There is no doubt that age-related macular degeneration (AMD) has a multifactorial aetiology with a variety of risk factors. Several epidemiological studies across the world have demonstrated how smoking is a major modifiable risk factor. It is frequently associated with more severe forms of AMD,1-4 and increases the likelihood of both eyes being affected.5
Does it matter how long someone has smoked?
Smoking status and history is important and, although currently lacking a standardised definition, generally falls into three categories:
- Current smoker
- Previous smoker
- Never smoked
Several studies have compared different levels of smoking, classified as pack-years, and most of them confirmed a dose-response effect for AMD.4 Pack-years smoked better reflects the amount of exposure over a lifetime of smoking.6 To calculate pack-years of smoking, the average of the number of cigarettes smoked per day is divided by 20 to give packs per day and multiplied by the total number of years of smoking.7,8
Current-smokers have two to four-fold increase in risk for developing AMD when compared to patients that never smoked.9-11 Current and former smokers were found to have an increased prevalence of late AMD, although a recent study also found an association of early AMD features with smoking.12 The increased AMD risk appears to be higher in those patients who have smoked 20 pack-years and more.4,13 There is bad news for people living with smokers too: passive smoking, ie living with a smoker for five years or more, increases the risk for AMD among non-smokers.9,14
Why is cigarette smoke so harmful for the retina?
Cigarette smoke is comprised of a gas and tar phase containing over 4,700 chemicals, with a high concentration of free radicals.15,16 Gas-phase radicals include reactive oxygen species (ROS), epoxides, peroxides, nitric oxide (NO), nitrogen dioxide and various other free radicals. It has been estimated that each puff of a cigarette contains 1,015 free radicals, including superoxide anion and nitric oxide, that combine to form peroxynitrite, which is a potent oxidant that initiates lipid peroxidation.17
Pathophysiology of Smoking
Cigarette smoke promotes molecular and pathological changes that may establish the ideal macular microenvironment for the development of AMD4 and has been linked to cellular changes in all retinal layers 3 particularly the retinal pigment epithelium (RPE).4,18 These cellular changes involve a variety of mechanisms including vascular inflammation and endothelial dysregulation,4,19 oxidative damage,20-22 toxic damage, and histopathological changes.4,23
Cigarette smoke also induces ‘pro-inflammatory’ changes in the RPE through an important part of the immune system: increased expression of complement activation products with reduced expression of complement regulators.24,25 Cigarette smoke contains a large number of pro-oxidant compounds that increase oxidative stress, resulting in damage to the RPE and the alterations in the metabolic support of the RPE, ultimately causing apoptosis of the photoreceptors.26 Oxidative stress is also thought to be pivotal in lipofuscin and drusen formation.4,27 Examples of pro-oxidant compounds in cigarette smoke include nicotine 28 and cadmium,29 though the most abundant is hydroquinone which is commonly found in processed foods, plastic containers and atmospheric pollutants.30,31 Nicotine specifically has been found to cause vasoconstriction and is thought to impair choroidal blood flow.19 Hydroquinone is thought to be a key factor in the pathogenesis of dry AMD22 as well in the development of CNV.32 Cigarette smoke can also cause toxic damage of the mitochondrial DNA within the RPE cell, contributing to the formation of drusen in individuals who are cigarette smokers.4,33,34
Although the role of genetic testing is considered a research rather than a clinical tool at this moment, studies show a correlation between smoking and late AMD which appears greater in patients with certain higher risk genetic profiles, particularly linked to genes encoding for complement factor H (CFH) and age-related maculopathy susceptibility (ARMS),35 and more recently in smokers with certain variants in nitric oxide synthase 2A (NOS2A).36
E-Cigarettes
A trend towards electronic cigarettes (ECs) is emerging as a potentially ‘safer alternative’ to conventional cigarettes. E-cigarettes were developed in China in the early 2000s and have gained in popularity.37,38 They are designed to deliver nicotine vapour without the toxic constituents of tobacco or tobacco combustion toxicants and carcinogens.39
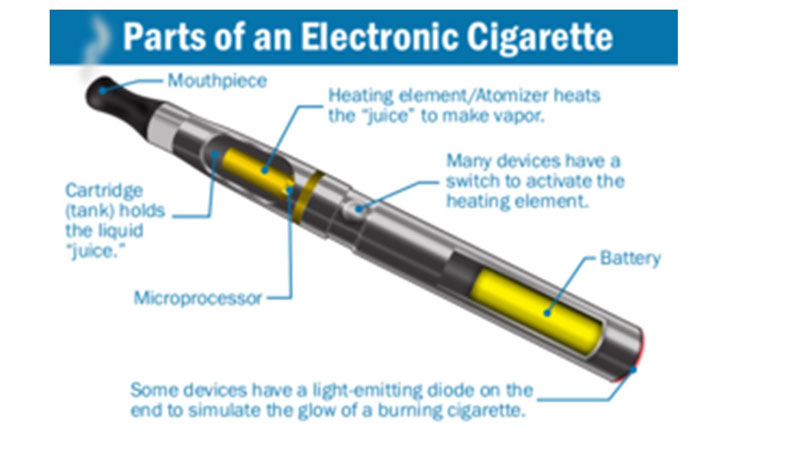
Figure 1: Components of an e-cigarette
E-cigarettes have three components: the battery, the heating element or atomizer, and a cartridge or tank (figure 1). The tank holds a nicotine solution dissolved in propylene glycol, glycerol, or both, plus nicotine and a vast array of flavourant chemicals, ranging from fruit, chocolate, gummy bear, to nicotine and others (figure 2).40 These elements are housed in a cylindrical container with some models looking very similar to traditional cigarettes, while others look like large pens with mouthpieces.

Figure 2: E-cigarettes suport a wide variety of flavours
When an EC user takes a puff, the nicotine solution is heated and the vapor can be inhaled into lungs, where nicotine is rapidly delivered to the brain.38,41 ECs are tobacco products that deliver nicotine and so carry the risk of addiction42 and the potential to harm adolescent brain development.40
Research regarding the benefits and risks of ECs are ongoing,43 There has been much debate recently over whether ECs offer the possibility of reducing the harm (of lung cancer, cardiovascular disease and respiratory disease) for people who cannot, or will not, quit smoking conventional cigarettes, or whether they threaten the goal of further reducing tobacco smoking with particular impact on the young population. There appear to be two schools of thought; that of harm minimisation and that of a precautionary approach. Although there is some commonality, advocates on both sides have drawn conclusions that are often conflicting, creating a global divide on policy strategies.44
Although ECs deliver lower levels of carcinogens than do conventional cigarettes, and therefore may pose less cancer risk to users (albeit not zero cancer risk),45,46,47 they still expose users to ultrafine particles and other toxins that may substantially increase cardiovascular48,49 and non-cancer lung disease risk50,51.
There is conclusive evidence that in addition to Nicotine, most EC products contain and emit numerous potentially toxic substances,42 although it emits fewer toxicants than combustible tobacco products.
Research is currently lacking on the effect of nicotine and eye health and EC use specifically, although lab studies have provided a link with wet macular degeneration 52 as well as risks of progression of wet AMD in passive smokers due to its effect on human retinal pigment epithelium (RPE).53 The College of Optometrists has called for greater research into the ocular effects of ECs.54
Public Health England (PHE) recently published a review of the evidence on ECs Feb 6, 2018.55 ECs are not risk free and appropriate regulation is important. Policy on ECs in countries in the Organisation for Economic Co-operation and Development (OECD) is converging on a cautiously permissive approach.56 The conclusions of a previous PHE report 2015 57 were controversial (often cited as the ‘95% safe’ debate) in providing qualified support for use of ECs, contributing to a shift in international opinion.58
The PHE recent review of the evidence on ECs covers regulation, patterns of EC use, effectiveness of ECs in smoking cessation, and safety of ECs, nicotine, and novel heat-not-burn tobacco products.55
Official guidance in the USA on ECs has been more precautionary, focusing on the potential risks, such as encouragement of youth smoking and the addictive nature of nicotine.59
Of general concern is rapid increase in ECs among adolescents. In the US, adolescents using EC has risen 900% from 2011 to 2015, with e-cigarettes becoming the most commonly used form of tobacco among the youth.40 Although ECs were designed to aid in smoking cessation, this is not the primary reason for use among youth and young adults.40 It is suggested that EC usage among youth in the US leads to progression (‘gateway’ behaviour) to traditional cigarette smoking,42,60 however this is not the conclusion drawn by PHE (2018).55
However, on core issues agreement is good between US59 and UK61 official communications on ECs: both state that ECs can benefit smokers who are able to switch completely from smoked tobacco; neither recommend ECs for young people or non-smokers, supporting regulations to limit sales and decrease the appeal of e-cigarettes to adolescents and young adults; both agree ECs are not risk free and that more research is needed.
Specifically, the PHE’s new review states that the evidence on ECs is incomplete but sufficient to guide current policy and continues to support the assumption of reduced risk of harm for ECs compared with smoking.56 This has been echoed by the National Academies of Science and Engineering and Medicine (NASEM) recent review where the chair of the committee that wrote the report concluded ‘E-cigarettes cannot be simply categorised as either beneficial or harmful. In some circumstances, such as their use by non-smoking adolescents and young adults, their adverse effects clearly warrant concern. In other cases, such as when adult smokers use them to quit smoking, they offer an opportunity to reduce smoking-related illness.62
In January this year, the US Annual Review of Public Health journal discussed evidence for a ‘gateway’ effect.63 Research into e-cigarettes experimentation in the young transitioning into established smoking began in 2011,64 with more recent studies as well as the US Surgeon General report (2016)40 supporting the gateway effect.’60,65,66 Some researchers have stated e-cigarettes lead young people to cigarette use and nicotine addiction. Of concern, is that this pathway appears to be is a ‘one-way street’, whereby e-cigarette products were not part of young people’s efforts to quit.67 It appears that lower-risk youth are being brought into the e-cigarette market, many of whom then transition to smoking cigarettes.66 The 2015 US National Youth Tobacco Survey 68 confirms this process may be starting.
Substantial evidence reveals that flavouring encourages adolescent use,69,70,71 some citing 95% first e-cigarettes use by adolescents being flavoured compared to 44.1% of older adults71 Recent reviews in the UK (Public Health England) and in the USA (National Academies of Sciences and Engineering and Medicine (NASEM) ) have supported more research into the potential risks from less well studied toxicants in many flavouring chemicals and device-specific metals.42,55
The NASEM report concluded substantial evidence of e-cigarettes acting a gateway to smoking among the youth and young adults,42 the PHE report agreed that while young people who use ECs may go on to try smoking, this had no detectable effect on youth smoking rates and has not interrupted declining trends in youth smoking in many countries.55
EC regulations have been revised recently. Falling into two categories, EC can be considered as a tobacco product and regulated under the newly revised Tobacco and Related Products Regulations 2016,72 with rules governing the manufacture, marketing and sale, or, as a medical product with licensing under the Medicines and Health Care products Regulation Agency (MHRA). Upon approval, successful applicants can license and market their product as a healthier alternative and effective at helping smokers quit,73 opening the possibility for prescribing under the NHS. An example being the MHRA’s approval of British American Tobacco’s (BAT) e-cigarette e-Voke (Nov 21, 2015),74 although BAT recently decided (Feb 2018) not bring the product to market.75
Studies have shown that adding ECs to tobacco smoking, or dual usage, did not facilitate smoking cessation or reduction. If e-cigarette safety is confirmed, the use of EC alone may facilitate smoking cessation.42,76,77,78 In terms of policy, all smokers should be supported to stop smoking completely, including those who smoke and use ECs. Support from smoking cessation clinics remains important in helping smokers to quit.61
There is at present little guidance offered by the Royal College of Ophthalmology or College of Optometrists79 regarding e-cigarettes. Recent guidance from the Royal College of Physicians (2016) however, supports the use of EC as a mode of harm prevention in smokers.39
More research is needed regarding EC. It is apparent that they are a growing part of the public health landscape and changing the way we view nicotine addiction. Health care providers should be more proactive in addressing nicotine and tobacco addiction with patients.41
The ‘95% Safer’ Debate
Public Health England, an agency of the Department of Health, produced a report entitled, E-cigarettes: an evidence update (2015)80 supported by many UK public health organisations (including Cancer Research, ASH, British Lung Foundation and Royal College of Physicians). The PHE report is built upon the idea that e-cigarettes may potentially represent a product that can reduce harm for established smokers. The report attracted international publicity39,44,80 stating that e-cigarettes are 95% less harmful than combustible cigarettes.
The ‘95% less harmful’ claim originated from a consensus meeting of 12 people convened by Professor DJ Nutt in 2014.81,82 They reached this conclusion without citing any specific evidence.83 The Nutt et al paper did include the caveat: ‘A limitation of this study is the lack of hard evidence for the harms of most products on most of the criteria’ (82 p. 224), which has generally been ignored by those quoting this report.84,85,86,87 Both the Lancet and the British Medical Journal questioned the PHE findings at the time.88,89
The ‘95% safer’ figure remains widely quoted, despite the fact that evidence of the potential dangers of e-cigarette use have accumulated since 2014. This new evidence indicates that the true risk of e-cigarette may be much higher than the ‘95% safer’ claim would indicate.81
What’s the future for smoking?

Figure 3: The IQOS device
Heat-not-burn (HNB) tobacco products are the latest nicotine-containing innovation offered to smokers by the tobacco industry.90 The most well-known device being the IQOS (I Quit Ordinary Smoking) by Philip Morris International (PMI).91 This sleek electronic device (figure 3) became available in England in December 2016 92 being available from dedicated stores that look more like premium electronics outlets stores,93 and some independent retailers (figure 4).

Figure 4: Apple store, left, and IQOS store, right
These devices consist of disposable tobacco sticks soaked in propylene glycol, which are inserted in a holder in the HNB cigarette. The tobacco is heated with an electric blade at 350°C (compared to 600°C to burn tobacco). The cigarettes are marketed by PMI as a ‘revolutionary technology that heats tobacco without burning it, giving you the true taste of tobacco, with no smoke, no ash and less smell’,94 thereby producing less toxins overall than conventional cigarettes. There are currently few independent studies on heat not burn products. When levels of these toxins and carcinogens were measured independently, they appeared to be a lot higher than the manufacturer had claimed.91,95
The FDA rejected the claim that PMI should be allowed to claim its iQOS electronic tobacco device can reduce the risk of tobacco-related diseases compared with traditional cigarettes.96 PHE states the limited evidence on environmental emissions from use of these products suggests that harmful exposure from heated tobacco products is higher than from e-cigarettes, but further evidence is needed to be able to compare products.55
What Can I Do as an Eye Care Professional?
Smoking is a major risk factor and that is something that we should discuss with all patients. Given that smoking increases the risk of AMD at least two to four-fold,4,97 discussing its cessation is an important recommendation due to its potential to alter AMD progression.98,99
As previously mentioned, ex-smokers still have an increased risk of developing AMD compared with never smokers,4 as does living with a smoker for at least five years.9 Perhaps rather alarmingly, studies have shown that the risk of late AMD remains present to some degree for up to 20 years after stopping smoking.4,13,100
Recently, genetic testing has arisen as an option to provide patients with a certain risk profile based on high-risk genes for AMD.4 Such information might motivate patients to quit smoking.101-104 At present, the majority of the peer-reviewed evidence suggests that genetic testing is more useful as a research tool than in Clinical management of patients although this may change in the future.105 Even patients with neovascular AMD who smoke should be advised to quit, as their response to anti-VEGF intravitreal therapy is poorer than in non-smokers.106
Public awareness of the link between smoking and ocular health is lacking.107-109 The role of eye care professionals is
therefore critical role in educating the public and encouraging smokers to quit.110 Awareness of the risk of blindness from smoking is low among teenagers, but fear of blindness may be more likely to motivate teenagers to stop smoking than fear of lung or heart disease.108
A recent survey into patients perceptions and experience of an eye examination revealed they expected their eye care professional to examine their eye health, ask them about their smoking and diet habits, and indicated feeling comfortable discussing these topics with their primary eye care provider.111 These findings suggest that brief momentary interventions relating to tobacco use and diet are likely to be acceptable to deliver in both ophthalmology and optometry settings.111 As nicotine can be considered a drug, any discussions about tobacco use needs to begin with understanding that smoking is generally not a ‘bad habit’, but a physical addiction112 with a high percentage (70%) of relapse.113 Most people who smoke require several ‘quit attempts’ before they are successful.114,115 Some studies quote patient compliance as low as 0%.116 In a 10 year study only one in four current smokers with any AMD at baseline quit smoking at five years and were still not smoking at 10 years; with 50% still smoking after 10 years.97 Almost one third of adults smokers were either unaware or had difficulty understanding the effects of smoking on ocular health. It is therefore unlikely that any single health care provider or one single discussion will manage a patient’s addiction, and that patients will likely receive ongoing support from a range of providers including optometrists.112 There is strong evidence that such advice and support can be effective in helping patients quit, and for many healthcare professional bodies, advice on smoking and cessation forms part of a duty of care.117,118
Time constraints and a perceived need for further training in this area4,119 may be why there is no consistent approach among clinicians in documenting patients’ smoking history or advising on smoking cessation.110,111,120
The College of Optometrists has raised smoking awareness through its Lifestyles and eyes, Cataracts and Macular degeneration leaflets. This, coupled with guidelines for other primary care providers, can be adapted into an optometric setting.112 Some focus on connecting a patient with a specific cessation support service, while other approaches rely on the provider to personally support patient cessation.112 Studies in UK optometric settings have demonstrated the feasibility of delivering brief smoking cessations with good success 122 and optometrists and trainees 123 should be encouraged to find out what cessation supports are available in their community.112
A variety of training programmes exist for all levels of healthcare professionals (see below). Of particular interest to optometrists is the National Centre for Smoking Cessation and Training (NCSCT) which offers a ‘Very Brief Advice’ module aimed at clinicians who provide smoking cessation and support as a small part of their wider role.124 These web-based programmes include video simulations of discussing smoking and its cessation in a variety of patient scenarios. See table 1.

Table 1: Very Brief Advice (VBA – ‘Ask, Advise, Act’)
Stopping smoking is tough. Smokers know they should stop, yet often they do not know how, or that support is available. There are many factors influencing a patient’s ability to quit, and although offering VBA can be influential, it may not result in a quit attempt at that time, however that should not prevent the clinician presenting it at subsequent visits.124
With the proper training and resources, it is certain that the optometric community can and will play a greater role in addressing tobacco-use cessation among patients. Many community pharmacists are knowledgeable and can offer advice over the counter. Strategies such as VBA, used as part of our everyday routine, can influence a patient’s potential success in quitting.112,124
In a case of smoking history alone, smoking cessation with a discussion of toward the possible benefit(s) of dietary modification, including natural consumption of the xanthophyll pigments has been advocated.125,126 AREDS formulations are only appropriate in intermediate or late AMD stage.127 If considering an AREDS based approach in a smoker, one must balance the possible risks with the benefits of the intervention.128 Smokers who fit the AREDS criteria should be cautioned against consumption of the original AREDS formulation containing high-dose beta-carotene supplementation, because of the potential increased risk of lung cancer in current smokers and former smokers.129-131 More recently, The AREDS2 Study suggested that lutein and zeaxanthin could be a safe alternative carotenoid substitute in the AREDS formulation.132
Summary
- Smoking is a major modifiable risk factor associated with AMD
- Smoking increases the risk of AMD two-four fold.
- Pack-years smoked better reflects the amount of exposure.
- Cigarette smoke is comprised of a gas and tar phase containing chemicals with a high concentration of free radicals
- Cigarette smoke induces ‘pro-inflammatory’ changes in the RPE, along with oxidative damage.
- Risk of AMD remains present up to 20 years after cessation of smoking.
- Passive smoking carries risk of AMD.
- Public awareness of the link between smoking and ocular health is lacking.
- Eye care providers have a duty of care to inform patients of the long-term ocular risk of smoking and AMD.
- Electronic cigarettes are considered ‘safer’ than conventional cigarettes, although more research is needed.
- Smoking cessation (using VBA) with a discussion of dietary modification is recommended.
- AREDS and AREDS II formulations only indicated in intermediate/late stage AMD.
- AREDS formula (beta carotene) carries increased risk of lung cancer in smokers.
- AREDS II: lutein+zeaxanthin a better substitute for beta carotene.
Dr Rohit Narayan is a therapeutic optometrist based in the Midlands.
Useful Websites
• Local stop smoking service (NHS): https://www.nhs.uk/smokefree
• Information on treatment options: https://www.nhs.uk/conditions/stop-smoking-treatments/aspx
• www.ncsct.co.uk
• www.makingeverycontactcount.co.uk
References
- JE Ardourel, “Risk factors associated with age-related macular degeneration: a case control study in the Age-Related Eye Disease Study: age-related eye disease Study report number 3,” Ophthalmology, vol. 107, no. 12, pp. 2224–2232, 2000
- Ângela Carneiro and José Paulo Andrade Nutritional and Lifestyle Interventions for Age-Related Macular Degeneration: A Review. Oxid Med Cell Longev. 2017; 2017: 6469138.
- Marisol Cano et al. Cigarette smoking, oxidative stress, the anti-oxidant response through Nrf2 signaling, and Age-related Macular DegenerationVision ResearchVolume 50, Issue 7, 31 March 2010, Pages 652–664
- Sara Velilla, José Javier García-Medina, Alfredo García-Layana, et al, “Smoking and Age-Related Macular Degeneration: Review and Update,” Journal of Ophthalmology, vol. 2013, Article ID 895147, 11 pages, 2013. doi:10.1155/2013/895147
- Joachim N, Colijn JM, Kifley A, et al Five-year progression of unilateral age-related macular degeneration to bilateral involvement: the Three Continent AMD Consortium report British Journal of Ophthalmology Published Online First: 20 January 2017. doi: 10.1136/bjophthalmol-2016-309729
- Cigarette Smoking and the Natural History of Age-related Macular Degeneration: the Beaver Dam Eye Study. Chelsea E Myers, Barbara EK Klein, Ronald Gangnon, Theru A Sivakumaran, Sudha K Iyengar, Ronald Klein. Ophthalmology. 2014 Oct; 121(10): 1949–1955.
- Bernaards CM, Twisk JW, Snel J et al Is calculating pack-years retrospectively a valid method to estimate life-time tobacco smoking? A comparison between prospectively calculated pack-years and retrospectively calculated pack-years. Addiction 2001961653–1661
- JCKhan, DA Thurlby, H Shahid, DG Clayton, JRW Yates, M Bradley, AT Moore, AC Bird. Smoking and age related macular degeneration: the number of pack years of cigarette smoking is a major determinant of risk for both geographic atrophy and choroidal neovascularisation. Br J Ophthalmol. 2006 Jan; 90(1): 75–80. doi: 10.1136/bjo.2005.073643
- W Smith, J Assink, R Klein et al, “Risk factors for age-related macular degeneration: pooled findings from three continents,” Ophthalmology, vol. 108, no. 4, pp. 697–704, 2001
- U Chakravarthy, C Augood, GC Bentham et al, “Cigarette smoking and age-related macular degeneration in the EUREYE Study,” Ophthalmology, vol. 114, no. 6, pp. 1157–1163, 2007.
- RD Jager, WF Mieler, and JW Miller, “Age-related macular degeneration,” The New England Journal of Medicine, vol. 358, no. 24, pp. 2606–2617, 2008
- Brandl C, Breinlich V, Stark KJ, Enzinger S, Aßenmacher M, Olden M, et al (2016) Features of Age-Related Macular Degeneration in the General Adults and Their Dependency on Age, Sex, and Smoking: Results from the German KORA Study. PLoS ONE 11(11): e0167181. doi:10.1371/journal.pone.0167181
- C Delcourt, J-L Diaz, A Ponton-Sanchez, and L Papoz, “Smoking and age-related macular degeneration: the POLA study,” Archives of Ophthalmology, vol. 116, no. 8, pp. 1031–1035, 1998.
- Smith W, Mitchell P, Leeder S R. Smoking and age-related maculopathy. The Blue Mountains Eye Study. Arch Ophthalmol 19961141518–1523
- T Rangasamy, CY Cho, et al Genetic ablation of Nrf2 enhances susceptibility to cigarette smoke-induced emphysema in mice. Journal of Clinical Investigation, 114 (9) (2004), pp. 1248–1259
- CJ Smith, C Hansch. The relative toxicity of compounds in mainstream cigarette smoke condensate. Food and Chemical Toxicology, 38 (7) (2000), pp. 637–646
- I Rahman, W MacNee. Role of oxidants/antioxidants in smoking-induced lung diseases. Free Radical Biology and Medicine, 21 (5) (1996), pp. 669–681
- F Roth, A Bindewald, and FG Holz, “Keypathophysiologic pathways in age-related macular disease,” Graefe's Archive for Clinical and Experimental Ophthalmology, vol. 242, no. 8, pp. 710–716, 2004.
- BQ Zhu and WW Parmley, “Hemodynamic and vascular effects of active and passive smoking,” American Heart Journal, vol. 130, no. 6, pp. 1270–1275, 1995
- C Heeschen, JJ Jang, M Weis et al, “Nicotine stimulates angiogenesis and promotes tumor growth and atherosclerosis,” Nature Medicine, vol. 7, no. 7, pp. 833–839, 2001.
- M Pons and ME Marin-Castaño, “Cigarette smoke-related hydroquinone dysregulates MCP-1, VEGF and PEDF expression in retinal pigment epithelium in vitro and in vivo,” PLoS ONE, vol. 6, no. 2, Article ID e16722, 2011.
- M Pons and ME Marin-Castaño Nicotine Increases the VEGF/PEDF Ratio in Retinal Pigment Epithelium: A Possible Mechanism for CNV in Passive Smokers with AMD. Invest Ophthalmol Vis Sci. 2011 May; 52(6): 3842–3853. Published online 2011 May 31. doi: 10.1167/iovs.10-6254
- WP Patton, MN Routledge, GD Jones et al, “Retinal pigment epithelial cell DNA is damaged by exposure to benzo[a]pyrene, a constituent of cigarette smoke,” Experimental Eye Research, vol. 74, no. 4, pp. 513–522, 2002
- Kijlstra A1, Berendschot TT. Age-related macular degeneration: a complementopathy?.Ophthalmic Res. 2015;54(2):64-73. doi: 10.1159/000432401
- Wang L, Kondo N, Cano M, Ebrahimi K, Yoshida T, Barnett BP, Biswal S, Handa JT: Nrf2 signaling modulates cigarette smoke-induced complement activation in retinal pigmented epithelial cells. Free Radic Biol Med 2014;70:155-166.
- S Beatty, HH Koh, M Phil, D Henson, and M Boulton, “The role of oxidative stress in the pathogenesis of age-related macular degeneration,” Survey of Ophthalmology, vol. 45, no. 2, pp. 115–134, 2000
- JR Sparrow and M Boulton, “RPE lipofuscin and its role in retinal pathobiology,” Experimental Eye Research, vol. 80, no. 5, pp. 595–606, 2005.
- J Lee and JP Cooke, “Nicotine and pathological angiogenesis,” Life Sciences, vol. 91, pp. 1058–1064, 2012.
- NK Wills, VMS Ramanujam, J Chang et al, “Cadmium accumulation in the human retina: effects of age, gender, and cellular toxicity,” Experimental Eye Research, vol. 86, no. 1, pp. 41–51, 2008
- PJ Deisinger, TS Hill, and JC English, “Human exposure to naturally occurring hydroquinone,” Journal of Toxicology and Environmental Health, vol. 47, no. 1, pp. 31–46, 1996.
- AP DeCaprio, “The toxicology of hydroquinone—relevance to occupational and environmental exposure,” Critical Reviews in Toxicology, vol. 29, no. 3, pp. 283–330, 1999
- IA Bhutto, DS McLeod, T Hasegawa et al, “Pigment epithelium-derived factor (PEDF) and vascular endothelial growth factor (VEGF) in aged human choroid and eyes with age-related macular degeneration,” Experimental Eye Research, vol. 82, no. 1, pp. 99–110, 2006.
- AH Conney, “Induction of microsomal enzymes for foreign chemicals and carcinogenesis by polycyclic aromatic hydrocarbons: GHA Clowes Memorial Lecture,” Cancer Research, vol. 42, no. 12, pp. 4875–4917, 1982
- TR Rajalakshmi, “DNA adducts-chemical addons” J Pharm Bioallied Sci. 2015 Apr; 7(Suppl 1): S197–S199
- Despriet DDG, Klaver CCW, Witteman JCM, Bergen AAB, Kardys I, de Maat MPM, Boekhoorn SS, Vingerling JR, Hofman A, Oostra BA, Uitterlinden AG, Stijnen T, van Duijn CM, de Jong P: Complement factor H polymorphism, complement activators, and risk of age-related macular degeneration. JAMA 2006; 296: 301–309.
- Ayala-Haedo JA, Gallins PJ, Whitehead PL, et al Analysis of single nucleotide polymorphisms in the NOS2A gene and interaction with smoking in age-related macular degeneration. Ann Hum Genet. 2010;74(3):195–201
- Nickels et al Ann Allergy Asthma Immunol. Jun 2014;112(6):481-3
- Nicotine Tob Res (2013) 16 (6): 655-662.
- 2016 Royal College of Physicians https://www.rcplondon.ac.uk/projects/outputs/nicotine-without-smoke-tobacco-harm-reduction-0
- 2016 Surgeon General’s Report: E-Cigarette Use Among Youth and Young Adults https://www.cdc.gov/tobacco/data_statistics/sgr/e-cigarettes/index.htm
- Nickels S. E-cigarettes, tobacco cessation, and the role of HCPs. Optometry Times 2015. http://optometrytimes.modernmedicine.com/optometrytimes/news/e-cigarettes-tobacco-cessation-and-role-hcps?page=full
- National Academies of Sciences and Engineering and Medicine. Committee on the Review of the Health Effects of Electronic Nicotine Delivery Systems. Public health consequences of e-cigarettes. ((accessed March 29th, 2018).)The National Academies Press, Washington, DC; 2018 https://www.nap.edu/catalog/24952/public-health-consequences-of-e-cigarettes
- Lerner CA et al. Electronic cigarette aerosols and copper nanoparticles induce mitochondrial stress and promote DNA fragmentation in lung fibroblasts. Biochem Biophys Res Commun 2016 477(4):620-625
- Green S et al. Evidence, Policy, and E-Cigarettes — Will England Reframe the Debate? N Engl J Med 2016; 374:1301-1303
- Combes RD, Balls M. 2015. On the safety of e-cigarettes: “I can resist anything except temptation.” Altern. Lab. Anim. 43:417–25
- Goniewicz ML, Knysak J, Gawron M, Kosmider L, Sobczak A, et al. 2014. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob. Control 23:133–39
- Shahab L, Goniewicz ML, Blount BC, Brown J, McNeill A, et al. 2017. Nicotine, carcinogen, and toxin exposure in long-term e-cigarette and nicotine replacement therapy users: a cross-sectional study. Ann.Intern. Med.166:390–400
- Bhatnagar A. 2016. E-cigarettes and cardiovascular disease risk: evaluation of evidence, policy implications, and recommendations. Curr. Cardiovasc. Risk Rep.10:24
- Schweitzer RJ, Wills TA, Behner D. 2017. E-cigarette use and indicators of cardiovascular disease risk. Curr. Epid. Rep. 4:248–57
- Moazed F, Calfee CS. 2017. The canary in the coal mine is coughing: electronic cigarettes and respiratory symptoms in adolescents.Am. J. Respir. Crit. Care Med.195:974–76
- Moretto N, Volpi G, Pastore F, Facchinetti F. 2012. Acrolein effects in pulmonary cells: relevance to chronic obstructive pulmonary disease.Ann. N. Y. Acad. Sci.1259:39–46
- Joint Statement of RNIB, EBU, AMD Alliance International and Royal College of Ophthalmology 8 March 2013 http://www.rnib.org.uk/aboutus/mediacentre/mediareleases/mediareleases2012/Pages/pressrelease8march2012e.aspx
- Suner IJ, Cousins SW (2006) The Biology of Smoking and AMD, Review of Ophthalmology, available online at http://www.revophth.com/content/d/retinal_insider/i/1303/c/25086/
- https://www.college-optometrists.org/the-college/media-hub/college-in-the-news/the-increased-risk-of-blindness-in-smokers.html
- McNeill, A, Brose, LS, Calder, R et al. Evidence review of e-cigarettes and heated tobacco products: a report commissioned by Public Health England. ((accessed March 29th, 2018).)Stationery Office, London; 2018 https://www.gov.uk/government/publications/e-cigarettes-and-heated-tobacco-products-evidence-review
- Newton, John N et al. (2018) Making sense of the latest evidence on electronic cigarettes The Lancet , Volume 391 , Issue 10121 , 639 - 642
- McNeill, A, Brose, LS, Calder, R, Hitchman, SC, Hajek, P, and McRobbie, H. E-cigarettes: an evidence update. a report commissioned by Public Health England. Stationery Office, London; 2015
- Fairchild, AL, Lee, JS, Bayer, R et al. E-cigarettes and the harm-reduction continuum. New Engl J Med. 2018; 378: 216–219
- US Centers for Disease Control and Prevention. Electronic cigarettes.(2017) https://www.cdc.gov/tobacco/basic_information/e-cigarettes/index.htm((accessed March 19, 2018).)
- Primack et al. Progression to Traditional Cigarette Smoking After Electronic Cigarette Use Among US Adolescents and Young Adults. JAMA Pediatr. 2015;169(11):1018-1023.
- Public Health England. E-cigarettes: a developing public health consensus: joint statement on e-cigarettes by Public Health England and other UK public health organisations.(2016)https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/534708/E-cigarettes_joint_consensus_statement_2016.pdf ((accessed March 29, 2018).)
- 2018 USA PHE press release following report (Jan 23rd, 2018) http://www8.nationalacademies.org/onpinews/newsitem.aspx?RecordID=24952
- Glantz SA and Bareham DW. E-Cigarettes: Use, Effects on Smoking, Risks, and Policy Implications Annual Review of Public Health 2018 39:1, 215-235 https://www.annualreviews.org/doi/abs/10.1146/annurev-publhealth-040617-013757
- Lee S, Grana R, Glantz S. 2014. Electronic cigarette use among Korean adolescents: a cross-sectional study of market penetration, dual use, and relationship to quit attempts and former smoking. J. Adolesc. Health 54:684–90
- DutraLM,Glantz SA. 2014. Electronic cigarettes and conventional cigarette use among U.S. adolescents: a cross-sectional study. JAMA Pediatr. 168:610–17
- Soneji S, Barrington-Trimis JL, Wills TA, Leventhal A, Unger JB, et al. 2017. Association between initial use of e-cigarettes and subsequent cigarette smoking among adolescents and young adults: a systematic review and meta-analysis. JAMA Pediatr. 171:788–97
- Jonathan D. Klein. (2017) E-Cigarettes: A 1-Way Street to Traditional Smoking and Nicotine Addiction for youth. http://pediatrics.aappublications.org/content/early/2017/11/30/peds.2017-2850
- Singh T, Arrazola RA, Corey CG, Husten CG, Neff LJ, et al. 2016. Tobacco use among middle and high school students–United States, 2011–2015. MMWR 65:361–67
- Trajectories of e-cigarette and conventional cigarette use among youth. Pediatrics.2017;141(1):e20171832
- Courtemanche CJ, Influence of the flavored cigarette ban on adolescent tobacco use. Am J Prev Med. 2017;52(5):e139–e146pmid:28081999
- Harrell MB et al,Flavored e-cigarette use: characterizing youth, young adult, and adult users. Prev Med Rep. 2016;5:33–40
- 2016 Tobacco and Related Products Regulations https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/440989/SI_tobacco_products_acc.pdf
- 2016 GOV.UK https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/534708/E-cigarettes_joint_consensus_statement_2016.pdf
- http://www.mhra.gov.uk/home/groups/par/documents/websiteresources/con616843.pdf
- http://www.dailymail.co.uk/health/article-5351331/NHS-plan-prescribe-e-cigarettes-goes-smoke.html
- Manzoli L et al. Electronic Cigarettes Efficacy and Safety at 12 Months: Cohort Study. http://journals.plos.org/plosone/article?id=10.1371/journal.pone.0129443
- Manzoli L et al. Cohort study of electronic cigarette use: effectiveness and safety at 24 monthsTob Control BMJ (2016) doi:10.1136/tobaccocontrol-2015-052822
- Nathan K Cobb and Rajiv Sonti. E-Cigarettes: The Science Behind the Smoke and Mirrors. Respiratory Care August 2016, 61 (8) 1122-1128; DOI: https://doi.org/10.4187/respcare.04944
- https://www.college-optometrists.org/the-college/media-hub/news-listing/no-smoking-day-2016.html
- https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/457102/Ecigarettes_an_evidence_update_A_report_commissioned_by_Public_Health_England_FINAL.pdf 2015
- Stanton A Glantz and David W Bareham. E-Cigarettes: Use, Effects on Smoking, Risks, and Policy Implications. Annual Review of Public Health 2018 39:1, 215-235
- Nutt DJ, Phillips LD, Balfour D, CurranHV, Dockrell M, et al. 2014. Estimating the harms of nicotine containing products using the MCDA approach. Eur. Addict. Res. 20:218–25
- Combes RD, Balls M. 2015. On the safety of e-cigarettes: “I can resist anything except temptation.” Altern. Lab. Anim. 43:417–25
- McNeill A, Brose LS, Calder R, Hitchman SC, Hajek P, McRobbie H. 2015. E-Cigarettes: An Evidence Update. A Report Commissioned by Public Health England. London: Public Health Engl. https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/457102/Ecigarettes_an_evidence_update_A_report_commissioned_by_Public_Health_England_FINAL.pdf
- Natl. Health Serv. 2017. Smokefree NHS: electronic cigarettes. Natl. Health Serv., London. https://www.nhs.uk/smokefree/help-and-advice/e-cigarettes
- R Coll Phys. 2016. Nicotine Without Smoke: Tobacco Harm Reduction. London: R Coll Phys.
- R Soc Public Health. 2016. Growing consensus on the harm-reduction potential of e-cigarettes. April 28. R Soc Public Health, London
- 2015 The Lancet http://thelancet.com/journals/lancet/article/PIIS0140-6736(15)00042-2/fulltext
- (2015)Evidence about electronic cigarettes: a foundation built on rock or sand? http://www.bmj.com/content/351/bmj.h4863/rr
- Jonathan D Klein. E-Cigarettes: A 1-Way Street to Traditional Smoking and Nicotine Addiction for youth.http://pediatrics.aappublications.org/content/early/2017/11/30/peds.2017-2850
- Auer R et al,Heat-not-burn tobacco cigarettes: smoke by any other name. JAMA Intern Med. 2017;177(7):1050–1052pmid:28531246
- Philip Morris International. 2017 Second-quarter results 2017. Available from: http://phx.corporateir.net/External.File?item=UGFyZW50SUQ9Njc1NjE3fENoaWxkSUQ9MzgzNzY5fFR5cGU9MQ==&t=1.
- Glantz SA.(2017) PMI’s MRTP Application for IQOS Does Not Consider IQOS’s Appeal to Youth or Adolescents https://tobacco.ucsf.edu/pmi%E2%80%99s-mrtp-application-iqos-does-not-consider-iqos%E2%80%99s-appeal-youth-or-adolescents
- https://www.iqos.co.uk/about-iqos
- Katz MH No smoke-just cancer-causing chemicals. JAMA Intern Med. 2017;177(7):1052pmid:28531245
- https://www.theguardian.com/us-news/2018/jan/25/us-panel-rejects-philip-morris-claim-iqos-tobacco-device-cuts-disease-risk
- Gopinath B, Flood VM, Kifley A, Liew G, Mitchell P (2015) Smoking, Antioxidant Supplementation and Dietary Intakes among Older Adults with Age-Related Macular Degeneration over 10 Years. PLoS ONE 10(3): e0122548. doi:10.1371/journal.pone.0122548
- Shah SU, Pilli S, Telander DG, Morse LS, Park SS. Survey of patients with age-related macular degeneration: knowledge and adherence to recommendations. Can J Ophthalmol. 2013;48: 204–209
- Risk factors associated with age-related macular degeneration. A case-control study in the age-related eye disease study: Age-Related Eye Disease Study Report Number 3 Ophthalmology. 2000;107: 2224–2232
- JR Vingerling, A Hofman, DE Grobbee, and PTVM De Jong, “Age-related macular degeneration and smoking: the Rotterdam study,” Archives of Ophthalmology, vol. 114, no. 10, pp. 1193–1196, 1996.
- CA Rennie, A Stinge, EA King, S Sothirachagan, C Osmond, and A.J Lotery, “Can genetic risk information for age-related macular degeneration influence motivation to stop smoking A pilot study,” Eye, vol. 26, no. 1, pp. 109–118, 2012.
- RR Priya, EY Chew, and A Swaroop, “Genetic studies of age-related macular degeneration: lessons, challenges, and opportunities for disease management,” Ophthalmology, vol. 119, pp. 2526–2536, 2012
- EM Stone, AJ Aldave, AV Drack et al, “Recommendations for genetic testing of inherited eye diseases. Report of the American Academy of Ophthalmology Task Force on Genetic Testing,” Ophthalmology, vol. 119, pp. 2408–2410, 2012
- CA Rennie, A Stinge, EA King, S Sothirachagan, C Osmond, and AJ Lotery, “Can genetic risk information for age-related macular degeneration influence motivation to stop smoking A pilot study,” Eye, vol. 26, no. 1, pp. 109–118, 2012
- Stone EM. Genetic testing for age-related macular degeneration: not indicated now. JAMA Ophthalmol. Epub 2015 Marc 19.
- S Lee, SJ Song, and HG Yu, “Current smoking is associated with a poor visual acuity improvement after intravitreal ranibizumab therapy in patients with exudative age-related macular degeneration,” Journal of Korean Medical Science, vol. 28, pp. 769–774, 2013.
- Bidwell G, Sahu A, Edwards R, Harrison RA, Thornton J, Kelly SP: Perceptions of blindness related to smoking: a hospital based cross-sectional Study. Eye. 2005, 19: 945-948. 10.1038/sj.eye.6701955.
- Moradi P, Thornton J, Edwards R, Harrison RA, Washington SJ, Kelly SP: Teenagers’ perceptions of blindness related to smoking: a novel message to a vulnerable group. Br J Ophthalmol. 2007, 91: 605-607. 10.1136/bjo.2006.108191
- Kennedy RD, Spafford MM, Parkinson CM, Fong GT: Knowledge about the relationship between smoking and blindness in Canada, the United States, the United Kingdom, and Australia: results from the International Tobacco Control Four-Country Project. Optometry. 2011, 82: 310-317. 10.1016/j.optm.2010.10.014.
- Lawrenson JG et al. Advice about diet and smoking for people with or at risk of age-related macular degeneration: a cross-sectional survey of eye care professionals in the UK. BMC Public Health 2013, 13: 564
- Downie LE, Douglass A, Guest D & Keller PR. What do patients think about the role of optometrists in providing advice about smoking and nutrition?. Ophthalmic Physiol Opt 2017; 37: 202–211. doi: 10.1111/opo.12353
- Kennedy, RD, & Douglas, O (2015). Strategies to help patients stop smoking: The optometrist’s perspective. Clinical Optometry, 7, 103-113. DOI: 10.2147/OPTO.S63185
- Agboola SA, Coleman TJ, McNeill AD. Relapse prevention in UK Stop Smoking Services: a qualitative study of health professionals’ views and beliefs. BMC Health Services Research. 2009;9:67. doi:10.1186/1472-6963-9-67.
- Hughes JR, Solomon L, Naud S, Fingar JR, Helzer JE, Callas PW. Natural history of attempts to stop smoking. Nicotine Tob Res. 2014;16(9):1190–1198.
- Berg CJ, An LC, Kirch M, et al. Failure to report attempts to quit smoking. Addict Behav. 2010;35:900–904.
- Shah SU, Pilli S, Telander DG, Morse LS, Park SS. Reprint of: Survey of patients with age-related macular degeneration: knowledge and adherence to recommendations. Can J Ophthalmol. 2015 Jun;50 Suppl 1:S23-8. doi: 10.1016/j.jcjo.2015.04.005.
- Stead LF, Bergson G, Lancaster T. Physician advice for smoking cessation. Cochr Database Syst Rev. 2008;(2):CD000165.
- Canadian Association of Occupational Therapists, Canadian Association of Social Workers, Canadian Dental Association, Canadian Medical Association, Canadian Nurses Association, Canadian Pharmacists Association, Canadian Physiotherapy Association, Canadian Psychological Association, and Canadian Society of Respiratory Therapists. Tobacco: the role of health professionals in smoking cessation. Joint Statement. J Can Dental Assoc. 2001;67(3):134–135.
- Brûlé J, Abboud C, Deschambault E: Smoking cessation counselling practices among Québec optometrists: evaluating beliefs, practices, barriers and needs. Clin Exp Optom. 2012, 95: 599-605
- Downie LE, Keller PR (2015) The Self-Reported Clinical Practice Behaviors of Australian Optometrists as Related to Smoking, Diet and Nutritional Supplementation. PLoS ONE 10(4): e0124533. doi:10.1371/journal.pone.0124533
- https://www.college-optometrists.org/membership/free-patient-resources/patient-leaflets.html
- Lawrenson JG, Roberts CA, Offord L. A pilot study of the feasibility of delivering a brief smoking cessation intervention in community optometric practice. Public Health. 2015;129(2):149–151.
- Lorencatto F, Harper AM, Francis JJ et al. (2016) A survey of UK optometry trainees’ smoking cessation training, Ophthalmic and Physiological Optics
- National Centre for Smoking Cessation and Training www.ncsct.co.uk
- Royal College of Ophthalmologists. Age-Related Macular Degeneration—Guidelines for Management—Update; 2009. Available at: http://www.rcophth.ac.uk/page.asp?section=451§ionTitle=Clinical+Guidelines
- College of Optometrists. Healthy lifestyle, healthy eyes. Available at: http://lookafteryoureyes.org/eye-care/healthy-lifestyle-healthy-eyes/
- AAO Retina/Vitreous PPP Panel, Hoskins Center for Quality Eye Care. Age-Related Macular Degeneration PPP - Updated 2015
- Chew EY, Clemons T. Vitamin E and the age-related eye disease study supplementation for age-related macular degeneration. Arch Ophthalmol 2005; 123: 395–6.
- O. P. Heinonen and D. Albanes, “The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers,” New England Journal of Medicine, vol. 330, no. 15, pp. 1029–1035, 1994.
- Alpha-Tocopherol, Beta Carotene Cancer Prevention Study Group. The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. N Engl J Med 1994; 330: 1029–35.
- Omenn GS, Goodman GE, Thornquist MD, Balmes J, Cullen MR, Glass A, Keogh JP, Meyskens FL, Valanis B, Williams JH, Barnhart S, Hammar S. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. N Engl J Med 1996; 334: 1150–5.
- Age-Related Eye Disease Study 2 Research Group, “Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration: the Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial,” Journal of the American Medical Association, vol. 309, pp. 2005–2015, 2013.
