination of eyelid pressure and silicone hydrogel material properties causing excessive ‘frictional’ pressure and shear force on the epithelial surface.13 The increase in modulus resulted in non-optimal fit of early silicone hydrogel lenses in some cases, with one study reporting more than three-quarters (77%) required the steeper of two base curves to achieve good comfort and fit.14 A further illustration of the effects of increased modulus and non-optimal fit of early silicone hydrogel materials were the occurrence of mucin balls. Thought to be caused by shear forces as the contact lens moved during the blink, they were most commonly seen with extended wear of first generation materials.15,16
Inflammatory reactions, such as CIEs and contact lens-related acute red eye (CLARE) occur with silicone hydrogels. In fact, daily wear of reusable silicone hydrogel contact lenses has an almost two-fold increase in the relative risk of infiltrative keratitis (IK).17-21 A number of explanations have been postulated for this increase, including poorer wettability, different deposition patterns between hydrogel and silicone hydrogel materials, higher modulus, and varying interactions with both care systems22-31 and contact lens cases.32-35
A further example concerned the ability of some patients to wear a silicone hydrogel material successfully. We can probably all recall patients who struggled to achieve comfortable wear when switched from hydrogel to silicone hydrogel contact lenses. Along with an increase in modulus from a hydrogel, lens wettability has been implicated as a possible reason for this. With reference to wettability, although reports have linked comfort and in-eye wettability,36 it has proved difficult to show a difference in wettability between material types when worn,37 although differences in vitro have been demonstrated.8,38-41
The deposition profile is markedly different between silicone hydrogels and hydrogels. Silicone hydrogels, containing hydrophobic elements of silicone, deposit more lipid38,42-46 and less protein47-54 than hydrogels. It is worth bearing in mind that although protein deposition on silicone hydrogels is minimal, the proportion of the deposited protein found to be denatured is typically much higher.55 However, a link between comfort and deposition has yet to be unequivocally demonstrated.56 Similar questions still also surround the link between comfort and the corneal staining seen as a result from interactions between silicone hydrogel materials and cleaning solutions, with a difference of opinion existing across a number of studies.22,26,57-59
How silicone hydrogel materials have changed over the past two decades
Initial focus on overnight continuous wear of silicone hydrogel contact lenses broadened into a desire to produce silicone hydrogel materials and designs suitable for comfortable daily, open eye wear. It is interesting to note that since the first silicone hydrogel in 1999, all subsequent silicone hydrogel lenses have had a lower Dk/t. Despite that change, all silicone hydrogel materials have been shown to deliver enough oxygen to meet the minimum requirements of the cornea during open eye wear.1,60
Balancing the properties of silicone hydrogel materials has been important for optimising comfortable daily wear. A general trend exists for silicone hydrogels such that as Dk/t reduces, so water content typically increases. This has an effect on modulus, and figure 1 illustrates the strong inverse relationship between modulus and water content. In general, modulus rises as percentage water content reduces. First generation silicone hydrogel materials, as expected, have the highest modulus and lowest water content. In contrast, a number of more recently developed silicone hydrogel materials sit towards the other end of the graph, with higher water content and lower moduli. Given the drive to develop silicone hydrogel materials for comfortable daily wear, it is interesting to note how close some of the more recent silicone hydrogel materials compare to hydrogel materials (figure 1, red dots). Reducing modulus, with the associated increase in water content, has helped to minimise mechanical complications such as CLIPC. In addition, changes made to the contact lens profile, base curve and edge design have helped to avoid mechanically-induced complications such as SEALs.
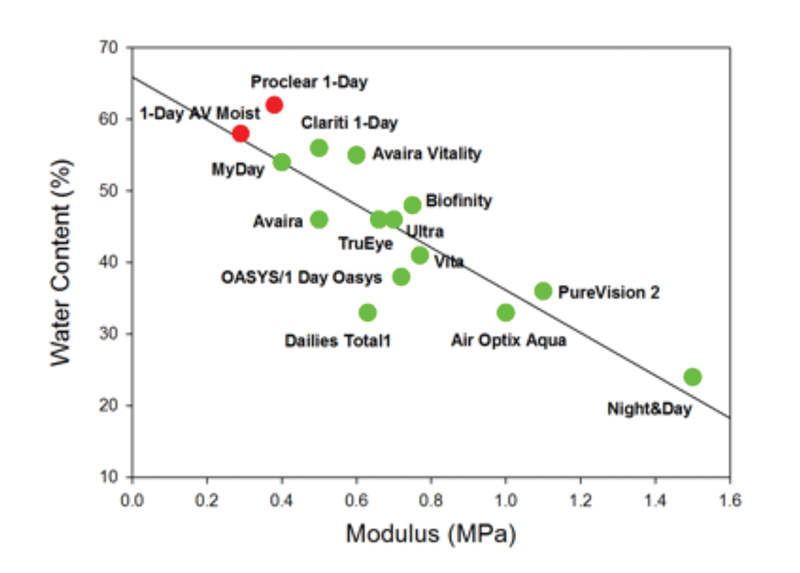
Figure 1: Modulus and water content for silicone hydrogel (green) and two representative hydrogel (red) materials
Material technology has, of course, changed over the last twenty years. Early materials such as lotrafilcon A (Alcon Focus (now Air Optix) Night & Day) and balafilcon A (Bausch & Lomb PureVision) overcame the hydrophobic nature of silicone by using a gas plasma reactive chamber as an additional step during manufacture to render the CL surface wettable. Galyfilcon A (Johnson & Johnson Acuvue Advance) was the first material manufactured without a surface treatment. Instead, a wetting agent, polyvinyl pyrollidone (PVP), was incorporated into the material to confer wettability.
Further innovation saw comfilcon A (CooperVision Biofinity) and stenfilcon A (CooperVision MyDay) being manufactured and marketed as materials which were naturally wettable, requiring no surface treatment or internal wetting agent. Materials such as delefilcon A (Alcon Dailies Total1) take a different approach, with its water gradient technology wrapping a silicone core with an essentially hydrogel-like surface. The aim of all these technologies is to deliver a wettable, hydrophilic surface in a material which contains the naturally hydrophobic component of silicone. As previously mentioned, it has proved difficult to demonstrate differences in wettability between materials on-eye, however, some links between wettability and comfort have been shown.36
Additional evidence of the evolution and refinements of silicone hydrogel design and manufacture over the years is demonstrated by examples such as the redesign of balafilcon A to produce a thinner lens (Bausch & Lomb PureVision 2HD), and the addition of wetting agents to the packaging solution of lotrafilcon B to aid initial comfort on application (Alcon Air Optix Aqua plus HydraGlyde).
Daily disposable silicone hydrogel materials
The benefits of shorter replacement frequencies have been understood for a long time, with lower patient satisfaction reported in three-month planned replacement hydrogels compared to monthly hydrogel lenses for example.61 Daily disposables offer the shortest replacement frequency, and since 2008, silicone hydrogel options have been available in this modality.
A number of the complications highlighted in table 1 could be considered related, or at least exacerbated, by the fact that many CLs are reusable. Comfort may reduce, and lipid deposition will continue to accumulate over the life of the lens.43,46 The use of daily disposable silicone hydrogels can therefore help to alleviate some complications. There are currently five different silicone hydrogel materials available as daily disposables, with designs to correct spherical, astigmatic and presbyopic prescriptions. The increased choice in this segment has naturally helped to facilitate an increase in daily disposable silicone hydrogel fits. The most recent annual report on international contact lens prescribing reports for the first time more fits in daily disposable silicone hydrogels than hydrogels.62 For fit data collected in the UK in 2017, nearly two-thirds (62%) of all soft lens fits used silicone hydrogel materials, with just under half of those going into daily disposable silicone hydrogel lenses (48%).62
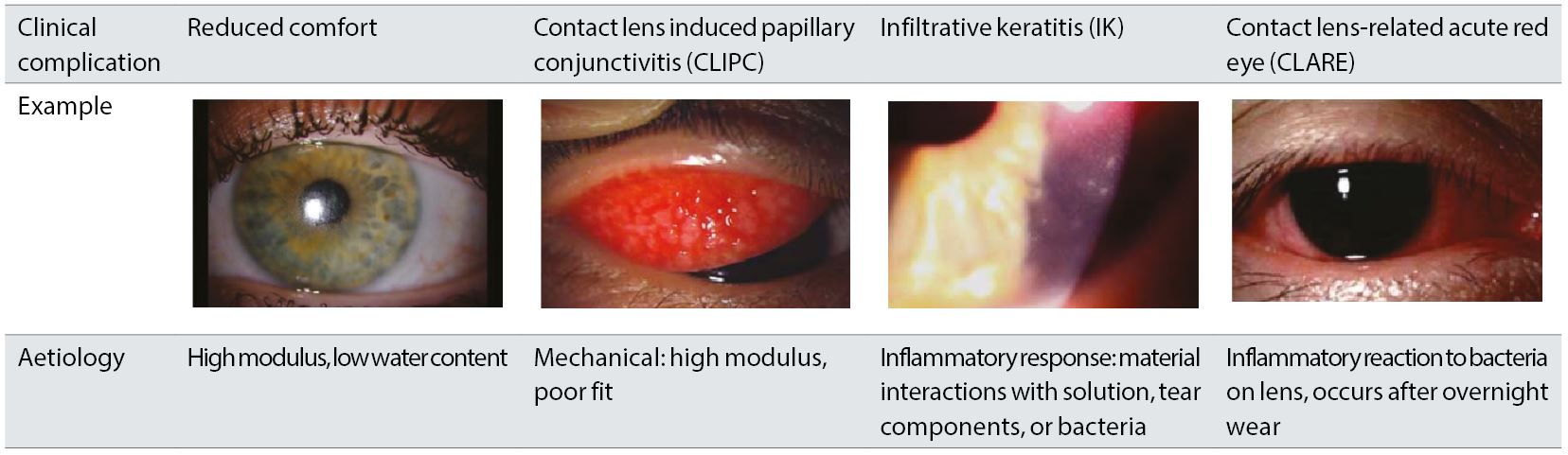 Table 1: Clinical complications associated with early silicone hydrogel contact lenses
Table 1: Clinical complications associated with early silicone hydrogel contact lenses
The evidence base for the performance of daily disposable silicone hydrogel lenses is growing, and this appears to be matched by the increase in prescribing by eye care professionals. Published data show comparable performance to daily disposable hydrogel lenses,63,64 along with excellent physiological responses and comfort levels.63-69 Of interest is the fact that levels of IK are no different between silicone hydrogel and hydrogel materials when used as daily disposables.63,65 Given the increased risk in CIEs present with reusable silicone hydrogels, this is an important finding. It helps both in our understanding of the potential causes for increased CIEs in reusable silicone hydrogels, and, more practically, gives the eye care professional good options for reducing CIE risk through the simple mechanism of recommending a daily disposable modality. Finally, although the mechanisms and impact of solution induced corneal staining (SICS) is not fully understood, clearly moving to a daily disposable lens removes the need of the silicone hydrogel material to interact with any care solution. This may enable the patient who has been previously labelled with an ‘incompatibility to silicone hydrogels’ due to previous signs of SICS or inflammatory complications to now successfully wear silicone hydrogels.
Conclusion
Twenty years is a long time in terms of technology. With respect to silicone hydrogel materials much has been learned over that time. Initial breakthrough technology paved the way for future innovation. The positive effects of increased oxygen transmission eliminating hypoxic complications of contact lens wear remain. Early undesirable sequelae have been understood and addressed in a number of different ways over time and across manufacturers. Material properties have been balanced to achieve more optimal combinations of modulus, water content, and wettability in order to promote comfortable daily wear. Changes to lens profile, base curve and edge design, along with the addition of comfort enhancing agents to packaging solutions have also been used by some manufacturers. New materials and proprietary manufacturing technology have become available. Wide availability across spherical, astigmatic and multifocal powers enables eye care professionals to recommend these materials to the majority of their patients.

These innovations are important and help to address a number of the complications seen with early silicone hydrogel materials. Other complications are reduced by exposing the eye to a fresh lens every day; comfort is enhanced, deposition limited, and the need for care solutions and contact lens cases eliminated. Increased availability of daily disposable silicone hydrogel lenses across all types of prescriptions affords the eye care professional more choice. Oxygen transmissibility is an important consideration in the fitting of all patients but especially important in patients with high spherical prescriptions or those needing toric designs where the thickness of their hydrogel lens may reduce oxygen transmissibility and cause hypoxic changes. We can trial different materials to establish the best option for a patient and offer different price points. After nearly twenty years we have more knowledge about silicone hydrogel materials than ever before. Keep in mind that silicone allergy is not biologically possible, and that the numerous changes which have occurred over time enable as many patients as possible to find a silicone hydrogel material that works well for them.
Lyndon Jones is Professor at the School of Optometry & Vision Science, Director, Centre for Ocular Research & Education, University of Waterloo where Karen Walsh is a research scientist.
This article has been supported by CooperVision UK
References
- Sweeney DF. Have silicone hydrogel lenses eliminated hypoxia? Eye Contact Lens 2013;39:53-60.
- Hall BJ, Jones LW, Dixon B. Silicone allergies and the eye: fact or fiction? Eye Contact Lens 2014;40:51-7.
- Iwasaki A, Medzhitov R. Regulation of adaptive immunity by the innate immune system. Science 2010;327:291-5.
- Ballow M, Donshik PC, Rapacz P, Maenza R, Yamase H, Muncy L. Immune responses in monkeys to lenses from patients with contact lens induced giant papillary conjunctivitis. CLAO J 1989;15:64-70.
- Porazinski AD, Donshik PC. Giant papillary conjunctivitis in frequent replacement contact lens wearers: a retrospective study. CLAO J 1999;25:142-7.
- Sorbara L, Jones L, Williams-Lyn D. Contact lens induced papillary conjunctivitis with silicone hydrogel lenses. Cont Lens Anterior Eye 2009;32:93-6.
- Skotnitsky CC, Naduvilath TJ, Sweeney DF, Sankaridurg PR. Two presentations of contact lens-induced papillary conjunctivitis (CLPC) in hydrogel lens wear: local and general. Optom Vis Sci 2006;83:27-36.
- Jones L, Subbaraman LN, Rogers R, Dumbleton K. Surface treatment, wetting and modulus of silicone hydrogels. Optician 2006;232:28 - 34.
- Horst CR, Brodland B, Jones LW, Brodland GW. Measuring the modulus of silicone hydrogel contact lenses. Optom Vis Sci 2012;89:1468-76.
- Tighe BJ. A decade of silicone hydrogel development: surface properties, mechanical properties, and ocular compatibility. Eye Contact Lens 2013;39:4-12.
- Dumbleton K. Noninflammatory silicone hydrogel contact lens complications. Eye Contact Lens 2003;29:S186-9; discussion S90-1, S92-4.
- Dumbleton K. Adverse events with silicone hydrogel continuous wear. Cont Lens Anterior Eye 2002;25:137-46.
- Holden BA, Stephenson A, Stretton S, Sankaridurg PR, O'Hare N, Jalbert I, Sweeney DF. Superior epithelial arcuate lesions with soft contact lens wear. Optom Vis Sci 2001;78:9-12.
- Dumbleton KA, Chalmers RL, McNally J, Bayer S, Fonn D. Effect of lens base curve on subjective comfort and assessment of fit with silicone hydrogel continuous wear contact lenses. Optom Vis Sci 2002;79:633-7.
- Dumbleton K, Jones L, Chalmers R, Williams-Lyn D, Fonn D. Clinical characterization of spherical post-lens debris associated with lotrafilcon high-Dk silicone lenses. CLAO J 2000;26:186-92.
- Pritchard N, Jones L, Dumbleton K, Fonn D. Epithelial inclusions in association with mucin ball development in high-oxygen permeability hydrogel lenses. Optom Vis Sci 2000;77:68-72.
- Chalmers RL, Keay L, McNally J, Kern J. Multicenter case-control study of the role of lens materials and care products on the development of corneal infiltrates. Optom Vis Sci 2012;89:316-25.
- Chalmers RL, Wagner H, Mitchell GL, Lam DY, Kinoshita BT, Jansen ME, Richdale K, Sorbara L, McMahon TT. Age and other risk factors for corneal infiltrative and inflammatory events in young soft contact lens wearers from the Contact Lens Assessment in Youth (CLAY) study. Invest Ophthalmol Vis Sci 2011;52:6690-6.
- Chalmers RL, Keay L, Long B, Bergenske P, Giles T, Bullimore MA. Risk factors for contact lens complications in US clinical practices. Optom Vis Sci 2010;87:725-35.
- Szczotka-Flynn L, Diaz M. Risk of corneal inflammatory events with silicone hydrogel and low dk hydrogel extended contact lens wear: a meta-analysis. Optom Vis Sci 2007;84:247-56.
- Radford CF, Minassian D, Dart JK, Stapleton F, Verma S. Risk factors for nonulcerative contact lens complications in an ophthalmic accident and emergency department: a case-control study. Ophthalmology 2009;116:385-92.
- Jones L, MacDougall N, Sorbara LG. Asymptomatic corneal staining associated with the use of balafilcon silicone-hydrogel contact lenses disinfected with a polyaminopropyl biguanide-preserved care regimen. Optom Vis Sci 2002;79:753-61.
- Andrasko G, Ryen K. A series of evaluations of MPS and silicone hydrogel lens combinations. Rev Cornea and Contact Lenses 2007;143:36 - 42.
- Carnt N, Jalbert I, Stretton S, Naduvilath T, Papas E. Solution toxicity in soft contact lens daily wear is associated with corneal inflammation. Optom Vis Sci 2007;84:309-15.
- Papas EB, Carnt N, Willcox MD, Holden BA. Complications associated with care product use during silicone daily wear of hydrogel contact lens. Eye Contact Lens 2007;33:392-3; discussion 9-400.
- Andrasko G, Ryen K. Corneal staining and comfort observed with traditional and silicone hydrogel lenses and multipurpose solution combinations. Optometry 2008;79:444-54.
- Carnt N, Evans V, Holden B, Naduvilath T, Tilia D, Papas E, Willcox M. IER matrix update: adding another silicone hydrogel. Contact Lens Spectrum 2008;23:28 - 35.
- Carnt NA, Evans VE, Naduvilath TJ, Willcox MD, Papas EB, Frick KD, Holden BA. Contact lens-related adverse events and the silicone hydrogel lenses and daily wear care system used. Arch Ophthalmol 2009;127:1616-23.
- Willcox MD, Phillips B, Ozkan J, Jalbert I, Meagher L, Gengenbach T, Holden B, Papas E. Interactions of lens care with silicone hydrogel lenses and effect on comfort. Optom Vis Sci 2010;87:839-46.
- Woods J, Jones LW. Pilot Study to Determine the Effect of Lens and Eye Rinsing on Solution-Induced Corneal Staining (SICS). Optom Vis Sci 2016;93:1218-27.
- Zhang X, Marchetti C, Lee J, Sun Y, Debanne S, Jiang Y, Kern J, Harrod M, Benetz BA, Pearlman E, Szczotka-Flynn L. The impact of lens care solutions on corneal epithelial changes during daily silicone hydrogel contact lens wear as measured by in vivo confocal microscopy. Cont Lens Anterior Eye 2017;40:33-41.
- Willcox MD, Carnt N, Diec J, Naduvilath T, Evans V, Stapleton F, Iskandar S, Harmis N, Lazon de la Jara P, Holden BA. Contact lens case contamination during daily wear of silicone hydrogels. Optom Vis Sci 2010;87:456-64.
- Wu YT, Teng YJ, Nicholas M, Harmis N, Zhu H, Willcox MD, Stapleton F. Impact of lens case hygiene guidelines on contact lens case contamination. Optom Vis Sci 2011;88:E1180-7.
- Dantam J, McCanna DJ, Subbaraman LN, Papinski D, Lakkis C, Mirza A, Berntsen DA, Morgan P, Nichols JJ, Jones LW, Performance of Contact Lens Solutions Study G. Microbial Contamination of Contact Lens Storage Cases During Daily Wear Use. Optom Vis Sci 2016;93:925-32.
- Willcox MD. Solutions for care of silicone hydrogel lenses. Eye Contact Lens 2013;39:24-8.
- Truong TN, Graham AD, Lin MC. Factors in contact lens symptoms: evidence from a multistudy database. Optom Vis Sci 2014;91:133-41.
- Keir N, Jones L. Wettability and silicone hydrogel lenses: a review. Eye Contact Lens 2013;39:100-8.
- Lorentz H, Rogers R, Jones L. The impact of lipid on contact angle wettability. Optom Vis Sci 2007;84:946-53.
- Read ML, Morgan PB, Kelly JM, Maldonado-Codina C. Dynamic contact angle analysis of silicone hydrogel contact lenses. J Biomater Appl 2011;26:85-99.
- Menzies KL, Jones LW. Sessile drop contact angle analysis of hydrogel and silicone hydrogel daily disposable and frequent replacement contact lenses. Contact Lens and Anterior Eye 2012;35, Supplement 1:e12-e3.
- Lira M, Silva R. Effect of Lens Care Systems on Silicone Hydrogel Contact Lens Hydrophobicity. Eye Contact Lens 2017;43:89-94.
- Carney FP, Nash WL, Sentell KB. The adsorption of major tear film lipids in vitro to various silicone hydrogels over time. Invest Ophthalmol Vis Sci 2008;49:120-4.
- Walther H, Lorentz H, Kay L, Heynen M, Jones L. The effect of in vitro lipid concentration on lipid deposition on silicone hydrogel and conventional hydrogel contact lens materials. Contact Lens and Anterior Eye 2011;34, Supplement 1:S21.
- Lorentz H, Heynen M, Trieu D, Hagedorn SJ, Jones L. The impact of tear film components on in vitro lipid uptake. Optom Vis Sci 2012;89:856-67.
- Pucker AD, Thangavelu M, Nichols JJ. In vitro lipid deposition on hydrogel and silicone hydrogel contact lenses. Invest Ophthalmol Vis Sci 2010;51:6334-40.
- Walther H, Subbaraman L, Jones LW. In Vitro Cholesterol Deposition on Daily Disposable Contact Lens Materials. Optom Vis Sci 2016;93:36-41.
- Senchyna M, Jones L, Louie D, May C, Forbes I, Glasier MA. Quantitative and conformational characterization of lysozyme deposited on balafilcon and etafilcon contact lens materials. Curr Eye Res 2004;28:25-36.
- Subbaraman LN, Glasier MA, Senchyna M, Sheardown H, Jones L. Kinetics of in vitro lysozyme deposition on silicone hydrogel, PMMA, and FDA groups I, II, and IV contact lens materials. Curr Eye Res 2006;31:787-96.
- Luensmann D, Jones L. Protein deposition on contact lenses: the past, the present, and the future. Cont Lens Anterior Eye 2012;35:53-64.
- Hall B, Jones L, Forrest JA. Kinetics of Competitive Adsorption between Lysozyme and Lactoferrin on Silicone Hydrogel Contact Lenses and the Effect on Lysozyme Activity. Curr Eye Res 2015;40:622-31.
- Omali NB, Subbaraman LN, Coles-Brennan C, Fadli Z, Jones LW. Biological and Clinical Implications of Lysozyme Deposition on Soft Contact Lenses. Optom Vis Sci 2015;92:750-7.
- Heynen M, Babaei Omali N, Fadli Z, Coles-Brennan C, Subbaraman LN, Jones L. Selectivity and localization of lysozyme uptake in contemporary hydrogel contact lens materials. J Biomater Sci Polym Ed 2017;28:1351-64.
- Nichols JJ. Deposition on silicone hydrogel lenses. Eye Contact Lens 2013;39:19-22.
- Suwala M, Glasier MA, Subbaraman LN, Jones L. Quantity and conformation of lysozyme deposited on conventional and silicone hydrogel contact lens materials using an in vitro model. Eye Contact Lens 2007;33:138-43.
- Subbaraman LN, Jones L. Kinetics of lysozyme activity recovered from conventional and silicone hydrogel contact lens materials. J Biomater Sci Polym Ed 2010;21:343-58.
- Jones L, Brennan NA, Gonzalez-Meijome J, Lally J, Maldonado-Codina C, Schmidt TA, Subbaraman L, Young G, Nichols JJ, members of the TIWoCLD. The TFOS International Workshop on Contact Lens Discomfort: report of the contact lens materials, design, and care subcommittee. Invest Ophthalmol Vis Sci 2013;54:TFOS37-70.
- Garofalo RJ, Dassanayake N, Carey C, Stein J, Stone R, David R. Corneal staining and subjective symptoms with multipurpose solutions as a function of time. Eye Contact Lens 2005;31:166 - 74.
- Diec J, Evans VE, Tilia D, Naduvilath T, Holden BA, Lazon de la Jara P. Comparison of ocular comfort, vision, and SICS during silicone hydrogel contact lens daily wear. Eye Contact Lens 2012;38:2-6.
- Lazon de la Jara P, Papas E, Diec J, Naduvilath T, Willcox MD, Holden BA. Effect of lens care systems on the clinical performance of a contact lens. Optom Vis Sci 2013;90:344-50.
- Morgan PB, Brennan NA, Maldonado-Codina C, Quhill W, Rashid K, Efron N. Central and peripheral oxygen transmissibility thresholds to avoid corneal swelling during open eye soft contact lens wear. J Biomed Mater Res B Appl Biomater 2010;92:361-5.
- Jones L, Franklin V, Evans K, Sariri R, Tighe B. Spoilation and clinical performance of monthly vs. three monthly Group II disposable contact lenses. Optom Vis Sci 1996;73:16-21.
- Morgan PB, Woods C, Tranoudis I, Helland M, Efron N, Jones L, Nelson L, Merchan B, Ing M, van Beusekom M, Grupcheva CN, Jones D, Beeler-Kaupke M, Krasnanska J, Pult H, Tast P, Ravn O, Santodomingo J, Malet F, Plakitis A, Vegh M, Shing C, Erdinest N, Jafari A, Montani G, Itoi M, Bendoriene J, Ziziuchin V, van der Worp E, Lam W, Ystenaes AE, Romualdez-Oo J, Abesamis-Dichoso C, Gonzalez-Meijome JM, Sim D, Silih M, Hsiao J, Nichols J. International Contact Lens Prescribing in 2017. Contact Lens Spectrum 2018;33:28-33.
- Diec J, Tilia D, Thomas V. Comparison of Silicone Hydrogel and Hydrogel Daily Disposable Contact Lenses. Eye Contact Lens 2017;In press.
- Ruiz-Alcocer J, Monsalvez-Romin D, Garcia-Lazaro S, Albarran-Diego C, Hernandez-Verdejo JL, Madrid-Costa D. Impact of contact lens material and design on the ocular surface. Clin Exp Optom 2017;In press.
- Chalmers RL, Hickson-Curran SB, Keay L, Gleason WJ, Albright R. Rates of adverse events with hydrogel and silicone hydrogel daily disposable lenses in a large postmarket surveillance registry: The TEMPO Registry. Invest Ophthalmol Vis Sci 2015;56:654-63.
- Varikooty J, Keir N, Richter D, Jones LW, Woods C, Fonn D. Comfort response of three silicone hydrogel daily disposable contact lenses. Optom Vis Sci 2013;90:945-53.
- Varikooty J, Schulze MM, Dumbleton K, Keir N, Woods CA, Fonn D, Jones LW. Clinical performance of three silicone hydrogel daily disposable lenses. Optom Vis Sci 2015;In press.
- Szczesna-Iskander DH. Comparison of tear film surface quality measured in vivo on water gradient silicone hydrogel and hydrogel contact lenses. Eye Contact Lens 2014;40:23-7.
- Wolffsohn JS, Mroczkowska S, Hunt OA, Bilkhu P, Drew T, Sheppard A. Crossover Evaluation of Silicone Hydrogel Daily Disposable Contact Lenses. Optom Vis Sci 2015;92:1063-8.
