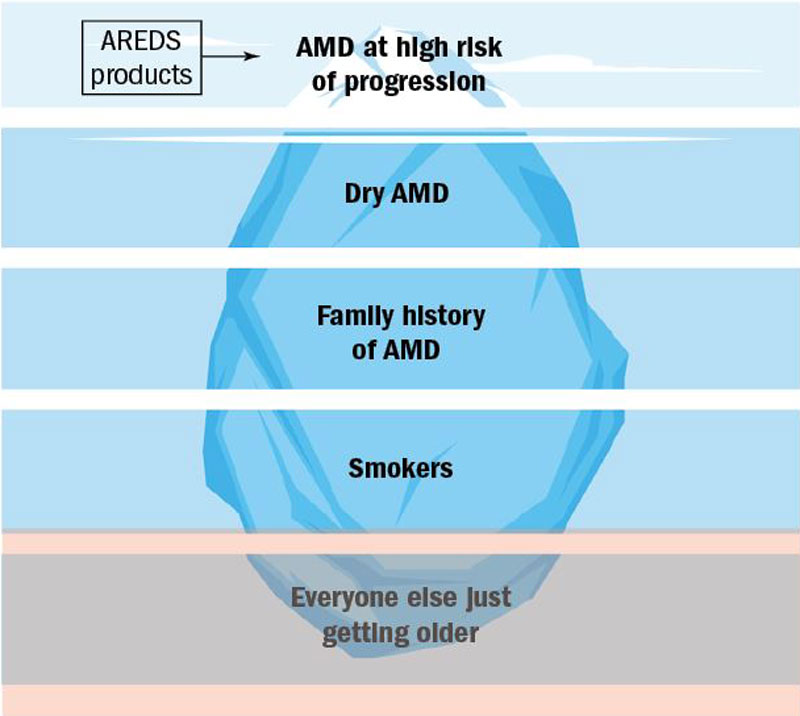
As eye care professionals, we routinely offer advice to patients with risk factors for macular degeneration. From a hierarchy of prevalence perspective, it becomes apparent that the majority of patients fall into the category of having age as their only risk factor, or simply put, the ‘everyone else’ group – what advice can we offer them?
Initially, one may think that there is little advice needed, as there is no obvious ‘disease’. Figure 1 shows clearly how age influences ocular structure even in the absence of retinal disease.
As part of natural aging, the retina suffers from chronic low grade oxidative insult, increasing in level with advancing age.1,65 In response, the retinal innate immune system undergoes low levels of activation, or para-inflammation.66 This para-inflammatory response maintains homeostasis in the healthy aging eye.4 However, it is thought that for some individuals, this para-inflammatory response is dysregulated, due in part to genetic predisposition, old age67 and lifestyle risk factors.68 This promotes a more chronic inflammatory response (figure 2), resulting in macular damage.4
Figure 1 A swept source OCT scan of patients aged 18 (a), 41 (b) and 82 (c) years old. Notice how both the retina and the underlying choroid thins with age
A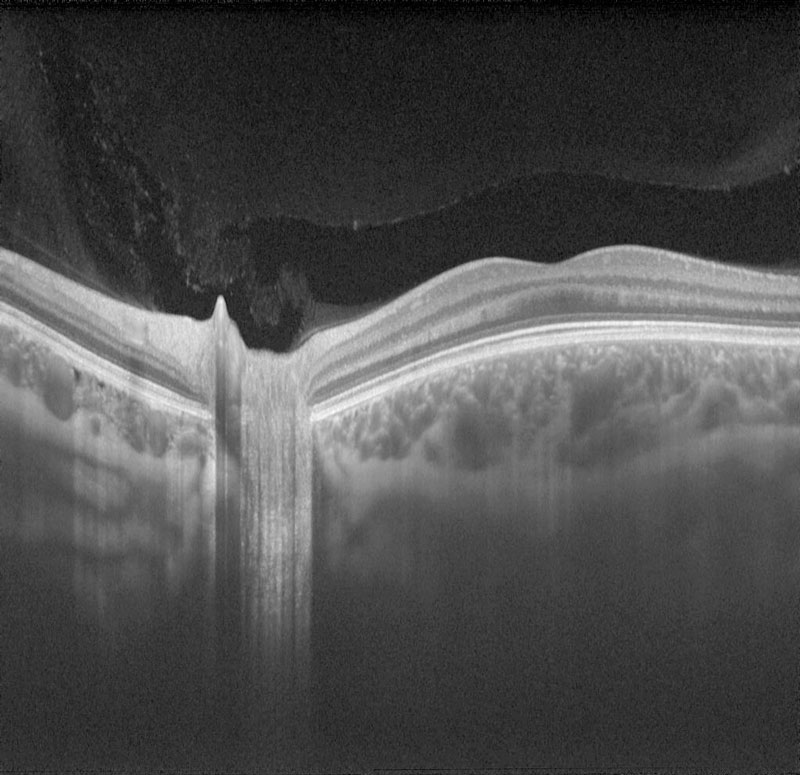 B
B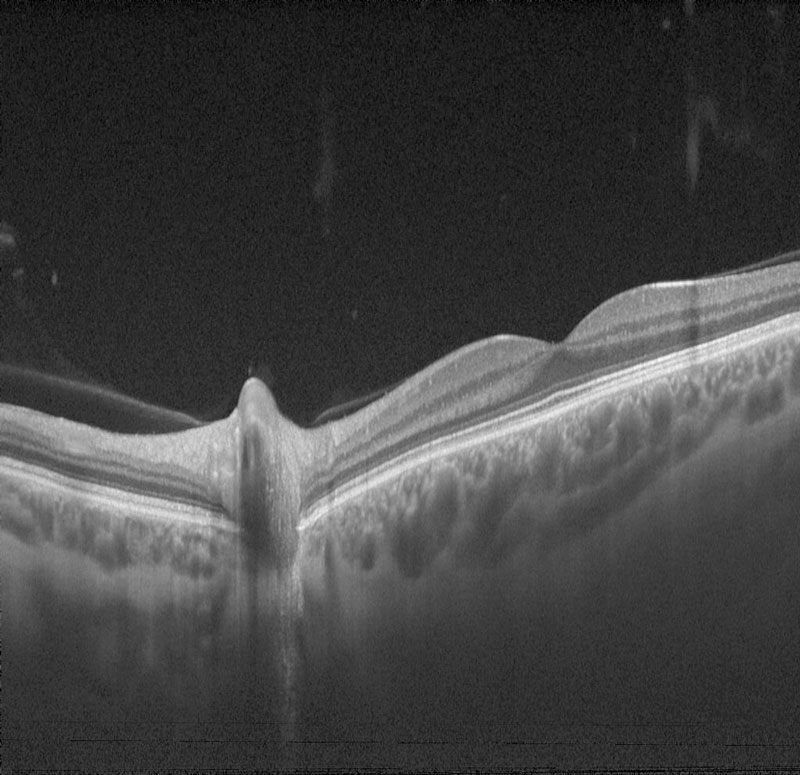 C
C
Studies have not established the benefits and safety of long term high-dose antioxidants use in the prevention or delay of the progression of AMD in early stages.3,4 Although diet is a significant modifiable factor in AMD progression, it is often difficult to dissociate a particular nutrient from other aspects of the diet. The concept of dietary lifestyle or patterns, rather than individual components, are growing in popularity and increasingly being studied in relation to AMD.5 It has also been proposed that synergistic relationships of food components exist.6,7,8 Numerous studies show that the combination of a healthy diet, the maintenance of an adequate body weight, and an active lifestyle are important to maintaining health and avoiding the physical and cognitive degeneration associated with aging.6,7,9
The evaluation of dietary habits has been studied for a number of years, indeed data from the baseline AREDS identified the ‘Oriental’ and ‘Western’ patterns10 and more recently, the ‘Prudent’ pattern.11
- Western diet: High intake of fried foods, salty snacks, eggs, and red meat.
- Oriental diet: High in tofu and sauces like soy and others.
- Prudent diet: High intake of fruits and vegetables with some characteristics of the Mediterranean diet.
It was found that the Western diet increased the population risk for acute myocardial infarction and stroke by approximately 30%. In contrast, the prudent diet decreased the same risk by 30%. The oriental diet was neutral probably because the high intake of fruits, fish, and vegetables was offset by the high salt intake and other factors.7,12,13
It has been reported that an ‘oriental diet’ as well as a diet rich in fruits, vegetables, nuts, and chicken (rather than red meat14) was associated with a lower prevalence of AMD.15,16 Conversely, the ‘Western diet’ was associated with increased odds of AMD.16
Studies have also shown that adherence to a Mediterranean diet was associated with a lower prevalence of early AMD and reduced risk of progression to advanced AMD.5,8
The Mediterranean Diet
The Mediterranean diet is one of the most studied healthy dietary patterns. This diet is rich in vegetables and fruits, thereby providing high amounts of bioactive antioxidant compounds. Furthermore, olive oil and fatty fish rich in omega-3 fatty acids are present in the Mediterranean dietary pattern.7,17
Defining a Mediterranean diet is difficult11 considering that the geographical region includes more than 17 countries, although it is thought to be inspired by the traditional diet found in Greece and southern Italy.7 It has the following characteristics:
- High consumption of fruits, legumes, and other vegetables, bread and other cereals, potatoes, beans, nuts, and seeds.
- Olive oil is the main source of monounsaturated fat.
- Dairy products, fish, and poultry are consumed in low to moderate amounts, only low quantities of red and processed meat are present.
- Wine is consumed during meals in low to moderate amounts.7,18
Overall, greater adherence to a Mediterranean diet was associated with an improvement in health status, namely, a major reduction in overall mortality (9%), mortality from cardiovascular diseases (CVD) (9%), and incidence of mortality from cancer (6%). The reduced incidence of Parkinson’s and Alzheimer’s diseases (13%) demonstrated that the adherence to the Mediterranean diet prevented these major chronic neurodegenerative diseases associated with aging.7,19,20
The Cochrane Heart Group published a review analyzing the Mediterranean dietary pattern for the primary prevention of CVD, and concluded that the Mediterranean diet may reduce some cardiovascular risk factors (total cholesterol levels, LDL cholesterol levels).21
Data from the AREDS trial and dietary information collected from food-frequency questionnaires and an alternate Mediterranean diet score was obtained.8 The consumption of fish22 and vegetables was associated with a lower risk of AMD progression. The administration of AREDS supplements did not change the protective effect of the Mediterranean diet on the risk of progression.8
Figure 2 Natural retinal aging involves the shift towards greater oxidative stress and a pro-inflammatory environment. (Adapted from Ardeljan)1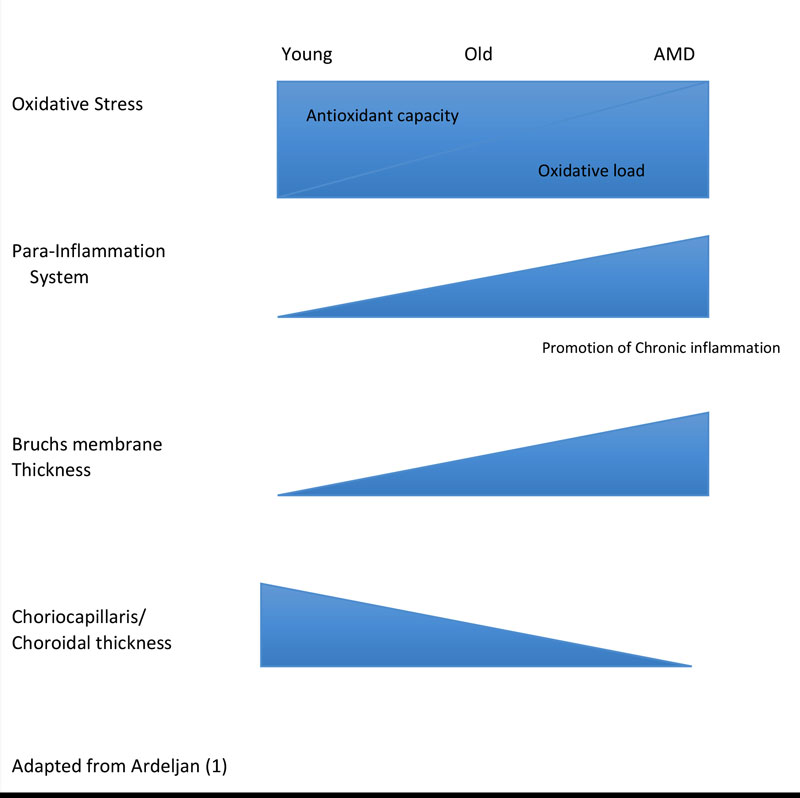
Olive oil is a component of the Mediterranean diet and exhibits strong antioxidant and anti-inflammatory properties.8 Studies suggest a protective role of olive oil consumption for late AMD,23 although the exact mechanism requires further study.24
Omega-3 has been in the news recently for its apparently disappointing effects in a recent RCT investigating its benefit in dry eye management,25 although numerous observational studies have concluded its beneficial effect in dry eye management. The apparent conflicting findings between Observational and RCT’s has been mirrored previously when considering omega-3 and its role in AMD progression, and is worthy of further discussion.
OMEGA 3 – THE ONE THAT GOT AWAY?
Although there have been relatively few interventional studies, a large number of epidemiological studies have taken place, examining the effects of diets rich in omega-3 on the progression of AMD over a number of years where incident AMD is recorded. In these studies, nutritional intake is often estimated from food frequency questionnaires. In the majority of studies there is a high degree of consistency with regard to a preventative effect of omega-3 in AMD.26-34
The Eye Disease Case Control Study in the United States demonstrated an association between the higher intake of omega-3 fatty acids and a lower risk of AMD.29 The Blue Mountains Eye Study, demonstrated a protective effect of omega-3 fatty acids in late AMD.27 Of particular interest are the subjects in the original AREDS trial who reported the highest consumption of n-3 fatty acids were also less likely to have neovascular AMD at baseline.35 Other studies have also reported a beneficial effect of consumption of omega-3 fatty acids and neovascular AMD.32,36
Some studies suggesting that genetic predispositions to the development of AMD can be countered by dietary intake of omega-3,37,38 opening up the possible future role of epigenetics and genetic testing in a clinical setting.
In light of these observational studies supporting the beneficial effects of omega-3 in AMD progression, there is a clear need for the results of good-quality interventional studies. Such studies face a number of challenges, specifically, a large number of subjects in order to achieve sufficient statistical power and the studies need to be long term in order for effects to be realised on what is a progressive disease. Earlier interventional studies, used less rigorous methodologies or multiple supplements making it challenging to differentiate the effects of omega-3 from other components.39,40 There have been, however, two well-conducted randomised, double-blind controlled trials of omega-3 in the prevention of AMD, although both studies raise some questions.
AREDS2
Although the protocol and general conclusions have been earlier discussed it is worth refreshing some important considerations as they relate to the effect of omega-3.
The study objective was to identify a reduction in AMD risk of 25% or more over the original AREDS study.41 It is important to keep in mind that the majority of the enrolled participants were taking AREDS1 formula, so the 25% effect size would need to exceed the beneficial effect of the AREDS1 formula. This means that if the beneficial effect is lower than 25% it would not be detected by AREDS2 study design.42 Despite an extensive protocol, no real placebo group was included, the control group comprising subjects taking the AREDS1.43
The subjects in AREDS2 were well educated, predominantly white and well nourished. It can also be inferred that they were health conscious; more than 40% were taking cholesterol lowering drugs and less than 7% were smokers (compared with the US average of 19%).44 Moreover, more than 10% of subjects in the control groups took nutritional supplements contravening the protocol guidelines. Indeed, a number of key baseline nutritional parameters, including serum lutein, zeaxanthin and DHA/EPA, were significantly better in the AREDS subjects than for the US population as a whole, which is unexpected in AMD subjects according to the epidemiological evidence exposed above,45 raising further questions as to how representative the test subjects were of the general population.26
The results regarding dietary supplementation with omega-3 fatty acids are of interest. In total, 1,749 patients were randomised to DHA/EPA supplementation and 1,691 to reference treatment. There were 507 advanced AMD events in the DHA/EPA supplementation group and 493 in the reference treatment groups, suggesting that DHA/EPA does not significantly protect against AMD.26
NAT2
The only other prospective randomised study of the prophylactic effect of omega-3 on AMD was reported almost simultaneously with AREDS2.46 Although this double-blind study had a relatively smaller sample size to AREDS2 (263 patients with early signs of AMD in the study eye and neovascular AMD in the other eye), the study specifically focused on a potential prophylactic effect of omega-3, mainly DHA, and therefore only two groups were required, one of which was a true placebo group. No background treatment was allowed in this study. In common with AREDS2, around half of all subjects were taking cholesterol-lowering drugs and the proportion of smokers was also relatively low. The study continued for three years and looked at the time taken for choroidal neovascularisation (CNV). In contrast to AREDS2, subjects in NAT2 who reported taking supplements not permitted in the protocol were not included in the efficacy analysis. The time to occurrence of CNV in the study eye was not significantly different between the DHA group and the placebo group.
Initially, the results of NAT2 do not appear different to those of AREDS2. There are complementarities between the results of AREDS2 and NAT2 that need to be analysed in order to form a view on the utility of omega-3 prophylaxis in AMD. Although superficially similar, the two studies have a number of important methodological differences.
In NAT2 Red Blood Cell Membrane (RBCM) analysis of EPA/DHA levels were studied rather than serum blood analysis as found in AREDS2. This difference in methodology is important as serum DHA/EPA reflects levels over a period of days47 rather than RBCM DHA/EPA which reflects levels over a period of months, and is considered to be a more reliable indicator of ongoing DHA/EPA status.47,48 In the treated group, patients who maintained consistently high RBCM EPA/DHA levels were significantly protected against AMD compared with those with permanently low EPA/DHA levels, a finding somewhat analogous to that observed in the epidemiological studies.26
A subsequent analysis of the NAT2 data indicates yet more strongly the relationship between polyunsaturated fatty acids and AMD incidence. Patients from the NAT2 study with neovascular AMD in one eye and early lesions in the other eye were compared with controls without AMD. Not only was dietary oily fish and seafood consumption significantly lower in patients with AMD than in controls, but serum red blood cell EPA and EPA + DHA were associated with a substantial and significantly lower risk of neovascular AMD.49
While AREDS2 enrolled an impressive 4,203 subjects, a complex protocol distributed them among 20 separate treatment groups in two randomisations that evaluated the effects of lutein/zeaxanthin, beta carotene and a lower dose of zinc, as well as that of DHA/EPA.
In contrast, the NAT2 was focused specifically on the effects of DHA on the progression of AMD.
NAT2 included a true placebo group (olive oil), while the control group in AREDS2 received the nutritional supplement already found effective in AREDS1 (the control group was also randomised in the second randomisation), but no placebo for the DHA/EPA treatment. Patients in both studies appeared to be both well educated and health conscious, although the nutritional status of the AREDS2 group for DHA/EPA and some other parameters was significantly above the national average. In AREDS, 11% of the subjects reported taking DHA/EPA supplements in violation of the protocol but remained in the study. In this context it is worthwhile noting that the serum level of DHA in the control group had increased by 14% by the end of the study. In NAT2, the protocol required that such noncompliant subjects were withdrawn from the study.46
Such differences in methodology might account for some of the differences in the results between the two studies; the control group in AREDS2 received supplements already shown to be effective in the prevention of AMD progression and, moreover, more than 10% added DHA/EPA supplements to their diet. This could make it more difficult to demonstrate a real effect of DHA/EPA.26 Such a hypothesis is supported by the finding in NAT2 that only those patients who had consistently raised DHA/EPA levels had delayed progression of their disease. Such an analysis for the AREDS2 data is not currently available for the 244 patients whose DHA/EPA levels were measured at three years.
There are further formulation differences between the two studies. Although the overall dose of omega-3 used was the same (1g per day), the formulations used were different; in AREDS2 ethyl-esters of the fatty acids were administered with a DHA/EPA ratio of 1:2 compared with the triglyceride formulation with a DHA/EPA ratio of 3:1 in NAT2.26 Studies suggest that the bioavailability of the triglyceride formulation is significantly higher than that of the ethyl-ester fatty acid formulation.50 Moreover, the differences in the proportion of DHA in the supplements may also be significant; DHA comprises around 50% of the fatty acids in retinal photoreceptor membranes (compared with EPA that comprises merely 0.1%) and DHA has proven anti-oxidant, anti-inflammatory, anti-apoptotic, neuroprotective and anti-angiogenic properties.51 Health claims regarding the role of DHA and EPA in the maintenance of vision have received approval from the European Food Safety Authority.52
In short, the majority of evidence suggesting a positive effect of dietary omega-3 intake on the development and progression of AMD comes from observational studies and remains to be demonstrated in randomized clinical trials.11,53 Given the conflicting findings and potential limitations in methodology from the most recent interventional studies, further investigation towards clarifying the role of omega-3 is warranted.26 The recent Cochrane review53 acknowledges that unanswered questions still exist, suggesting further investigation. Since RCTs are expensive to conduct, a more cost effective approach would be to include AMD outcomes in large trials of other morbidities. One such trial is the ongoing VITAL-AMD study (VITamin D and OmegA 3 triaL). This ancillary study to the VITamin D and OmegA-3 TriaL 54 will examine whether vitamin D3 and omega-3 fatty acids can help prevent the onset or progression of AMD.
Vitamin D
There is growing interest in the role of Vitamin D and its association with reducing the progression of AMD. Studies suggests that higher intake of dietary vitamin D is associated with a lower risk of progression to advanced AMD.2,55
Vitamin D is produced by skin following sunlight exposure,56 as well as from food. As it is not yet activated, it can be stored in fat cells for later use. Regardless of how it is derived, it is activated in the liver and kidneys.2
Vitamin D is well known for its importance in bone health57 and other potential benefits have been suggested through observational studies58,59 as shown below, although these have yet to confirmed through RCT.60
Figure 3 Major biological functions of Vitamin D2
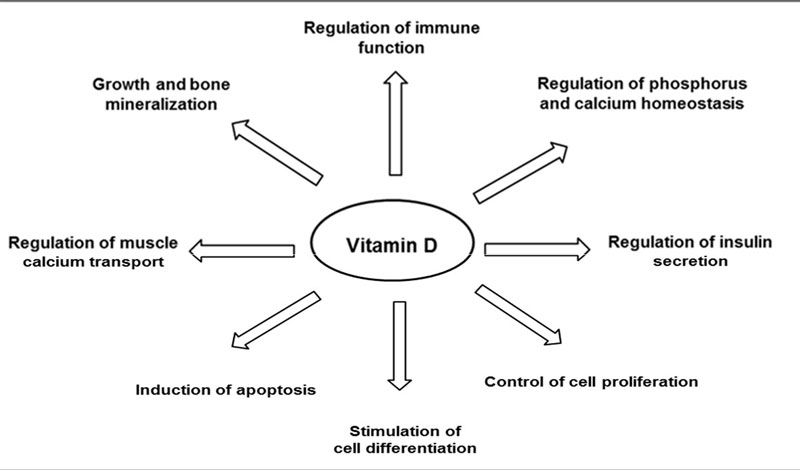
Vitamin D performs a variety of functions beneficial to the retina including reducing oxidative stress,2 reducing chronic inflammatory pathways as well reducing the potential for angiogenesis (figure 3).55 The potential benefits of Vitamin D, come largely through observational studies which suggest an association between vitamin D deficiency and a higher risk of early and/or late AMD. To date, RCT’s assessing the effect of vitamin D supplementation in preventing the onset or progression of AMD are lacking, and consequently there are no specific dietary recommendations regarding vitamin D for prevention of AMD.2.As mentioned earlier, one such trial is the ongoing VITAL-AMD study (VITamin D and OmegA 3 triaL). This ancillary study to the VITamin D and OmegA-3 TriaL will examine whether vitamin D3 and omega-3 fatty acids can help prevent the onset or progression of age-related macular degeneration.54
The several mechanisms of the healthy lifestyle leading to protection are difficult to disentangle from one another.6 The role of exercise has been put forward as part of a generic plan of improved wellbeing. The rationale being that physical activity positively impact enzymatic systems related to antioxidant protection with the effect of reducing oxidative events.61 Although more research is needed, most studies recommend all levels of exercise as being beneficial6,61 however, there is recent evidence that vigorous exercise is of greater benefit to women in reducing progression to the late stage than in men.62
The energy expenditure of the physical exercise leads to an increase of daily nutrient intake. Both physical activity and diet may contribute to a better vitamin D status, also associated with lower risk of AMD.63,64
In general, a healthy diet, avoiding food which is high in sugar, fat, red meat, alcohol, refined starch, and oils, coupled with physical activity and an absence of smoking were associated with reduced occurrence of early or advanced AMD, or both.69 This risk reduction was greater when combining these positive lifestyles modifications.10
SUMMARY
The majority of the population have age as their only AMD risk factor.
Discussions of positive lifestyle modifications including:
- Mediterranean diet
- Greater consumption of chicken rather than red meat
- Absence of smoking
- Regular physical exercise
- Vitamin D awareness
AREDS based supplements not indicated.
Dr Rohit Narayan is a therapeutic optometrist based in the Midlands.
This CET series was supported by Thea Pharmaceuticals UK
REFERENCES
- Ardeljan, D., & Chan, C. C. (2013). Aging is not a disease: distinguishing age-related macular degeneration from aging. Progress in retinal and eye research, 37, 68-89.
- Layana, A. G., Minnella, A. M., Garhöfer, G., Aslam, T., Holz, F. G., Leys, A., ... & Seddon, J. M. (2017). Vitamin D and Age-Related Macular Degeneration. Nutrients, 9(10).
- AAO Retina/Vitreous PPP Panel, Hoskins Center for Quality Eye Care. Age-Related Macular Degeneration PPP - Updated 2015
- D. C. Musch, “Evidence for including lutein and zeaxanthin in oral supplements for age-related macular degeneration,” JAMA Ophthalmology, vol. 132, no. 2, pp. 139–141, 2014.
- Hogg, Ruth E. et al.2017. Mediterranean Diet Score and Its Association with Age-Related Macular Degeneration. Ophthalmology , Volume 124 , Issue 1 , 82 – 89
- J. A. Mares, R. P. Voland, S. A. Sondel et al., “Healthy lifestyles related to subsequent prevalence of age-related macular degeneration,” Archives of Ophthalmology, vol. 129, no. 4, pp. 470–480, 2011.
- I. Tomada and J. P. Andrade, “Science-based anti-ageing nutritional recommendations,” in Anti-Ageing Nutrients Evidence-Based Prevention of Age-Associated Diseases, D. Neves, Ed., pp. 335–390, John Wiley & Sons, Oxford, UK, 2015
- B. M. J. Merle, R. E. Silver, B. Rosner, and J. M. Seddon, “Adherence to a Mediterranean diet, genetic susceptibility, and progression to advanced macular degeneration: a prospective cohort study,” American Journal of Clinical Nutrition, vol. 102, no. 5, pp. 1196–1206, 2015.
- M. Bernstein, N. Munoz, and Academy of Nutrition and Dietetics, “Position of the Academy of Nutrition and Dietetics: food and nutrition for older adults: promoting health and wellness,” Journal of the Academy of Nutrition and Dietetics, vol. 112, no. 8, pp. 1255–1277, 2012.
- Chakravarthy U, Augood C, Bentham GC, et al. Cigarette smoking and age-related macular degeneration in the EUREYE Study. Ophthalmology 2007;114(6):1157-63.
- Ângela Carneiro and José Paulo Andrade, “Nutritional and Lifestyle Interventions for Age-Related Macular Degeneration: A Review,” Oxidative Medicine and Cellular Longevity, vol. 2017, Article ID 6469138, 13 pages, 2017. doi:10.1155/2017/6469138
- R. Iqbal, S. Anand, S. Ounpuu et al., “Dietary patterns and the risk of acute myocardial infarction in 52 countries: results of the INTERHEART study,” Circulation, vol. 118, no. 19, pp. 1929–1937, 2008.
- O’Donnell M,D.Xavier,C.Dieneretal.,“Rationale and design of interstroke: a global case-control study of risk factors for stroke,” Neuroepidemiology, vol. 35, no. 1, pp. 36–44, 2010.
- Elaine W.-T. Chong Julie A. Simpson Luibov D. Robman Allison M. Hodge Khin Zaw Aung Dallas R. English Graham G. Giles Robyn H. Guymer. 2009 Red Meat and Chicken Consumption and Its Association With Age-related Macular Degeneration. Am J Epidemiol (2009) 169 (7): 867-876.
- F. M. Amirul Islam, E. W. Chong, A. M. Hodge et al., “Dietary patterns and their associations with age-related macular degeneration: the Melbourne collaborative cohort study,” Ophthalmology, vol. 121, no. 7, pp. 1428.e2–1434.e2, 2014.
- C.-J. Chiu, M.-L. Chang, F. F. Zhang et al., “The relationship of major american dietary patterns to age-related macular degeneration,” American Journal of Ophthalmology, vol. 158, no. 1, pp. 118.e1–127.e1, 2014.
- K. Pallauf, K. Giller, P. Huebbe, and G. Rimbach, “Nutri- tion and healthy ageing: calorie restriction or polyphenol- rich ‘MediterrAsian’ diet?” Oxidative Medicine and Cellular Longevity, vol. 2013, Article ID 707421, 14 pages, 2013.
- M.DeLorgeril,“Mediterranean diet and cardiovascular disease: historical perspective and latest evidence,” Current Atherosclerosis Reports, vol. 15, no. 12, article 370, 2013.
- F. Sofi, F. Cesari, R. Abbate, G. F. Gensini, and A. Casini, “Adherence to Mediterranean diet and health status: meta- analysis,” British Medical Journal, vol. 337, Article ID a1344, 2008.
- F. Sofi, R. Abbate, G. F. Gensini, and A. Casini, “Accruing evidence on benefits of adherence to the Mediterranean diet on health: an updated systematic review and meta-analysis,” American Journal of Clinical Nutrition, vol. 92, no. 5, pp. 1189– 1196, 2010.
- K. Rees, L. Hartley, N. Flowers et al., “’Mediterranean’ dietary pattern for the primary prevention of cardiovascular disease,” The Cochrane Database of Systematic Reviews, vol. 8, Article ID CD009825, 2013.
- Eunyoung Cho, Shirley Hung, Walter C Willett, Donna Spiegelman, Eric B Rimm, Johanna M Seddon, Graham A Colditz, Susan E Hankinson; Prospective study of dietary fat and the risk of age-related macular degeneration, The American Journal of Clinical Nutrition, Volume 73, Issue 2, 1 February 2001, Pages 209–218, https://doi.org/10.1093/ajcn/73.2.209
- E. W.-T. Chong, L. D. Robman, J. A. Simpson et al., “Fat consumption and its association with age-related macular degeneration,” Archives of Ophthalmology, vol. 127, no. 5, pp. 674–680, 2009.
- 24 A. Cougnard-Gre ́goire, B. M. J. Merle, J.-F. K. M.-B. Rougier et al., “Olive oil consumption and age-related macular degenera- tion: the ALIENOR Study,” PLoS ONE, vol. 11, no. 7, Article ID e0160240, 2016.
- Dry Eye Assessment and Management Study Research Group. "n− 3 Fatty Acid Supplementation for the Treatment of Dry Eye Disease." New England Journal of Medicine 378.18 (2018): 1681-1690.
- E. H. Souied, T. Aslam, A. Garcia-Layana et al., “Omega-3 fatty acids and age-related macular degeneration,” Ophthalmic Research, vol. 55, no. 2, pp. 62–69, 2016.
- Chua B, Flood V, Rochtchina E, Wang JJ, Smith W, Mitchell P: Dietary fatty acids and the 5-year incidence of age-related maculopathy. Arch Ophthalmol 2006;124:981–986.
- Delcourt C, Carriere I, Cristol JP, Lacroux A, Gerber M: Dietary fat and the risk of age-re- lated maculopathy: the POLANUT study. Eur J Clin Nutr 2007;61:1341–1344.
- Seddon JM, George S, Rosner B: Cigarette smoking, fish consumption, omega-3 fatty acid intake, and associations with age-related macular degeneration: the US Twin Study of Age-Related Macular Degeneration. Arch Ophthalmol 2006;124:995–1001.
- Tan JS, Wang JJ, Flood V, Mitchell P: Dietary fatty acids and the 10-year incidence of age- related macular degeneration: the Blue Mountains Eye Study. Arch Ophthalmol 2009;127:656–665.
- Swenor BK, Bressler S, Caulfield L, West SK: The impact of fish and shellfish consumption on age-related macular degeneration. Ophthalmology 2010;117:2395–2401.
- J. P. SanGiovanni, E. Y. Chew, T. E. , E. Argon et al, “The relationship of dietary w-3 long-chain polyunsaturated fatty acid intake with incident age-related macular degeneration: AREDS report no. 23,” Archives of Ophthalmology, vol. 126, no. 9, pp. 1274–1279, 2008.
- J. P. SanGiovanni, E. Argon, T. E. Clemons and E. Y. Chew, “w-3 Long-chain polyunsaturated fatty acid intake inversely associated with 12-year progression to advanced age-related macular degeneration,” Archives of Ophthalmology, vol. 127, no. 1, pp. 110–112, 2009.
- Chong EW, Robman LD, Simpson JA, Hodge AM, Aung KZ, Dolphin TK, English DR, Giles GG, Guymer RH: Fat consumption and its association with age-related macular de- generation. Arch Ophthalmol 2009;127:674– 680.
- J. P. SanGiovanni, E. Y. Chew, T. E. Clemons et al., “The relationship of dietary lipid intake and age-related macular degeneration in a case-control study: AREDS report no. 20,” Archives of Ophthalmology, vol. 125, no. 5, pp. 671–679, 2007.
- SanGiovanni JP, Agrón E, Meleth AD, Reed GF, Sperduto RD, Clemons TE, Chew EY: Omega-3 long-chain polyunsaturated fatty acid intake and 12-year incidence of neovascular age-related macular degeneration and central geographic atrophy: a prospective cohort study from the Age-Related Eye Disease Study. Am J Clin Nutr 2009;90:1601–1607.
- Ho L, van Leeuwen R, Witteman JC, van Duijn CM, Uitterlinden AG, Hofman A, de Jong PT, Vingerling JR, Klaver CC: Reducing the genetic risk of age-related macular degeneration with dietary antioxidants, zinc, and omega-3 fatty acids: the Rotterdam study. Arch Ophthalmol 2011;129:758–766.
- Reynolds R, Rosner B, Seddon JM: Dietary omega-3 fatty acids, other fat intake, genetic susceptibility, and progression to incident geographic atrophy. Ophthalmology 2013; 120:1020–1028.
- Feher J, Kovacs B, Kovacs I, Schveoller M, Papale A, Balacco Gabrieli C: Improvement of visual functions and fundus alterations in ear- ly age-related macular degeneration treated with a combination of acetyl-L-carnitine, n-3 fatty acids, and coenzyme Q10. Ophthalmo- logica 2005;219:154–166.
- Cangemi FE: TOZAL Study: an open case control study of an oral antioxidant and ome- ga-3 supplement for dry AMD. BMC Oph- thalmol 2007;7:3.
- Sackett CS, Schenning S: The age-related eye disease study: the results of the clinical trial. Insight 2002;27:5–7.
- Elisabeth M. van Leeuwen, Eszter Emri, Benedicte M.J. Merle, Johanna M. Colijn, Eveline Kersten, Audrey Cougnard-Gregoire, Sascha Dammeier, Magda Meester-Smoor, Frances M. Pool, Eiko K. de Jong, Cécile Delcourt, Eduardo Rodrigez-Bocanegra, Marc Biarnés, Philip J. Luthert, Marius Ueffing, Caroline C.W. Klaver, Everson Nogoceke, Anneke I. den Hollander, Imre Lengyel, A new perspective on lipid research in age-related macular degeneration,Progress in Retinal and Eye Research, 2018.
- AREDS Age-Related Eye Disease Study Group: A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E and beta carotene for age-related cata- ract and vision loss: AREDS report No 9. Arch Ophthalmol 2001;119:1439–1452.
- Centres for Disease Control and Prevention: Current cigarette smoking among adults – United States, 2011. MMWR Morb Mortal Wkly Rep 2012;61:889–894.
- AREDS2 Research Group: Lutein + zeaxan- thin and omega-3 fatty acids for age-related macular degeneration: the Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA 2013;309:2005–2015.
- Souied EH, Delcourt C, Querques G, Bassols A, Merle B, Zourdani A, Smith T, Benlian P; Nutritional AMD Treatment 2 Study Group: Oral docosahexaenoic acid in the prevention of exudative age-related macular degeneration: the Nutritional AMD Treatment 2 study. Ophthalmology 2013;120:1619–1631.
- Arab L: Biomarkers of fat and fatty acid in- take. J Nutr 2003;133(suppl 3):925S–932S.
- Baylin, Ana & Campos, Hannia. (2006). The use of fatty acid biomarkers to reflect dietary intake. Current opinion in lipidology. 17. 22-7. 10.1097/01.mol.0000199814.46720.83.
- Merle BM, Benlian P, Puche N, Bassols A, Delcourt C, Souied EH: Circulating omega-3 fatty acids and neovascular age-related macular degeneration. Invest Ophthalmol Vis Sci 2014;55:2010–2019.
- Dyerberg J, Madsen P, Møller JM, Aardestrup I, Schmidt EB: Bioavailability of marine n-3 fatty acid formulations. Prostaglandins Leukot Essent Fatty Acids 2010;83:137–141.
- SanGiovanni JP, Chew EY: The role of omega-3 long-chain polyunsaturated fatty acids in health and disease of the retina. Prog Retin Eye Res 2005;24:87–138.
- EFSA Panel on Dietetic Products, Nutrition and Allergies: Scientific opinion on the sub- stantiation of health claims related to docosa- hexaenoic acid (DHA), eicosapentaenoic acid (EPA) and brain, eye and nerve development (ID 501, 513, 540), maintenance of normal brain function (ID 497, 501, 510, 513, 519, 521, 534, 540, 688, 1323, 1360, 4294), mainte- nance of normal vision (ID 508, 510, 513, 519, 529, 540, 688, 2905, 4294), maintenance of normal cardiac function (ID 510, 688, 1360), ‘maternal health; pregnancy and nursing’ (ID 514), ‘to fulfil increased omega-3 fatty acids need during pregnancy’ (ID 539), ‘skin and digestive tract epithelial cells maintenance’ (ID 525), enhancement of mood (ID 536), ‘membranes cell structure’ (ID 4295), ‘anti- inflammatory action’ (ID 4688) and maintenance of normal blood LDL-cholesterol con- centrations (ID 4719) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J 2011;9:2078.
- Lawrenson JG, Evans JR. Omega 3 fatty acids for preventing or slowing the progression of age-related macular degeneration. Cochrane Database of Systematic Reviews 2015, Issue 4. Art. No.: CD010015. DOI: 10.1002/14651858.CD010015.pub3.
- VITAL (2017) https://clinicaltrials.gov/ct2/show/NCT01782352
- B. M. J. Merle, Rachel E. Silver, Bernard Rosner, Johanna M. Seddon; Associations Between Vitamin D Intake and Progression to Incident Advanced Age-Related Macular Degeneration. Invest. Ophthalmol. Vis. Sci. 2017;58(11):4569-4578. doi: 10.1167/iovs.17-21673.
- Holick, M.F. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am. J. Clin. Nutr. 2004, 80, 1678S–1688S.
- Lips, P.; van Schoor, N.M. The effect of vitamin D on bone and osteoporosis. Best Pract. Res. Clin. Endocrinol. Metab. 2011, 25, 585–59
- Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281.
- Bikle, D.D. Vitamin D metabolism, mechanism of action, and clinical applications. Chem. Biol. 2014, 21, 319–329.
- Scientific Advisory Committee on Nutrition. Vitamin D and Health (2016). Avaliable online: https://www.gov.uk/ government/publications/sacn-vitamin-d-and-health-report
- P. D. Loprinzi, B. K. Swenor, and P. Y. Ramulu, “Age-related macular degeneration is associated with less physical activity among US adults: cross-sectional study,” PLoS ONE, vol. 10, no. 5, Article ID e0125394, 2015.
- McGuinness MB, Karahalios A, Simpson JA, et al Past physical activity and age-related macular degeneration: the Melbourne Collaborative Cohort Study British Journal of Ophthalmology 2016;100:1353-1358.
- N. Parekh, R. J. Chappell, A. E. Millen, D. M. Albert, and J. A. Mares, “Association between Vitamin D and age-related macular degeneration in the third National Health and Nutrition Examination Survey, 1988 through 1994,” Archives of Ophthalmology, vol. 125, no. 5, pp. 661–669, 2007.
- Annweiler, Cedric, et al. "Circulating vitamin D concentration and age-related macular degeneration: Systematic review and meta-analysis." Maturitas 88 (2016): 101-112.
- Xu H, Chen M, Forrester JV. Para-inflammation in the aging retina. Prog.Retin.Eye Res. 2009; 28:348–368.
- Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008; 454:428–435.
- Desai A, Grolleau-Julius A, Yung R. Leukocyte function in the aging immune system. J.Leukoc.Biol. 2010; 87:1001–1009.
- Chen, M., & Xu, H. (2015). Parainflammation, chronic inflammation, and age‐related macular degeneration. Journal of leukocyte biology, 98(5), 713-725
