Contact lens discomfort (CLD) is an issue that eye care practitioners and patients are all too aware of, and one that requires attention in practice during contact lens (CL) aftercare on an almost daily basis. The experience of sub-optimal comfort can range from subtle end of day lens awareness, through to more noticeable discomfort that may drive early removal of lenses. Left unattended, the progression of CLD can of course lead to dropping out of contact lens wear. It is widely understood that discomfort is the primary factor associated with ceasing CL wear with estimates of drop out attributable to CLD ranging from 12-51%.1
The Tear Film and Ocular Surface (TFOS) society’s report on the management of CLD recognises the multifactorial nature of the issue, and presents the potential causes and management as a flow diagram which can be followed to address the problem in a logical order.2 A number of common management strategies are highlighted in this approach which include changing care solution, material or lens design, adjusting replacement frequency, including changing to daily disposable lenses, tear supplementation and giving tips for improving the environment the lenses are worn in. These are all factors which eye care practitioners consider when trying to improve the wearing experience for their CL patients.
One further consideration is the eyelid, specifically the lid margin. Acute presentations of meibomian gland dysfunction (MGD) or blepharitis are covered in the TFOS management summary under the ‘eliminate any co-existing factors’ part of the strategy. What though of more subtle lid changes? How may these influence CLD, and how prominently should they feature in the practitioner’s mind as one of the options to help improve comfort? This article reviews the evidence for the effect the eyelids have on CL comfort, and provides insight into the potential for managing some presentations of CLD by addressing the health and condition of the lids rather than reaching for a different CL or solution.
Overview of the eyelids
The eyelids are highly specialised structures which provide physical protection to the eye via the blink reflex and eyelashes. They are responsible, via the motion of the blink, for refreshing the tear layer which resides over the surface of the eye. Within the tarsal plates of the eyelids are 30-40 (upper lid) and 20-30 (lower lid) meibomian glands.3 Through their production of meibum these glands are the major contributor to the lipid layer of the tear film. The primary functions of the lipid layer are thought to be preventing tear evaporation and aiding lubrication,4,5 with additional roles in enabling spread and preventing collapse of the tear film.6
Clinical examination of the lid margin involves the assessment of their overall appearance in terms of shape, position, and regularity. The meibomian gland orifices can be viewed and by applying gentle pressure, the glands expressed to assess the quantity and quality of meibum.7 The condition of the skin, particularly around the base of the eyelashes can be determined, along with noting the overall position of the lids on eye closure. Diagnostic stains can be used to visualise tissue changes in the lid wiper region of the lid.
Meibomian gland dysfunction
Meibomian gland dysfunction (MGD) is defined as ‘a chronic, diffuse abnormality of meibomian glands, commonly characterised by terminal duct obstruction or qualitative or quantitative changes in glandular secretion.'8 More advanced symptomatic MGD can result in posterior blepharitis, but it should be noted that the terms for these two conditions are not synonymous. MGD is only one cause of posterior blepharitis, and early stage MGD can present without blepharitis.8
Contact lens wear and meibomian glands
Changes in meibum result in disruption of the tear film lipid layer leading to symptoms of dry eye. Some parallels can be drawn between MGD and CLD. Although CLD is multifactorial, one aspect of CL wear is that it disrupts the tear film, reducing tear break up time and destabilising the lipid layer.9-11 It has been suggested that it is particularly important to pay attention to the lipid layer and condition of the meibomian glands in CL wearers because they could be considered as having sub-clinical MGD and be at risk for this progressing.12 Indeed, a number of studies have found changes to the lid margins and meibomian glands of CL wearers. These include lid margin irregularity, vascular changes and blocked or plugged glands, plus changes to meibum quality and abnormal meibography findings.13-15 It should also be noted that some studies have not found the same associations between CL wear and meibomian gland changes.16,17 A recent study examined the relationship between CLD and the eyelids and tear film, finding that morphological irregularities of the glands and alterations to tear film that affected evaporation were associated with discomfort in symptomatic CL wearers.18
Figure 1 Lower lid margin appearance prior to expression of meibum (images used courtesy of N. Rumney)

A further point to consider is the association between MGD and the presence of bacteria on the lid margins. When compared to normal eyes, those with MGD have been found to have a higher bacterial load, typically of Gram positive organisms such as Staphylococcus aureus, on the lid margin.19,20 These higher levels of bacteria have been associated with a five-fold increased risk for the development of corneal infiltrative events (CIEs),21 particularly at 2, 4, 8 and 10 o’clock where the lids margins touch the cornea.22
Examination
In practice, the lid margins should be assessed carefully in every prospective and current CL wearer. The clinical signs most associated with MGD have been reported as gland dropout, altered meibum consistency, and changes to lid morphology along with plugging or pouting of the orifices.23 Any thickening, rounding, notching, or telangiectasia of the lid margin should be noted. Meibography allows the whole gland to be visualised and provides a useful method to monitor change over time. In routine practice, examination can be conducted visually with the slit lamp biomicroscope, and aided with gentle expression of the glands via either a clean finger, cotton applicator or meibomian gland evaluator (Johnson & Johnson Vision).7
Figure 2 Appearance of same lid margin following expression of meibum (images used courtesy of N. Rumney)
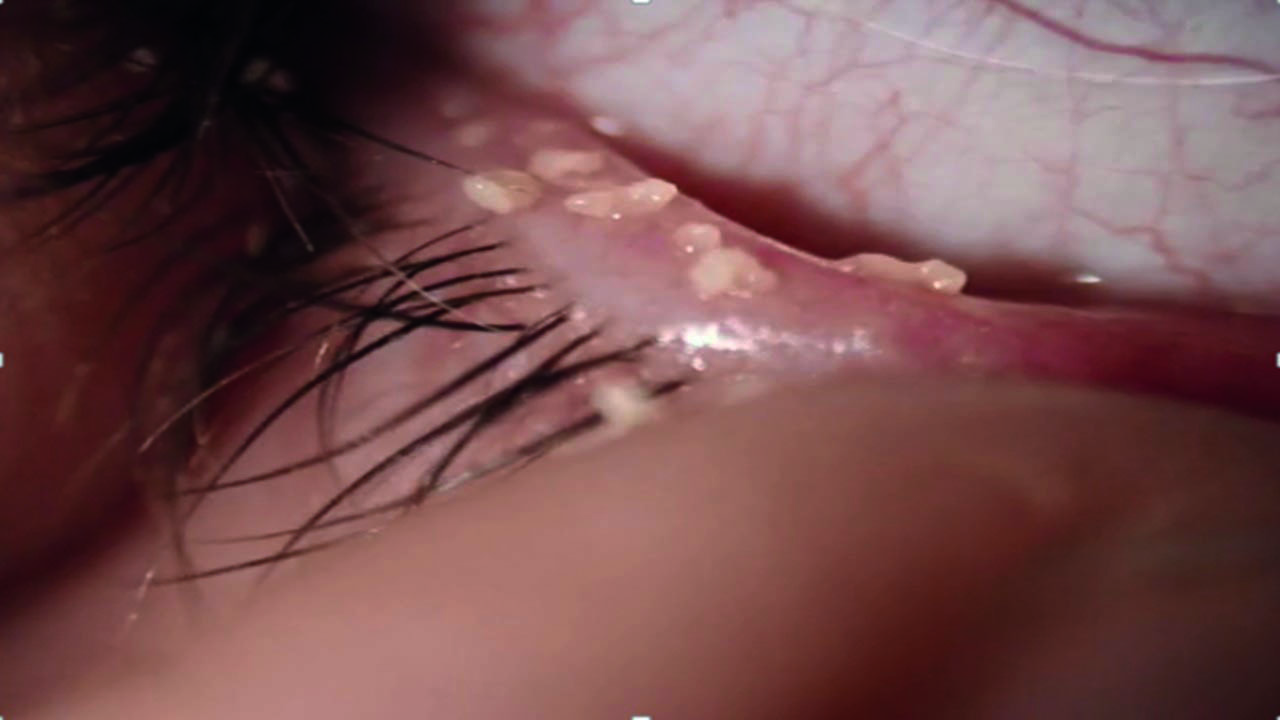
The number of glands that can express meibum and the quality of the secretion should be assessed. The importance of expressing is illustrated in figure 1 and figure 2, where the first image gives the appearance of a quiet lid margin and unremarkable meibomian glands, whereas the second image displays the thick, discoloured meibum that emerges through expression. The point here is that meibum quality cannot be determined by observation of the gland openings alone.
Management options
Treatment of more severe MGD is beyond the scope of this article, but is well summarised in the TFOS report on the management and treatment of MGD.24 When low grade and potentially sub-clinical MGD changes in a CL wearer are present it may be useful to introduce some early management such as lid cleansing, warm compresses and lid massage.
Some evidence also exists to show improvement in symptoms in non-contact lens wearers following use of omega-3 fatty acid dietary supplements.25
Low grade meibomian gland changes may respond well to these simple interventions. Active management at this stage may help to encourage meibum to be expelled, with the aim of improving the lipid layer and ultimately enhancing the stability of the pre-lens tear film for the patient.
Additional instrumentation is available for in-practice use to help treat MGD, and a recent study examined the use of such equipment in CL wearers with MGD.26 A single in-practice application of a vectored thermal pulsation treatment (LipiFlow thermal pulsation system, Johnson & Johnson Vision) significantly improved meibomian gland function, symptoms of dry eye and increased mean comfortable wearing time by four hours.26 Of interest is that the increase in comfortable wearing time after just one treatment was sustained for up to three months on average.
In terms of CL material, given that MGD involves changes in lipid secretions, it may seem logical to avoid silicone hydrogels with their known affinity for lipid deposition.27,28 However, although silicone hydrogels have been shown in vitro to accumulate lipid, in terms of on-eye performance it has proved difficult to link lipid deposition to reduced comfort or visual performance.29
So while the practitioner has an open choice of CL material, there is evidence available to suggest a preferred replacement schedule in these patients. To ameliorate the increased CIE risk associated with the higher numbers of Gram positive bacteria present on the lid margin, daily disposable CLs can be recommended. Replacing CLs every time they are worn has been shown to reduce the risk of CIEs,30,31 as well reducing the amount of lipid deposition compared to longer replacement frequencies.32
Figure 3 Cylindrical dandruff pathognomonic for Demodex folliculorum (image courtesy of Professor Etty Bitton)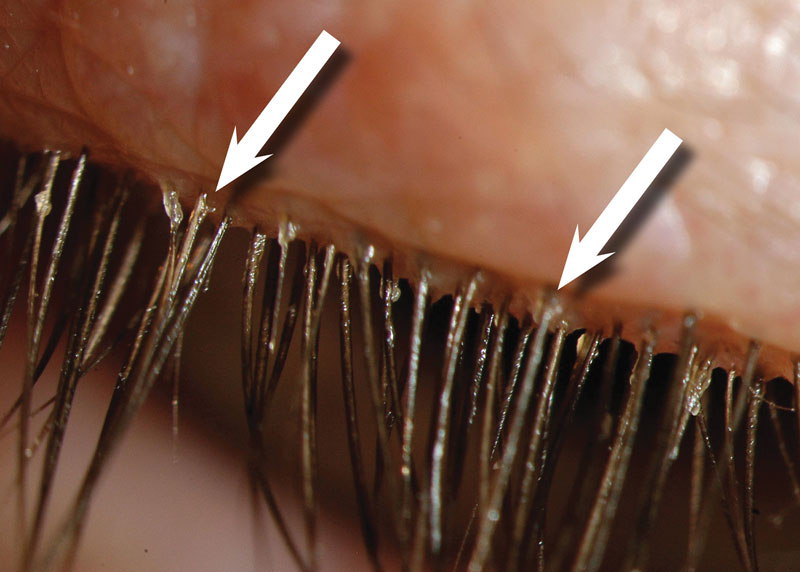
Figure 4 Base of lash prior to circular rotation (top) and post rotation (bottom) with tails of Demodex folliculorum visible (image courtesy of Professor Etty Bitton)
Anterior Blepharitis
Anterior blepharitis is commonly encountered in clinical practice. It is typically described as a chronic inflammatory condition and affects the base of the eyelashes, eyelash follicles and skin.33,34 Anterior blepharitis can be further subdivided into Staphylococcal and seborrhoeic forms. These forms present differently, with Staphylococcal blepharitis associated with changes to eyelash direction or loss of lashes, and seborrhoeic blepharitis characterised by oily, greasy lid margins, possibly in association with skin disorders.34 The clinical features of anterior blepharitis can overlap with those of dry eye,33 and it has been linked with aqueous deficient dry eye.34,35 In fact one theory directly linking the two conditions has been proposed, termed ‘dry eye blepharitis syndrome’ or DEBS, where dry eye is said to be a later manifestation of chronic blepharitis.36
Contact lens wear and anterior blepharitis
It is of interest to note that the TFOS report on CLD mentions blepharitis only in passing. It is referred to in the executive summary as a co-existing pathology which should be identified and treated prior to fitting.37 However its association with both dry eye and MGD suggest the presence of low grade blepharitis on the lid margins could well affect the tear film and in turn, CL comfort.
Additionally, blepharitis is also associated with higher numbers of bacteria on the lid margin.38-40 Similar to MGD, these tend to be Gram positive bacteria such as Staphylococcus species, which can increase the risk of marginal keratitis.
Management options
Higher grades of blepharitis may require topical or systemic use of both antibiotics and steroids and it would be advisable to avoid CL wear until the condition was reduced in severity. For early changes however, what options are available for the practitioner who is trying to help CL comfort? Given the association with higher levels of bacteria, advising on both lid hygiene and use of daily disposable CLs would be recommended.
There is a wide choice of products available to clean the lid margin, from lid wipes and washes, to in-practice ‘deep-clean’ treatments such as BlephEx (Scope Ophthalmics).41 If everything else is looking satisfactory with the CL fit and performance, the practitioner might explore addressing the condition of the lid margins to see if that alone could improve comfort. Further research in this area would be a welcome addition to the evidence base around whether treating blepharitis can impact CLD.
Demodex
Over the last few years infestation of the eyelash follicles by the ectoparasite Demodex folliculorum has received increasing attention. The presence of both Demodex folliculorum and the smaller Demodex brevis, which reside in human sebaceous glands, is considered normal. Prevalence increases with age, to a maximum of 100% by the age of 70,42 with higher numbers of mites associated with certain skin conditions such as rosacea.43
It has been suggested that Demodex become pathogenic once they are present in sufficient numbers. Whilst not causing infection themselves, they do cause damage, irritation and inflammation to the eyelash follicle.44,45 They can act as a vector to bring pathogenic microorganisms into the follicle, and when they die, they burst open, which releases their waste products, causing further irritation. Itching is the symptom most significantly associated with Demodex infestation,46,47 although many people are asymptomatic.
Contact lens wear and Demodex
It is of interest that an association has been found between CL wear and Demodex infestation. CL wearers have been found to have Demodex present in their eyelash follicles at least as frequently as non-wearers (90% vs. 65%, p=0.06), but in significantly higher numbers.48 A further study examined the difference in numbers of Demodex between asymptomatic CL wearers and those who had dropped out of wear. There was a significant difference between the two groups, with 6% of asymptomatic wearers found to have Demodex, compared to 93% of CL dropouts.49 These associations suggest a higher number of Demodex is possible in CL wearers, and that the presence of Demodex in these patients may contribute to discomfort and lead to wearers being unable to successfully wear their lenses.
Examination
Clear cylindrical dandruff or ‘collarettes’ around the base of the eyelashes is considered pathognomonic for Demodex folliculorum (figure 3).50 To confirm presence of the mite, the eyelash can be epilated, and the base of the lash examined under magnification. More practical in a clinical setting is to follow the technique described by Katherine Mastrota, where tweezers are used to hold and gently rotate an eyelash, while the base of that lash is observed for tails of the Demodex mites to appear (figure 4).51
Management
Given the potential for Demodex to affect comfort it would appear prudent to initiate treatment in CL wearers once their presence has been confirmed. In-practice lid debridement can help to remove cylindrical dandruff. To kill Demodex an agent containing terpineol-4-ol is typically used. This is the active ingredient in tree tea oil, with weekly lid scrubs using a 50% solution of tree tea oil recommended by the College of Optometrists.
It is advised this application is only undertaken by experienced practitioners and delivered in practice because this concentration of tea tree oil is toxic to the ocular surface. Use of lower tea tree oil concentrations has also been examined with a 5% ointment shown to be effective in reducing signs and symptoms.52 Options such as lid wipes containing 25% tea tree oil,45 and tea tree oil cleaning foams do exist for patients to use at home. A recent study examined the efficacy of two at-home treatments (OcuSoft Lid Scrub and Dr Organic Tea Tree Face Wash) and one in-practice treatment (BlephEx device) and found all three to reduce quantity of Demodex and improve symptoms in non-CL wearers.4
Lid wiper epitheliopathy
An article on the assessment of lid margins in CL wearers would not be complete without mention of the lid wiper. This is the area of the lid at the mucocutaneous junction that is in contact with the surface of the eye and CL during a blink. The presence of lid wiper epitheliopathy (LWE), as assessed with either fluorescein, lissamine green or combination staining of both has been associated with dry eye and CL wear (figure 5).53
Figure 5 Lid wiper epitheliopathy shown by lissamine green staining
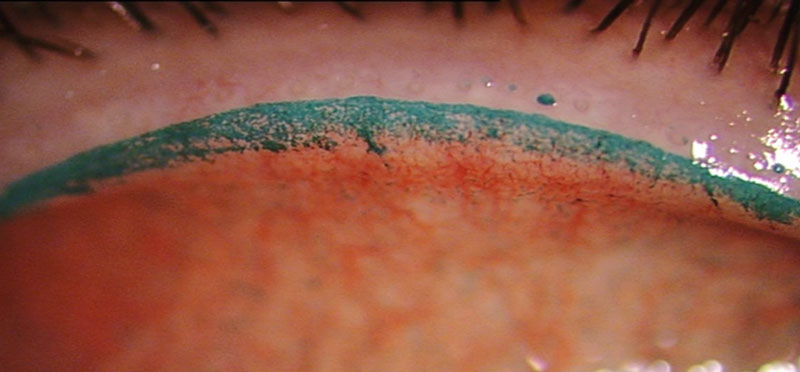
The mechanism for LWE has been postulated to be the result of frictional effects caused by a thin, unstable tear film or poorly wetting CL surface.53,54 For CL wearers, the evidence associating LWE with CLD remains equivocal.18,55-58 A recent large cross-sectional study attempted to better describe prevalence of LWE in certain populations, the characteristics linked to its severity and its association with ocular symptoms.59 LWE was found to be more prevalent in Asians and CL wearers. In terms of symptoms, no association was found between the severity of LWE in non-CL wearers, however in CL wearers width of LWE was associated with greater symptoms of ocular discomfort. It was noted that this was only clinically significant for moderate to severe grades of LWE.59
In terms of action to take, LWE is different to the lid margin issues discussed earlier in the article in that it would potentially necessitate changing the CL material. Higher levels of CL comfort have been linked to materials that have a lower coefficient of friction,60,61 and a recent study showed that for the same material used with, and without a surface enhancing coating, comfort was improved when the coating was used.62 So this is one instance where reaching for a different CL may be sensible. However, it is worth noting that to make a meaningful clinical difference to symptoms through the changing of a CL material it has been suggested that the grade of LWE seen would need to be at least moderate to severe to start with. That is, the wearer is unlikely to notice an improvement in symptoms with a different CL if only low grade LWE is present.59
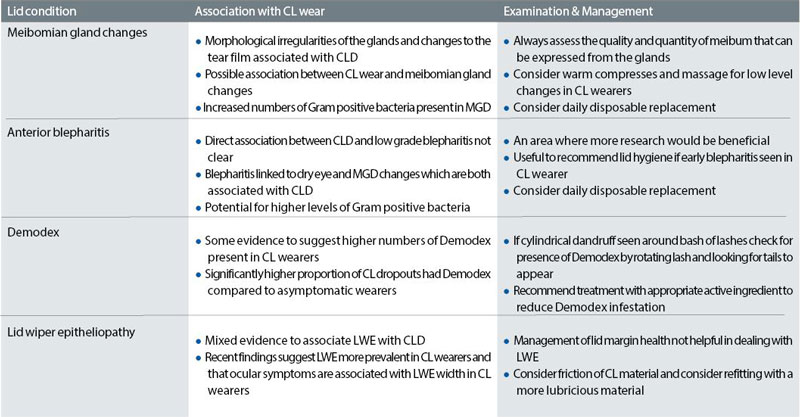
Table 1 summarises the assessment and management of common disorders of the eyelids.
Conclusion
So next time a CL wearer presents with symptoms of discomfort, the evidence summarised here suggests that in addition to the more usual considerations of the tear film, ocular surface, CL fit, material and care solution, that the practitioner should also pay close attention to the lid margins.
Minor and early changes to the meibomian glands, low levels of blepharitis and presence of any Demodex should all be addressed in a CL wearer. Given early intervention it may be possible to help manage some symptoms of CLD through improving lid margin health. It certainly provides the practitioner with an alternative consideration for the management of their patients, particularly when CL factors such as fit, material and care system have already been optimised.
Karen Walsh is a clinical scientist, Sruthi Srinivasan is research assistant professor and clinical research manager at the Centre for Ocular Research & Education University of Waterloo. Lyndon Jones is professor at the School of Optometry & Vision Science and Director at the Centre for Ocular Research & Education University of Waterloo, Waterloo, Ontario, Canada
References
- Nichols JJ, Jones L, Nelson JD, et al. The TFOS International Workshop on Contact Lens Discomfort: Introduction. Investigative ophthalmology & visual science 2013;54:TFOS1-6.
- Papas EB, Ciolino JB, Jacobs D, et al. The TFOS International Workshop on Contact Lens Discomfort: report of the management and therapy subcommittee. Investigative ophthalmology & visual science 2013;54:TFOS183-203.
- Andrews JS. The Meibomian secretion. International ophthalmology clinics 1973;13:23-8.
- Butovich IA. Tear film lipids. Experimental eye research 2013;117:4-27.
- Pucker AD, Haworth KM. The presence and significance of polar meibum and tear lipids. The ocular surface 2015;13:26-42.
- Millar TJ, Schuett BS. The real reason for having a meibomian lipid layer covering the outer surface of the tear film - A review. Experimental eye research 2015;137:125-38.
- Zhao Y, Xie J, Li J, et al. Evaluation of Monocular Treatment for Meibomian Gland Dysfunction with an Automated Thermodynamic System in Elderly Chinese Patients: A Contralateral Eye Study. J Ophthalmol 2016;2016:9640643.
- Nelson JD, Shimazaki J, Benitez-del-Castillo JM, et al. The international workshop on meibomian gland dysfunction: report of the definition and classification subcommittee. Investigative ophthalmology & visual science 2011;52:1930-7.
- Young G, Efron N. Characteristics of the pre-lens tear film during hydrogel contact lens wear. Ophthalmic & physiological optics : the journal of the British College of Ophthalmic Opticians 1991;11:53-8.
- Guillon M, Styles E, Guillon J, et al. Preocular tear film characteristics of nonwearers and soft contact lens wearers. Optometry and vision science 1997;74:273-9.
- Morris CA, Holden BA, Papas E, et al. The ocular surface, the tear film, and the wettability of contact lenses. Advances in experimental medicine and biology 1998;438:717-22.
- Arita R, Fukuoka S, Morishige N. Meibomian Gland Dysfunction and Contact Lens Discomfort. Eye & contact lens 2017;43:17-22.
- Alghamdi WM, Markoulli M, Holden BA, et al. Impact of duration of contact lens wear on the structure and function of the meibomian glands. Ophthalmic & physiological optics : the journal of the British College of Ophthalmic Opticians 2016;36:120-31.
- Cox SM, Berntsen DA, Chatterjee N, et al. Eyelid Margin and Meibomian Gland Characteristics and Symptoms in Lens Wearers. Optometry and vision science 2016;93:901-8.
- Machalinska A, Zakrzewska A, Adamek B, et al. Comparison of Morphological and Functional Meibomian Gland Characteristics Between Daily Contact Lens Wearers and Nonwearers. Cornea 2015;34:1098-104.
- Marren S. Contact lens wear, use of eye cosmetics, and meibomian gland dysfunction. Optometry and vision science 1994;71:60-2.
- Pucker AD, Jones-Jordan LA, Li W, et al. Associations with Meibomian Gland Atrophy in Daily Contact Lens Wearers. Optometry and vision science 2015;92:e206-13.
- Siddireddy JS, Vijay AK, Tan J, et al. The eyelids and tear film in contact lens discomfort. Contact lens & anterior eye 2017.
- Zhang SD, He JN, Niu TT, et al. Bacteriological profile of ocular surface flora in meibomian gland dysfunction. The ocular surface 2017;15:242-7.
- Watters GA, Turnbull PR, Swift S, et al. Ocular surface microbiome in meibomian gland dysfunction. Clinical & experimental ophthalmology 2017;45:105-11.
- Szczotka-Flynn L, Jiang Y, Raghupathy S, et al. Corneal inflammatory events with daily silicone hydrogel lens wear. Optometry and Vision Science 2014;91:3-12.
- Jayamanne DG, Dayan M, Jenkins D, et al. The role of staphylococcal superantigens in the pathogenesis of marginal keratitis. Eye (Lond) 1997;11 ( Pt 5):618-21.
- Tomlinson A, Bron AJ, Korb DR, et al. The international workshop on meibomian gland dysfunction: report of the diagnosis subcommittee. Investigative ophthalmology & visual science 2011;52:2006-49.
- Geerling G, Tauber J, Baudouin C, et al. The international workshop on meibomian gland dysfunction: report of the subcommittee on management and treatment of meibomian gland dysfunction. Investigative ophthalmology & visual science 2011;52:2050-64.
- Macsai MS. The role of omega-3 dietary supplementation in blepharitis and meibomian gland dysfunction (an AOS thesis). Trans Am Ophthalmol Soc 2008;106:336-56.
- Blackie CA, Coleman CA, Nichols KK, et al. A single vectored thermal pulsation treatment for meibomian gland dysfunction increases mean comfortable contact lens wearing time by approximately 4 hours per day. Clinical ophthalmology (Auckland, NZ) 2018;12:169-83.
- Lorentz H, Jones L. Lipid deposition on hydrogel contact lenses: how history can help us today. Optometry and vision science 2007;84:286-95.
- Walther H, Lorentz H, Kay L, et al. The effect of in vitro lipid concentration on lipid deposition on silicone hydrogel and conventional hydrogel contact lens materials. Contact Lens and Anterior Eye 2011;34, Supplement 1:S21.
- Jones L, Brennan NA, Gonzalez-Meijome J, et al. The TFOS International Workshop on Contact Lens Discomfort: report of the contact lens materials, design, and care subcommittee. Investigative ophthalmology & visual science 2013;54:TFOS37-70.
- Chalmers RL, Keay L, McNally J, et al. Multicenter case-control study of the role of lens materials and care products on the development of corneal infiltrates. Optometry and vision science 2012;89:316-25.
- Steele KR, Szczotka-Flynn L. Epidemiology of contact lens-induced infiltrates: an updated review. Clinical & experimental optometry 2017;100:473-81.
- Walther H, Lorentz H, Heynen M, et al. Factors that influence in vitro cholesterol deposition on contact lenses. Optometry and vision science 2013;90:1057-65.
- Wolffsohn JS, Arita R, Chalmers R, et al. TFOS DEWS II Diagnostic Methodology report. The ocular surface 2017;15:539-74.
- Pflugfelder SC, Karpecki PM, Perez VL. Treatment of blepharitis: recent clinical trials. The ocular surface 2014;12:273-84.
- Lienert JP, Tarko L, Uchino M, et al. Long-term Natural History of Dry Eye Disease from the Patient’s Perspective. Ophthalmology 2016;123:425-33.
- Rynerson JM, Perry HD. DEBS - a unification theory for dry eye and blepharitis. Clinical ophthalmology (Auckland, NZ) 2016;10:2455-67.
- Nichols JJ, Willcox MD, Bron AJ, et al. The TFOS International Workshop on Contact Lens Discomfort: Executive Summary. Investigative ophthalmology & visual science 2013;54:TFOS7-TFOS13.
- Jackson WB. Blepharitis: current strategies for diagnosis and management. Can J Ophthalmol 2008;43:170-9.
- Lindsley K, Matsumura S, Hatef E, et al. Interventions for chronic blepharitis. Cochrane Database Syst Rev 2012;5:CD005556.
- Teweldemedhin M, Gebreyesus H, Atsbaha AH, et al. Bacterial profile of ocular infections: a systematic review. BMC ophthalmology 2017;17:212.
- Murphy O, O’Dwyer V, Lloyd-McKernan A. The efficacy of tea tree face wash, 1, 2-Octanediol and microblepharoexfoliation in treating Demodex folliculorum blepharitis. Contact lens & anterior eye 2018;41:77-82.
- Roth AM. Demodex folliculorum in hair follicles of eyelid skin. Annals of ophthalmology 1979;11:37-40.
- Forton F, Germaux MA, Brasseur T, et al. Demodicosis and rosacea: epidemiology and significance in daily dermatologic practice. J Am Acad Dermatol 2005;52:74-87.
- Liu J, Sheha H, Tseng SC. Pathogenic role of Demodex mites in blepharitis. Curr Opin Allergy Clin Immunol 2010;10:505-10.
- Ng A, Bitton E, Jones L. Demodex infestation of the eyelashes. Contact Lens Spectrum 2014;29:36-41.
- Kabatas N, Dogan AS, Kabatas EU, et al. The Effect of Demodex Infestation on Blepharitis and the Ocular Symptoms. Eye & contact lens 2017;43:64-7.
- Sedzikowska A, Oseka M, Grytner-Ziecina B. Ocular symptoms reported by patients infested with Demodex mites. Acta parasitologica 2016;61:808-14.
- Jalbert I, Rejab S. Increased numbers of Demodex in contact lens wearers. Optometry and vision science 2015;92:671-8.
- Tarkowski W, Moneta-Wielgos J, Mlocicki D. Demodex sp. as a Potential Cause of the Abandonment of Soft Contact Lenses by Their Existing Users. BioMed research international 2015;2015:259109.
- Gao YY, Di Pascuale MA, Li W, et al. High prevalence of Demodex in eyelashes with cylindrical dandruff. Investigative ophthalmology & visual science 2005;46:3089-94.
- Mastrota KM. Method to identify Demodex in the eyelash follicle without epilation. Optometry and vision science 2013;90:e172-4.
- Gao YY, Xu DL, Huang l J, et al. Treatment of ocular itching associated with ocular demodicosis by 5% tea tree oil ointment. Cornea 2012;31:14-7.
- Korb DR, Greiner JV, Herman JP, et al. Lid-wiper epitheliopathy and dry-eye symptoms in contact lens wearers. The CLAO journal : official publication of the Contact Lens Association of Ophthalmologists, Inc 2002;28:211-6.
- Korb DR, Herman JP, Greiner JV, et al. Lid wiper epitheliopathy and dry eye symptoms. Eye & contact lens 2005;31:2-8.
- Pult H, Purslow C, Berry M, et al. Clinical tests for successful contact lens wear: relationship and predictive potential. Optometry and vision science 2008;85:E924-9.
- Best N, Drury L, Wolffsohn JS. Predicting success with silicone-hydrogel contact lenses in new wearers. Contact lens & anterior eye 2013;36:232-7.
- Efron N, Brennan NA, Morgan PB, et al. Lid wiper epitheliopathy. Prog Retin Eye Res 2016;53:140-74.
- Schulze MM, Srinivasan S, Hickson-Curran SB, et al. Lid Wiper Epitheliopathy in Soft Contact Lens Wearers. Optometry and vision science 2016;93:943-54.
- Li W, Yeh TN, Leung T, et al. The Relationship of Lid Wiper Epitheliopathy to Ocular Surface Signs and Symptoms. Investigative ophthalmology & visual science 2018;59:1878-87.
- Brennan NA, Coles C. Supportive data linking coefficient of friction and comfort. Contact Lens and Anterior Eye 2013;36:e10.
- Kern J, Rappon J, Bauman E, et al. Assessment of the relationship between contact lens coefficient of friction and subject lens comfort. Investigative ophthalmology & visual science 2013;54:e-abstract 494.
- Vidal-Rohr M, Wolffsohn JS, Davies LN, et al. Effect of contact lens surface properties on comfort, tear stability and ocular physiology. Contact lens & anterior eye 2018;41:117-21.
