The major advances in treatment options for macular neovascularisation have improved the future eye health of those with the condition, although delivery of these services is creating challenges within the NHS. Of the 100 million outpatient appointments between 2013 and 2014, 10 million were for ophthalmology services – 10% of all outpatient hospital attendances that year.¹
The number of patients with eye diseases is increasing in line with the ageing population. For the period 2010 to 2015, it was estimated that diagnoses of age-related macular degeneration (AMD) doubled to almost 890,000 people,² and that approximately 400,000 intravitreal injection procedures were performed in 2014 to 2015.³ The ‘service delivery’ challenges arising from chronic medical conditions are ‘the healthcare equivalent to climate change’.4 In light of this, the concept of self-management is growing in popularity.5
Background to nutritional influence
This leads to the topic of nutritional supplementation for eye health, a sometimes controversial subject amongst some eye care professionals (ECPs). The first clue that AMD may have association with dietary risk factors comes from the first National Health and Nutrition Examination Survey survey (NHANES), which was published in 1988.6
Diets rich in fruits and vegetables with vitamins, especially vitamin A, were inversely associated with AMD. Since then, numerous studies have suggested the importance of dietary risk factors, and clinical trials were conducted to evaluate specific antioxidant vitamins and zinc.7
Although the exact pathogenesis of AMD is not known, oxidative stress is considered to be involved as the retina is exposed to the production of free radicals leading to a pro-oxidative environment and inflammation,8,9 involving the immune system by virtue of the complement cascade.10,11
The light-initiated oxidative damage and the presence of oxidized metabolites in the outer segment of the photoreceptors of the retinal pigment epithelium (RPE) may contribute to the formation of drusen and the pigmentary disturbances around the macula.11,12,13 It is, therefore, conceivable that dietary antioxidants and/or supplements may be beneficial in preventing and/or delaying the progression of AMD due to the decrease of oxidative stress and reduction of inflammatory events.14
The role of nutrition, nutritional supplements and their potential protection against eye age-related disease has been highlighted in a number of observational and randomised clinical trials, focusing on the following nutritional factors:
- Antioxidants (mainly vitamins C, E, and provitamin beta-carotene)
- Zinc
- Carotenoids lutein and zeaxanthin
- Omega-3 polyunsaturated fatty acids (PUFAs)
Information comes from a variety of sources. Random controlled trials (RCT) are considered the ‘gold standard,’ although can be costly and prone to bias as health conscious people tend to respond to recruitment, producing insignificant results and perhaps less useful conclusions if not precisely designed and executed.15 RCTs are a powerful tool for health research, but may have limitations for diet-related studies.16 The alternative is observational or epidemiological studies, often relying on food diaries, and due to differences in their genetic and ethnic profile these may not be representative of the UK population. Additionally, observational data cannot detect causal effects and can only determine associations.
AREDS
With this in mind, the Age-related Eye Disease Study (AREDS) results have received the most attention: the studies are large and noteworthy for their study design and aims, but they are not perfect.2
AREDS was a large (4,757 patients) complex randomised clinical trial designed to evaluate the effect of higher than NRV (nutrient reference value, formerly known as recommended daily allowance, or RDA) dose supplements as follows:
- C (500 mg)
- E (400 IU)
- beta- carotene (15 mg)
- with or without zinc (80 mg)
- copper (2mg)
The aim was to gauge their impact on the progression of AMD.17 Most of the patients were taking nutritional supplements at the time of the study enrollment and, to standardize this occurrence, a daily dose of a (Centrum) multivitamin and minerals tablet was provided. The patients were followed up for a mean period of 6.3 years.14
The results showed that treatment with zinc alone or in combination with antioxidants reduced the risk of progression to advanced AMD in patients of categories 3 and 4. The AREDS results provide evidence that risk of progression in moderate, high risk AMD to the severe form of AMD can be reduced by up to 25% when taking a combination of high-dose antioxidants and zinc.² The treatment effect persisted following five additional years of follow-up after the end of the clinical trial.18
The AREDS formula showed no beneficial effect for any other stage of AMD. 18 Observational data from AREDS and other studies suggested that the increased dietary intake of lutein and zeaxanthin, and omega-3 long-chain polyunsaturated fatty acids (LCPUFAs) found in fish reduced the risk of late AMD.12,19,20-25
AREDS2 was designed to investigate whether other nutrients supplements, specifically lutein/ zeaxanthin and omega-3 LCPUFAs, in addition to the AREDS supplements might further reduce the risk of progression to advanced AMD.26,27
The AREDS2 was a multicentre phase 3 randomised, double-masked, placebo-controlled trial conducted between 2006 and 2012, which enrolled participants with either bilateral intermediate AMD, or advanced AMD in one eye. The primary randomisation included the daily AREDS supplement plus one of the following:
- Lutein (10mg)
- Zeaxanthin (2 mg)
- DHA/EPA (350/650 mg)
- a combination of these two
- or placebo.
Table 1 summarises the AREDS2 formulation:
- Vitamin C (500 mg)
- Vitamin E (273 mg/400 IU)
- Zinc 80 mg
- Copper 2 mg
- Lutein 10mg
- Zeaxanthin 2mg
The secondary randomisation consisted of the elimination of beta-carotene and/or lowering of zinc to 25 mg instead of the original 80 mg found in the AREDS formulation.18
The results, published in 2013, revealed that addition of lutein/ zeaxanthin, DHA/EPA or both to the AREDS1 formulation did not further reduce the risk of progression to advanced AMD in the primary analysis.18 As the design of AREDS2 was complex, any of the interventions need to perform 25% (or more) better than the AREDS1 formulation to reach statistical significance. This represents a very high threshold, and it seems reasonable to question whether this is an achievable target for such a study.
In addition, secondary analysis was performed regarding the effect of lutein/zeaxanthin, which showed effects in certain subgroups.27 Lutein/zeaxanthin was not related to increase in lung cancer incidence, contrary to beta-carotene that was associated with a rise in lung cancer in former smokers.28 Meso-zeaxanthin (a third macular pigment synthesized within the retina) is now available in the market but there are no randomised clinical trials supporting its superiority to lutein/zeaxanthin.29
There are a number of limitations to the AREDS2 study that are important to note. Firstly, given that almost all patients included took the original (or modified) AREDS1 supplement, no real placebo group is available. Thus, the question whether supplementation of lutein/zeaxanthin (L/Z) or DHA/EPA alone is superior to placebo is still unanswered. In addition, it needs to be stated that, on average, the individuals participating the AREDS2 trial were mostly very well-nourished Caucasians.
Whether this holds true for other populations or even is representative for the US population in general is uncertain.30 Further, a large proportion of the participants took additional supplements such as Centrum, which makes the interpretation of the results even more difficult. In summary, the AREDS2 led to the recommendation that lutein/zeaxanthin may be considered as a replacement for beta-carotene. Due to the complex design and the fact that no real placebo group was included, several other questions, such as the efficacy of omega 3 fatty acids, remain unanswered.27
There are currently no official recommended dietary intake levels for L/Z. However an intake of 6-10 mg of L/Z per day for men and women has been suggested as a dietary target to reduce the risk of age-related macular degeneration.31,32
Data on population L/Z intake is limited, but current evidence suggests the intake of carotenoids varies in different populations.33 Of the limited studies measuring L/Z consumption in populations, UK-based AMD patients had a reduced daily L/Z intake of 1.4 mg32 the US intake varies from 1 to 3mg.34,35
The Australian Blue Mountain Eye Study found that the average intake of L/Z was 0.9 mg in older adults.36 Based on these results, current intakes in older adults are lower than ideal. In countries including Australia, US and Great Britain, the national intakes of lutein may be declining. Overall, the available data on current intakes suggests they are considerably below the current suggested intake of 6 mg per day.37
A Cochrane review of prospective studies of dietary intake found no evidence that diets high in antioxidant vitamins prevent AMD, and more specifically, there is no evidence from clinical randomised trials that healthy people should take vitamin and antioxidant supplements to delay or prevent the onset of AMD.38 However a more recent systematic review perhaps provides a more pragmatic approach and will be discussed later.39
In fact, whilst much criticism has been levelled at supplements on sale that do not match the formula used in AREDS or AREDS 2,40 there is no evidence to show that an AREDS-type formula will benefit everyone else who may be simply concerned with eye health or have various levels of eye disease.2 Both AREDS and AREDS2 formulas contain high doses of vitamin E and zinc.
These high doses are of concern to many researchers and clinicians alike, referring to a ‘U-shaped response curve’ where very low or very high blood levels of a nutrient are thought to be harmful but more moderate levels are beneficial.41 Indeed, the dose of zinc was shown in the AREDS to lead to increased hospital admissions secondary to urinary tract infections.2,42 Some evidence suggests that high dose vitamin E may have long-term effects on prostate cancer risk.43
A bigger picture
Navigating the world of vitamin and mineral (dietary) supplements can be daunting, in large part due to the nuances of dietary supplement regulation. Oral products containing vitamins and nutrients are regulated as a general food product under the classification of ‘dietary supplements’ rather than as a medicine.
This level of categorisation provides considerable freedom in terms of the claims that can be made in relation to their health benefits. The regulation of dietary supplements contrasts significantly to scheduled medicines, which require high-quality evidence usually from randomised, controlled clinical trials to validate any claims regarding the safety and/or efficacy of an intervention.44
Suppliers range from leading ophthalmic pharmaceutical companies who invest in research to demonstrate benefit, to wholesalers and distributors whose primary concern might be margin and consumer promotion.2 As supplements are classed as ‘food products’, the responsibility of the manufacturer is to ensure the appropriate safety and presentation of the product. The regulatory authority is not required to approve, test, or analyse the supplement before it is available for sale to the public.44
Furthermore, manufacturer claims as to the potential health benefits of a particular vitamin are neither tested nor confirmed by the relevant authority. There is therefore the potential for claims to be made that are not supported by high-level evidence.
Many supplements are promoted as a potential means of delaying the onset of disease and/or as a reasonable means of improving health and well-being. Such claims are fundamental to the confusion regarding the actual benefits of antioxidant therapy in AMD.44
As supplements are less vigorously regulated than pharmaceuticals and medical devices, some manufacturers can, and do, create and market new formulations, muddying the water further.45
Often the key ingredients in such products may have some robust evidence behind them but the combined product may or may not, depending on its heritage.2 Guidance about allowable claims for any benefits for particular nutrients, like zinc or Vitamin A for example, is, however, provided by the European Food Standards Agency (EFSA).46
Clinical applications
So, what advice can the busy clinician give to a patient regarding diet and its effect on eye health? One possible approach is to make recommendations based on the risk factors of certain patient groups.
As clinicians, we see a large number of patients who present with a variety of AMD risk factors that can be represented broadly into five groups:
- Those whose AMD is at a high risk of progression
- Those with early (dry) AMD
- Those whose only have a family history of AMD
- Patients who smoke
- Those patients whose only risk factor is that of getting older
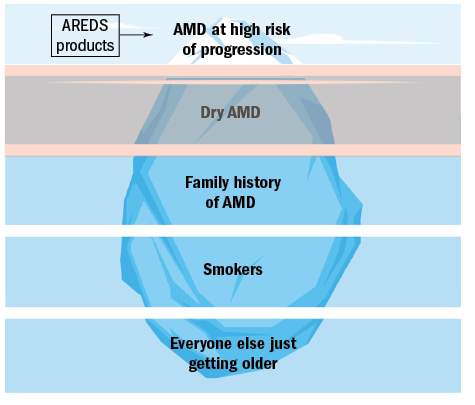
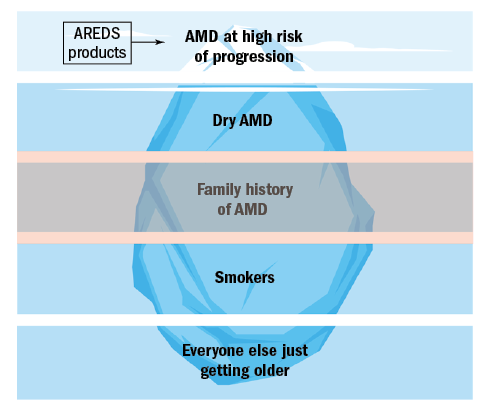
The prevalence of these groups varies and can be represented as an pyramidal iceberg, the tip of which represents the group highlighted by AREDS as benefitting from intervention (figure 1).

Figure 1: The five groups of AMD risk factors
As can be seen, those with a high risk of AMD progression and vision loss, represent the smallest group of the general patient population. The vast majority of patients we see in practice just have age as their only risk factor.
It may initially appear that those with AMD at high risk of progression are the only ones for which intervention exists. This article will look at the current evidence of where nutritional/ lifestyle recommendations can be used in each of these groups.
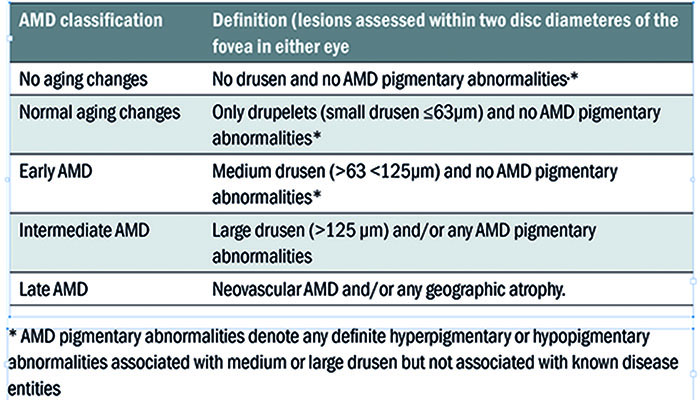
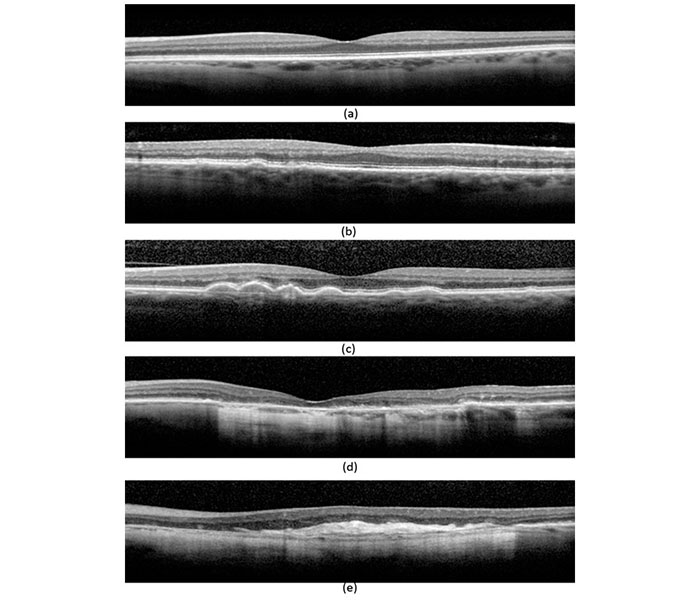
Table 2: Beckman 5-stage grading scheme
Layer 1: The tip of the iceberg
There are numerous classification schemes for AMD staging.47,48 However, the Beckman 5-stage grading scheme (see table 2) has been proposed for use in clinical settings,49 where drusen size and variation of pigmentation (hypo/hyper) are used to grade disease severity.44
Intermediate AMD is a critical distinction clinically as it identifies the individual at risk for progression to more advanced AMD and sight loss. It has been defined by the AREDS as having extensive medium drusen (63–124 µm) or one or more large drusen (>125 µm in diameter) in one or both eyes. 50
The progression to advanced AMD at five years in this group is approximately 18% according to the original AREDS, although this varies with presentation from 6-26% (based on the number of large drusen and whether they are unilateral or bilateral). 17,51
What evidence-based advice is appropriate for this group?
Eye care professionals should consider advice relating to any modifiable risk factors plus the potential merit of modifying their diet and/or taking some form of antioxidant supplementation. A recent review of nutrition and lifestyle, relating to the risk of AMD progression show beneficial effects in dietary patterns rich in green leafy vegetables and other sources of zeaxanthin and lutein, as well as omega-3 EFAs from marine sources (oily fish).39
If this is not achievable through diet alone, it reasonable to recommend dietary supplements that include these nutrients, with special consideration given to the form and dosage of omega-3 recommendation.52
In the original AREDS17 and in AREDS228, participants who benefited from antioxidant vitamin and mineral supplementation were those who had either intermediate AMD or advanced AMD in one eye. The rate of development of advanced AMD at five years was reduced by 25% in the participants using the combination treatment of high dose antioxidant vitamins with zinc and copper.17
There is no evidence from the AREDS results to support the use of antioxidant vitamin and mineral supplements for patients who have less than intermediate AMD, an important finding often overlooked when professionals refer to this source of evidence in their daily practice.
The AREDS2 study results demonstrated that in patients at high risk for progression, there was no statistically significant difference associated with supplementation with the original AREDS formula versus each of the other modifications on AMD progression.28 Interestingly, secondary analysis pointed to lutein and zeaxanthin playing a role for reducing the risk of progression to advanced AMD when given without beta carotene and may represent an appropriate substitute for beta-carotene.53
In the same analysis, patients with very low dietary intake of lutein plus zeaxanthin experienced a further 20% reduction in AMD risk by taking the AREDS2 formulation.28,53 Finally, there was no significant effect of lowering the zinc levels (25 mg) on the reported risk of progression to advanced disease.53
However, it needs to be kept in mind that the AREDS 2 formula is a deliberately high dose, where many of the components are greatly in excess of the NRV. When considering long-term supplementation, some people may have reason to avoid one or more of the supplements evaluated in the original AREDS or AREDS2. Of particular interest is the dosage of Vitamin E.
Vitamin E is a fat-soluble vitamin, possessing antioxidant properties which protects the cell membrane from free radical damage.54 The levels of Vitamin E in particular are controversial to some as the dosage found in AREDS formulations (400 IU) are significantly higher than the NRV (17.9 IU). The SELECT study demonstrated a 17% relative increased risk of developing prostate cancer in otherwise healthy men using Vitamin E at higher dosages.43
The SELECT study findings have been applied to AREDS patients and did not demonstrate a harmful effect of vitamin E on incidence of prostate cancer, due possibly to the interaction with other supplementation found in the AREDS formulations.55 Discussion between ECP and the patient of the potential adverse effects of high doses of antioxidant vitamins and minerals recommended by the original AREDS and AREDS256.57.58 including an increased rate of genitourinary conditions that may require hospitalization17,42 is warranted and may benefit from additional review by the patient’s GP.17,52
In summary:
- The progression from intermediate to advanced AMD within five years occurs in 18% of patients
- Strong evidence supports the use of an AREDS or AREDS2 formula antioxidant vitamin and mineral supplements only for patients who have intermediate AMD and are at risk of progression
- Awareness that AREDS formulations contain significantly higher dosages than NRV and carry the potential for adverse effects, which may benefit from a review by GP
- Discussion of lifestyle modification is important
- A lower zinc dose (25 mg) in the AREDS2 formulation could be considered
Figure 2: Early AMD. (Image courtesy of Alison Edwards)
Layer 2: Pateients with a few drusen
What should I be saying to people with just a few drusen (or early AMD – figure 2)? Again, we should consider giving advice on any modifiable risk factors such as smoking, but how relevant or appropriate is dietary and lifestyle advice in cases such as these?
In AREDS, only 1.3% of participants with early AMD as shown in figure 2 progressed to late AMD within five years.17 The AREDS demonstrated that there was no statistically significant evidence of a benefit in delaying the progression of eyes with early AMD to more significant drusen-related pathology (intermediate AMD) through the use of antioxidant vitamin and/or zinc supplementation.
There is only strong evidence to show that there is no benefit of an AREDS-type antioxidant vitamin and/or mineral supplements for patients who have less than intermediate AMD.17 A recent Cochrane review of five prospective studies of dietary intake found no evidence that diets high in antioxidant vitamins prevent AMD, and more specifically, there is no evidence from clinical randomised trials that healthy people should take vitamin and antioxidant supplements to delay or prevent the onset of AMD.38
However, these reviews were based only on a handful of studies that reached the criteria for inclusion so it is perhaps better to keep in mind that where evidence is not yet providing consensus, this doesn’t mean we can dismiss the rationale, just that more work needs to be done. Indeed, a more recent systematic review that includes 14 studies provides perhaps a more pragmatic approach.
This review highlights the multifactorial influences of diet and food intake on the incidence and progression of AMD.39 As diet is a modifiable risk factor for AMD, improvement to diet and food intake. coupled maintenance of a healthy body mass index, healthy diet, physical activity and stress management should be encouraged.59
A variety of dietary patterns were reviewed, with the mediterranean diet, known for reduction in oxidative stress,60 being recommended. This pattern is based on high consumption of fruits, vegetables, legumes, whole grains and nuts, moderate consumption of fish, poultry and dairy, use of olive oil in place of other oils/fats,61 optional low amounts of red wine, and limited consumption of red meat.62 The oriental diet pattern is preferred over a western diet pattern.
Figure 3: Healthy patient with a Family History of late AMD (Image courtesy of Alison Edwards)
When considering particular food groups, the studies in this review recommend the consumption of vegetables to increase lutein and zeaxanthin carotenoid intake. Consumption of fatty fish greater than twice a week is also advised to increase the omega-3 fatty acids, DHA and EPA has been linked with reducing the risk of early AMD.63 Omega-6 fatty acids, such as vegetable oils and animal fats should be kept to a minimum.63
Low glycaemic index food choices are preferred over high glycaemic index foods,64,65 and alcohol consumption should be limited to less than two standard drinks a day.66 Red meat deserves special consideration, and should be consumed less than four times a week, with specific recommendations that processed meat (salami or continental sausage) consumption should be reduced to less than once a month. Research has shown an increased risk of AMD progression although the exact mechanism needs more research.62
A potential public health concern is the greater risk of cancer associated with red meat (especially processed).67-69 The International Agency for Research on Cancer concluded that consumption of processed meat ‘is carcinogenic to humans’ and red meat was ‘probably carcinogenic to humans’, based on data from a substantial number of high-quality epidemiological studies.70 The recommendations from the Chapman review are presented in the summary.
As discussed earlier, nutrition is more than dietary supplementation, and the role of the eye care professional is first and foremost to assess risk. Some risks can’t be modified (age, sex, genetics) but some can, and this is where dietary and lifestyle modification is an important consideration for this patient.
Evidence for the role of diet in AMD is derived predominantly from real world evidence (RWE) including observational studies.22,71 It is worth noting that a number of professional guidelines make recommendations regarding the possible benefit(s) of dietary modification.72,73
Food sources and a discussion of lifestyle may be preferable to supplementation for improving nutritional status as they are sustainable, less expensive, and have a significantly lower risk of systemic toxicity.74 Conversely, specific interventions such as antioxidants at high dose with a risk for adverse effects are not justified in these cases.38
Although diet is a significant modifiable factor in AMD progression, it is often difficult to dissociate a particular nutrient from other aspects of the diet, the concept of dietary lifestyle or patterns, rather than individual components are growing in popularity and increasingly being studied in relation to AMD.74
It has also been proposed that synergistic relationships of food components exist.76,77,78 Numerous studies show that the combination of a healthy diet, the maintenance of an adequate body weight, and an active lifestyle are important to maintaining health and avoiding the physical and cognitive degeneration associated with aging.76,77,79 The concept of dietary lifestyle will be discussed in more detail later.
There is the challenge for patients in this group to obtain the nutrients they need from their daily life. If they smoke or acknowledge a poor diet and lifestyle, then taking a supplement may be beneficial in a general way.
In summation:
- The progression from early to advanced AMD within five years occurs in just over 1% of patients
- Specific studies on how the risk of progression in mild AMD is influenced by diet and lifestyle are few, but several population studies show benefit for overall risk
- Taking the pragmatic approach, all that we would say to someone to keep their eyes healthy can be applied here – smoking cessation, advice on sun protection
- The highlights of the population studies are summarised in table 3.
- Adopt a healthy lifestyle: a healthy body mass index, healthy diet, physical activity and stress management
- College of Optometrists Leaflet ‘Healthy lifestyle, healthy eyes’. Available at: http://lookafteryoureyes.org/eye-care/healthy-lifestyle-healthy-eyes/
- There is no evidence to support the use of high dose antioxidant vitamin and mineral supplements for patients who have less than intermediate AMD
Table 3: Recommendations on dietary intake
Layer 3: Patients with a family history
There are approximately 700,000 people living with moderate to severe AMD today. Although current ws are not available, it is estimated that 400,000 injections were performed as treatment in 2015 (3). Almost all patients will have relatives where we know risk of AMD will be increased. What can and should we be saying to these people as a duty of care?
Family history is a known risk factor for AMD.80 Although some variability exists,81-86 reports of at least a 12-fold increase in the odds of AMD for those with an affected first degree relative have been cited.80-85 Most of the risk to siblings towards advanced AMD was due to choroidal neovascularisation.87,88,89
These findings have important implications for eye care professionals in their management of patients with advanced AMD. These patients should be made aware of the increased risk to their siblings and children, who need to have scheduled eye examinations for detecting the intermediate stage of AMD50 and be encouraged to assess their own visual acuity using monocular vision testing (usually the Amsler grid) and seek medical advice and urgent intervention if they develop visual symptoms of distortion or reduced vision.
Coupled with this, AMD patients should also have an awareness to pass information to their first-degree relatives on the importance of risk reduction strategies including avoiding smoking, a healthy lifestyle with good nutrition.17,90 As previously stated, treatment with high dose antioxidants and minerals is recommended only for patients who have progressed to intermediate or advanced AMD in at least one eye.38,50 With this in mind, does nutrition play a part when the only risk factor is that of a family history?
Epidemiologic studies support the importance of nutrition in these relatives. The most well known being the Rotterdam study which looked into whether modifying diet could impact the chances of AMD in subjects whose only risk factor was a genetic predisposition to AMD.91
Previous research has shown a number of genetic modifications are related to AMD, however the majority (80%) of subjects with late AMD have either of two susceptible genes (CFH or LOC387715/HTRA1)91 The role of genetics can not be overstated as evidence exists that the risk of developing late stage AMD in the elderly who do not carry susceptible genes is less than 1%.92
The Rotterdam study looked at the impact of diet in over 2000 individuals who were at genetic risk and were AMD free at the start of the study.
The study found that the NRV (formerly RDA) intake of antioxidants, zinc, and Omega-3 fatty acids may reduce the risk of early AMD among those at high genetic risk. Suggestions for what to eat included was based on the sources of the three groups studied in the trial is summarised in table 4.
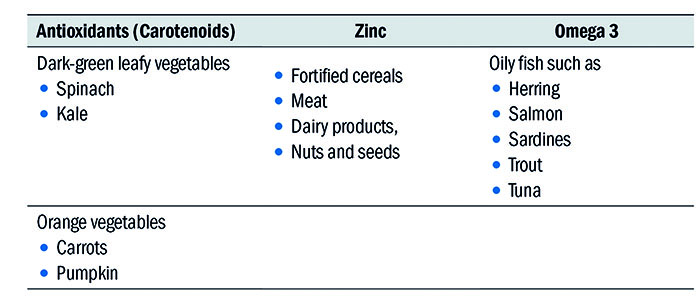
Table 4: Recommendations from the Rotterdam study
These nutrients should be part of a regular diet for susceptible individuals, to postpone or prevent the effects of late AMD.91 In cases where patients struggle to meet these requirements on a daily basis, it could be reasonable to suggest nutritional supplements that deliver the RDA of these 3 groups. Let’s not forget that a supplement is designed to do just that – supplement your daily diet.
In summation:
- More than 80% of patients with late stage AMD have a genetic predisposition
- First degree relatives, particularly siblings should have routine eye examinations
- Observational studies support the use of nutrition/ supplementation whose dosages are inline with the NRV
AMD and smoking
This has been reviewed in an earlier article93and can be summarised as:
- Smoking is a major modifiable risk factor associated with AMD
- Smoking increases the risk of AMD two to four-fold.
- Pack-years smoked better reflects the amount of exposure.
- Cigarette smoke is comprised of a gas and tar phase containing chemicals with a high concentration of free radicals
- Cigarette smoke induces ‘pro-inflammatory’ changes in the RPE, along with oxidative damage.
- Risk of AMD remains present for many years after cessation of smoking.
- Passive smoking carries risk of AMD.
- Public awareness of the link between smoking and ocular health is lacking.
- Eye care providers have a duty of care to inform patients of the long-term ocular risk of smoking and AMD.
- Electronic cigarettes are considered ‘safer’ than conventional cigarettes, although more research is needed.
- Smoking cessation (using VBA) with a discussion of dietary modification is recommended.
- AREDS and AREDS II formulations only indicated in intermediate/late stage AMD.
- AREDS formula (beta carotene) carries increased risk of lung cancer in smokers.
- AREDS II: lutein plus zeaxanthin a better substitute for beta carotene.
Conclusion
In the last of these articles looking at risk intervention and AMD, we will take a closer look at the ageing process within the eye in general, the lowest level of the iceberg.
Dr Rohit Narayan is a therapeutic optometrist based in the Midlands
References
1 MacEwen 2016 https://www.rcophth.ac.uk/2016/03/increasing-demand-on-hospital-eye-services-risks-patients-losing-vision/
2 Purslow, C. ‘Its not all about ARED- Time for a pragmatic approach to nutrition for eye health?’ Eyenews.co.uk (2016) https://www.eyenews.uk.com/features/ophthalmology/post/it-s-not-all-about-ared-time-for-a-pragmatic-approach-to-nutrition-for-eye-health
3 Hollingworth W, Jones T, Reeves BC, et al A longitudinal study to assess the frequency and cost of antivascular endothelial therapy, and inequalities in access, in England between 2005 and 2015 BMJ Open 2017;7:e018289. doi: 10.1136/bmjopen-2017-018289
4 Guardian (2014) http://www.theguardian.com/society/2014/jan/03/nhs-overwhelmed-long-term-medical-conditions
5 Selfcare http://www.selfcareforum.org/
6 Goldberg J, Flowerdew G, Smith E, Brody JA, Tso MO: Factors associated with age-related macular degeneration. An analysis of data from the first National Health and Nutrition Examination Survey. Am J Epidemiol 1988; 128:700–710.
7 Chew EY, Nutrition, Genes, and Age-Related Macular Degeneration: What Have We Learned from the Trials? Ophthalmologica 2017;238:1-5
8 Carneiro, A, ‘Nutrition and the ageing eye,’ in Anti-Ageing Nutrients Evidence-Based Prevention of Age-Related Diseases, D. Neves, Ed, pp. 277–297, John Wiley & Sons, Oxford, UK, 2015.
9 Kauppinen A, Paterno JJ, Blasiak J et al. Inflammation and its role in age-related macular degeneration. Cell Mol Life Sci 2016;Feb 6 [Epub ahead of print]
10 Kijlstra, A., & Berendschot, T. T. (2015). Age-related macular degeneration: a complementopathy?. Ophthalmic research, 54(2), 64-73.
11 Pujol-Lereis, Luciana M., et al. ‘Interrelation between oxidative stress and complement activation in models of age-related macular degeneration.’ Retinal Degenerative Diseases. Springer, Cham, 2016. 87-93.
12 J. P. SanGiovanni, E. Y. Chew, T. E. Clemons et al., ‘The relationship of dietary lipid intake and age-related macular degeneration in a case-control study: AREDS report no. 20,’ Archives of Ophthalmology, vol. 125, no. 5, pp. 671–679, 2007.
13 J. P. SanGiovanni, E. Y. Chew, T. E. , E. Argon et al, ‘The relationship of dietary w-3 long-chain polyunsaturated fatty acid intake with incident age-related macular degeneration: AREDS report no. 23,’ Archives of Ophthalmology, vol. 126, no. 9, pp. 1274–1279, 2008.
14 Ângela Carneiro and José Paulo Andrade, ‘Nutritional and Lifestyle Interventions for Age-Related Macular Degeneration: A Review,’ Oxidative Medicine and Cellular Longevity, vol. 2017, Article ID 6469138, 13 pages, 2017.
15 Ju ̈ni P, Altman DG, Egger M. Systematic reviews in health care: assessing the quality of controlled clinical trials. BMJ 2001;323:42Y6.
16 Hébert, J. R., Frongillo, E. A., Adams, S. A., Turner-McGrievy, G. M., Hurley, T. G., Miller, D. R., & Ockene, I. S. (2016). Perspective: randomised controlled trials are not a panacea for diet-related research. Advances in Nutrition, 7(3), 423-432.
17 AREDS Age-Related Eye Disease Study Research Group, ‘A random- ized, placebo-controlled, clinical trial of high-dose supplemen- tation with vitamins C and E, beta carotene, and zinc for age- related macular degeneration and vision loss: AREDS report no. 8,’ Archives of Ophthalmology, vol. 119, no. 10, pp. 1417–1436, 2001.
18 Chew EY, Clemons TE, Agron E et al. Long-term effects of vitamins C and E, beta-carotene and zinc on age-related macular degeneration. AREDS Reoprt no. 35. Ophthalmology 2013;120:1604-1611.
19 J. P. SanGiovanni, E. Argon, T. E. Clemons and E. Y. Chew, ‘w-3 Long-chain polyunsaturated fatty acid intake inversely associated with 12-year progression to advanced age-related macular degeneration,’ Archives of Ophthalmology, vol. 127, no. 1, pp. 110–112, 2009.
20 Seddon JM, Ajani UA, Sperduto RD, Hiller R, Blair N, Burton TC, Faber MD, Gragoudas ES, Haller J, Miller DT, et al: Dietary carotenoids, vitamins A, C, and E, and advanced age-related macular degeneration. JAMA 1994;272:1413–1420.
21 Augood C, Chakravarthy U, Young I, Vioque J, de Jong PT, Bentham G, Rahu M, Seland J, Soubrane G, Tomazzoli L, Topouzis F, Vingerling JR, Fletcher AE: Oily fish consumption, dietary docosahexaenoic acid and eicosapentaenoic acid intakes, and associations with neovascular age-related macular degeneration. Am J Clin Nutr 2008;88:398– 406.
22 Mares-Perlman JA, Fisher AI, Klein R, Palta M, Block G,Millen AE, Wright JD: Lutein and zeaxanthin in the diet and serum and their relation to age-related maculopathy in the third national health and nutrition examination survey. Am J Epidemiol 2001;153:424–432.
23 Moeller SM, Parekh N, Tinker L, Ritenbaugh C, Blodi B, Wallace RB, Mares JA; CAREDS Research Study Group: Associations between intermediate age related macular degeneration and lutein and zeaxanthin in the Carotenoids in Age-Related Eye Disease Study (CAREDS): ancillary study of the Women’s Health Initiative. Arch Ophthalmol 2006;124: 1151–1162.
24 Tan JS, Wang JJ, Flood V, Rochtchina E, Smith W, Mitchell P: Dietary antioxidants and the longer-term incidence of age-related macular degeneration: the Blue-Mountains Eye Study. Ophthalmol 2008;115:334–341.
25 Seddon JM, Cote J, Rosner B: Progression of age-related macular degeneration: association with dietary fat, transunsaturated fat, nuts, and fish intake. Arch Ophthalmol 2003; 121:1728–1737.
26 The AREDS2 Research Group, Chew EY, Clemons T, SanGiovanni JP, Danis RP, Domalpally A, McBee W, Sperduto R, Ferris FL: The Age-Related Eye Disease Study 2 (AREDS2): study design and baseline characteristics (AREDS2 report number 1). Ophthalmology 2012;119:2282–2289
27 Schmidl, D. , Garhöfer, G. and Schmetterer, L. (2015), Nutritional supplements in age‐related macular degeneration. Acta Ophthalmol, 93: 105-121. doi:10.1111/aos.12650
28 The Age-Related Eye Disease Study 2 (AREDS2) Research Group, ‘Lutein + zeaxanthin and omega-3 fatty acids for age- related macular degeneration: the Age-Related Eye Disease Study 2 (AREDS2) randomised clinical trial,’ JAMA, vol. 309, no.19, pp. 2005-2015, 2013
29 P. S. Bernstein, B. Li, P. P. Vachali et al., ‘Lutein, zeaxanthin, and meso-zeaxanthin: the basic and clinical science underlying carotenoid-based nutritional interventions against ocular dis- ease,’ Progress in Retinal and Eye Research, vol. 50, pp. 34–66, 2016.
30 Andreatta W, El-Sherbiny S. Evidence-Based Nutritional Advice for Patients Affected by Age-Related Macular Degeneration. Ophthalmologica 2014;231:185–190.
31 Rasmussen, H.M.; Johnson, E.J. Nutrients for the aging eye. Clin. Interv. Aging 2013, 8, 741–748.
32 Stevens R, Bartlett H, Cooke R. Dietary analysis and nutritional behaviour in people with and without age-related macular disease. Clin Nutr ESPEN 2015; 10: (3): 112-7.
33 Scott, K.J.; Thurnham, D.I.; Hart, D.J.; Bingham, S.A.; Day, K. The correlation between the intake of lutein, lycopene and beta-carotene from vegetables and fruits, and blood plasma concentrations in a group of women aged 50–65 years in the UK. Br. J. Nutr. 1996, 75, 409–418.
34 Tucker, K.L.; Chen, H.; Vogel, S.; Wilson, P.W.; Schaefer, E.J.; Lammi-Keefe, C.J. Carotenoid intakes, assessed by dietary questionnaire, are associated with plasma carotenoid concentrations in an elderly population. J. Nutr. 1999, 129, 438–445.
35 Mares-Perlman, J.A.; Millen, A.E.; Ficek, T.L.; Hankinson, S.E. The body of evidence to support a protective role for lutein and zeaxanthin in delaying chronic disease: Overview. J. Nutr. 2002, 132, 518S–524S.
36 Manzi, F.; Flood, V.; Webb, K.; Mitchell, P. The intake of carotenoids in an older Australian population: The blue mountains eye study. Public Health Nutr. 2002, 5, 347–352.
37 Bronwyn Eisenhauer, Sharon Natoli, Gerald Liew, Victoria Flood, Victoria M. Flood. Lutein and Zeaxanthin—Food Sources, Bioavailability and Dietary Variety in Age‐Related Macular Degeneration Protection. Nutrients 2017, 9(2), 120; https://doi.org/10.3390/nu9020120
38 Evans JR, Lawrenson JG. Antioxidant vitamin and mineral supplements for preventing age-related macular degeneration. Cochrane Database of Systematic Reviews 2017, Issue 7. Art. No.: CD000253. DOI: 10.1002/14651858.CD000253.pub4.
39 Chapman, N. A., Jacobs, R. J., & Braakhuis, A. J. (2018). Role of diet and food intake in age‐related macular degeneration: a systematic review. Clinical & experimental ophthalmology.
40 Yong JJ, Scott IU, Greenberg PB. Ocular nutritional supplements: are their ingredients and manufacturers' claims evidence-based? Ophthalmology. 2015 Mar;122(3):595-9.
41 Jansen, E., Viezeliene, D., Beekhof, P., Gremmer, E., & Ivanov, L. Effects of Higher Doses of Vitamin E on Toxicity and Inflammation. European Journal of Nutrition & Food Safety 8(2): 47-58, 2018
42 Johnson, A. R., Munoz, A., Gottlieb, J. L., & Jarrard, D. F. (2007). High dose zinc increases hospital admissions due to genitourinary complications. The Journal of urology, 177(2), 639-643.
43 EA Klein, IM Thompson, CM Tangen, et al. Vitamin E and the Risk of Prostate Cancer: Results of The Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA 2011; 306(14) 1549-1556.
44 Downie, Laura & Keller, Peter. (2014). Nutrition and Age-Related Macular Degeneration. Optometry and vision science : official publication of the American Academy of Optometry. 91. 10.1097/OPX.0000000000000285
45 J Knaub, What AREDS2 means in the clinic. Ophthalmology Management, Volume: 18, Issue: January 2014, page(s): 44 – 47
46 EFSA Panel on Dietetic Products, Nutrition and Allergies: Scientific opinion on the sub- stantiation of health claims related to docosa- hexaenoic acid (DHA), eicosapentaenoic acid (EPA) and brain, eye and nerve development (ID 501, 513, 540), maintenance of normal brain function (ID 497, 501, 510, 513, 519, 521, 534, 540, 688, 1323, 1360, 4294), mainte- nance of normal vision (ID 508, 510, 513, 519, 529, 540, 688, 2905, 4294), maintenance of normal cardiac function (ID 510, 688, 1360), ‘maternal health; pregnancy and nursing’ (ID 514), ‘to fulfil increased omega-3 fatty acids need during pregnancy’ (ID 539), ‘skin and digestive tract epithelial cells maintenance’ (ID 525), enhancement of mood (ID 536), ‘membranes cell structure’ (ID 4295), ‘anti- inflammatory action’ (ID 4688) and mainte- nance of normal blood LDL-cholesterol con- centrations (ID 4719) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J 2011;9:2078.
47 Davis MD, Gangnon RE, Lee LY, Hubbard LD, Klein BE, Klein R, Ferris FL, Bressler SB, Milton RC. The Age-Related Eye Disease Study severity scale for age-related macular degeneration: AREDS Report No. 17. Arch Ophthalmol 2005;123:1484Y98.
48 Seddon JM, Sharma S, Adelman RA. Evaluation of the clinical age- related maculopathy staging system. Ophthalmology 2006;113:260Y6.
49 Ferris FL, 3rd, Wilkinson CP, Bird A, Chakravarthy U, Chew E, Csaky K, Sadda SR. Clinical classification of age-related macular de-generation. Ophthalmology 2013;120:844Y51.
50 American Academy of Ophthalmology Retina/Vitreous Panel. Preferred Practice Pattern®Guidelines. Age-Related Macular Degeneration. San Francisco, CA: American Academy of Ophthalmology; 2015. Available at: www.aao.org/ppp.
51 Chew EY, Davis MD, Seddon JM, et al, Age-Related Eye Disease Study Research Group. The effect of antioxidant and zinc supplements on change in drusen size/area in the Age-Related Eye Disease Study (AREDS). Invest Ophthalmol Vis Sci 2002;43:E-Abstract 1903.
52 Mitchell. S. Fineman, MD ‘Nutritional Supplements for Age-Related Macular Degeneration’ Cataract & Refractive Surgery Today January 2016
53 Chew, Emily Y., et al. ‘Secondary analyses of the effects of lutein/zeaxanthin on age-related macular degeneration progression: AREDS2 report No. 3.’ JAMA ophthalmology 132.2 (2014): 142-149.
54 Austrailian Minstery of Health:https://www.nrv.gov.au/nutrients/vitamin-e
Accessed 30/06/2018)
55 Chew, E. Y., & Clemons, T. (2012). Vitamin E and Prostate Cancer. Ophthalmology, 119(9), 1938-1939.
56 Clemons TE, Kurinij N, Sperduto RD, AREDS Research Group. Associations of mortality with ocular disorders and an intervention of high-dose antioxidants and zinc in the Age-Related Eye Disease Study: AREDS report number 13. Arch Ophthalmol 2004;122:716-26.
57 Alpha-Tocopherol, Beta Carotene Cancer Prevention Study Group. The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. N Engl J Med 1994;330:1029-35.
58 Omenn GS, Goodman GE, Thornquist MD, et al. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. N Engl J Med 1996;334:1150-5.
59 Bodai BI NT, Wong WT, et al. Lifestyle medicine: A brief review of its dramatic impact on health and survival. Perm J 2018; 22: doi: 10.7812/TPP/17-025.
60 Dai J, Jones DP, Goldberg J, et al. Association between adherence to the Mediterranean diet and oxidative stress. Am J Clin Nutr 2008; 88: (5): 1364-70.
61 Cougnard-Grégoire A, Merle BM, Korobelnik J-F, et al. Olive oil consumption and age-related macular degeneration: The Alienor study. PLoS One 2016; 11: (7)
62 Chong EWT, Simpson JA, Robman LD, et al. Red meat and chicken consumption and its association with age-related macular degeneration. Am J Epidemiol 2009; 169: (7): 867-76.
63 Chong EWT, Robman LD, Simpson JA, et al. Fat consumption and its association with age-related macular degeneration. Arch Ophthalmol 2009; 127: (5): 674-80.
64 Kaushik S, Wang JJ, Flood V, et al. Dietary glycemic index and the risk of age- related macular degeneration. Am J Clin Nutr 2008; 88: (4): 1104-10.
65 Chiu C-J, Milton RC, Klein R, Gensler G, Taylor A. Dietary carbohydrate and the progression of age-related macular degeneration: a prospective study from the age-related eye disease study. Am J Clin Nutr 2007; 86: (4): 1210.
66 Adams MK, Chong EW, Williamson E, et al. 20/20 - Alcohol and age-related macular degeneration: The Melbourne collaborative cohort study. Am J Epidemiol 2012; 176: (4): 289-98.
67 Chan DS, Lau R, Aune D, et al. Red and processed meat and colorectal cancer incidence: meta-analysis of prospective studies. PLoS One 2011; 6: (6): e20456.
68 Choi Y, Song S, Song Y, Lee JE. Consumption of red and processed meat and esophageal cancer risk: meta-analysis. World J Gastroenterol 2013; 19: (7): 1020-9.
69 Larsson SC, Orsini N, Wolk A. Processed meat consumption and stomach cancer risk: A meta-analysis. J Natl Cancer Inst 2006; 98: (15): 1078-87.
70 Bouvard V, Loomis D, Guyton KZ, et al. Carcinogenicity of consumption of red and processed meat. Lancet Oncol 2015; 16: (16): 1599-600.
71 Van Leeuwen R, Boekhoorn S, Vingerling JR, Witteman JC, Klaver CC, Hofman A, de Jong PT. Dietary intake of antioxidants and risk of age-related macular degeneration. JAMA 2005; 294: 3101–7.
72 Royal College of Ophthalmologists. Age-Related Macular Degeneration—Guidelines for Management—Update; 2009.
73 College of Optometrists. Healthy lifestyle, healthy eyes. Available at: http://lookafteryoureyes.org/eye-care/healthy-lifestyle-healthy-eyes/ Accessed 30/06/2018
74 Thomson CD, Chisholm A, McLachlan SK, Campbell JM. Brazil nuts: an effective way to improve selenium status. Am J Clin Nutr 2008; 87: 379–84.
75 Hogg, Ruth E. et al.2017. Mediterranean Diet Score and Its Association with Age-Related Macular Degeneration. Ophthalmology , Volume 124 , Issue 1 , 82 – 89
76 J. A. Mares, R. P. Voland, S. A. Sondel et al., ‘Healthy lifestyles related to subsequent prevalence of age-related macular degen- eration,’ Archives of Ophthalmology, vol. 129, no. 4, pp. 470–480, 2011.
77 I. Tomada and J. P. Andrade, ‘Science-based anti-ageing nutritional recommendations,’ in Anti-Ageing Nutrients Evidence- Based Prevention of Age-Associated Diseases, D. Neves, Ed., pp. 335–390, John Wiley & Sons, Oxford, UK, 2015.
78 B. M. J. Merle, R. E. Silver, B. Rosner, and J. M. Seddon, ‘Adherence to a Mediterranean diet, genetic susceptibility, and progression to advanced macular degeneration: a prospective cohort study,’ American Journal of Clinical Nutrition, vol. 102, no. 5, pp. 1196–1206, 2015.
79 M. Bernstein, N. Munoz, and Academy of Nutrition and Dietetics, ‘Position of the Academy of Nutrition and Dietetics: food and nutrition for older adults: promoting health and wellness,’ Journal of the Academy of Nutrition and Dietetics, vol. 112, no. 8, pp. 1255–1277, 2012.
80 Shahid, Humma, et al. ‘Age-related macular degeneration: the importance of family history as a risk factor.’ British Journal of Ophthalmology 96.3 (2012): 427-431.
81 Hyman LG, Lilienfeld AM, Ferris FL 3rd., et al. Senile macular degeneration: a case-control study. Am J Epidemiol 1983;118:213–27.
82 Klein ML, Mauldin WM, Stoumbos VD. Heredity and age-related macular degeneration. Observations in monozygotic twins. Arch Ophthalmol 1994;112:932–7.
83 Meyers SM, Greene T, Gutman FA. A twin study of age-related macular degeneration. Am J Ophthalmol 1995;120:757–66.
84 Silvestri G, Johnston PB, Hughes AE. Is genetic predisposition an important risk factor in age-related macular degeneration? Eye (Lond) 1994;8:564–8.
85 Klein BE, Klein R, Lee KE, et al. Risk of incident age-related eye diseases in people with an affected sibling: the Beaver Dam Eye study. Am J Epidemiol 2001;154:207–11.
86 Seddon JM, Cote J, Page WF, et al. The US twin study of age-related macular degeneration: relative roles of genetic and environmental influences. Arch Ophthalmol 2005;123:321–7
87 Klein R, Klein BE, Linton KL. Prevalence of age-related maculopathy. The Beaver Dam Eye study. Ophthalmology 1992;99:933–43.
88 Mitchell P, Smith W, Attebo K. Prevalence of Age-related maculopathy in Australia. The Blue Mountains Eye Study. Ophthalmology 1995;102:1450–60.
89 Vingerling JR, Dielemans I, Hofman A. The prevalence of age-related maculopathy in the Rotterdam Eye study. Ophthalmology 1995;102:205–10.
90 K. J. Meyers, Z. Liu, A. E. Millen et al., ‘Joint associations of diet, lifestyle, and genes with age-related macular degeneration,’ Ophthalmology, vol. 122, no. 11, pp. 2286–2294, 2015.
91 Ho, L., van Leeuwen, R., Witteman, J. C., van Duijn, C. M., Uitterlinden, A. G., Hofman, A., ... & Klaver, C. C. (2011). Reducing the genetic risk of age-related macular degeneration with dietary antioxidants, zinc, and ω-3 fatty acids: the Rotterdam study. Archives of ophthalmology, 129(6), 758-766
92 Despriet DD, van Duijn CC, de Jong PTVM, Vingerling JR, Bergen AAB, Klaver CC. Genetic diagnosis of age-related macular degeneration: the role of molecular genetics in the identification of high risk eyes [ARVO abstract 1769/A498] Invest Ophthalmol Vis Sci. 2008
93 Narayan, R. Smoking and age related macular degeneration. Optician online (2018). https://www.opticianonline.net/cet-archive/4902. Accessed 4/7/2018