Despite many recent advances in diagnosis and management, congenital and childhood cataract can be difficult to manage and remains a major cause of lifelong visual impairment. In 1997, 200,000 children were estimated to be blind from cataract worldwide1 and it remains a global health priority.2 The UK incidence is just under 2.5 per 10,000 by the age of one year. This figure increases to around 3.5 out of 10,000 by age 15.3
Deprivation amblyopia and its sequelae (nystagmus, strabismus) causes most of the loss of vision apparent in affected children. Thus better understanding of the neurophysiology of the developing visual system has informed management of affected children.4
Early recognition of the abnormal pupil reflex (leukocoria) typically seen in infantile and congenital cataracts is crucial and it should be noted that the differential diagnosis of childhood leukocoria includes other disorders that are important to recognise promptly. These include retinoblastoma, retinal and choroidal coloboma and retinal detachment resulting from conditions such as Coats’ disease and retinopathy of prematurity.
Figure 1 Examples of dense nuclear congenital cataracts of differing morphologies viewed with the pupils dilated before surgery
A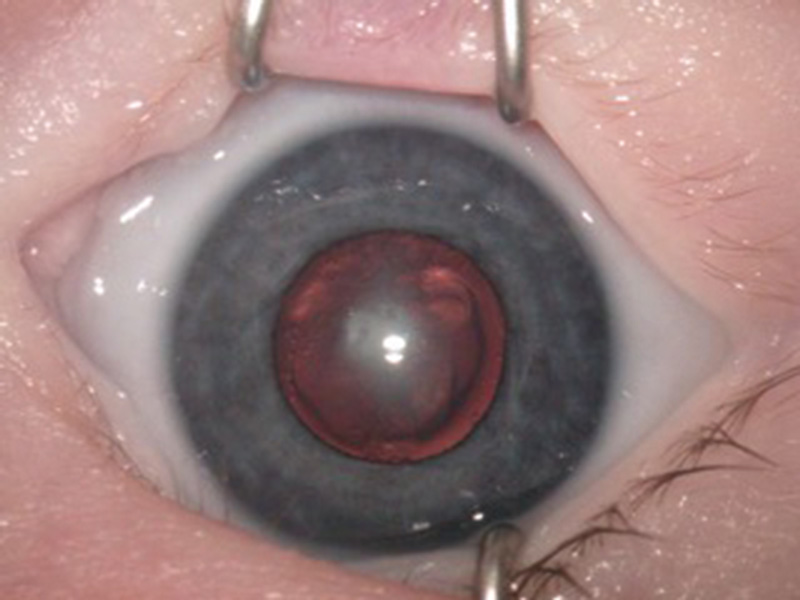
B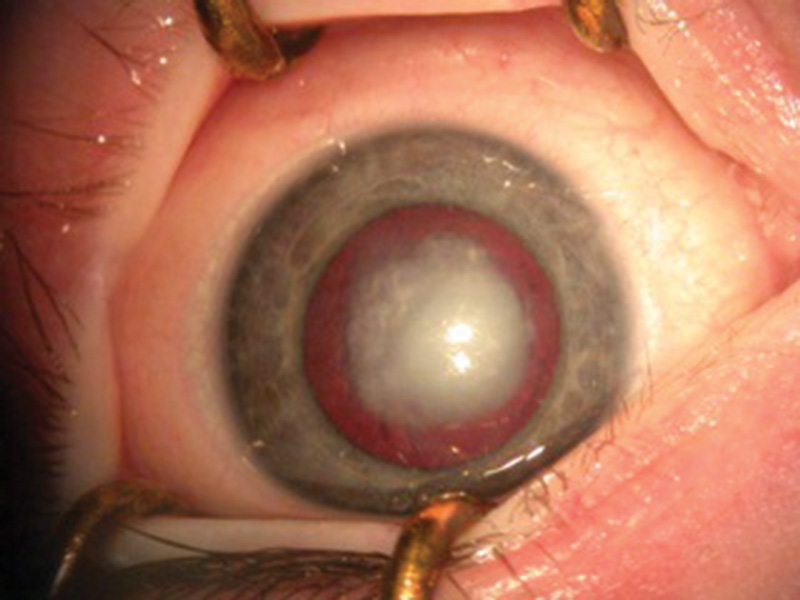
C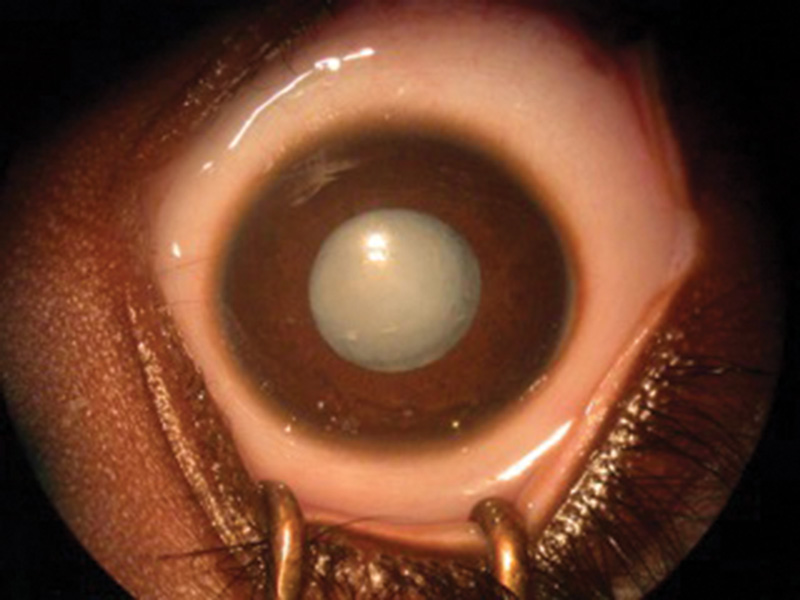 The presence of dense cataracts (figures 1a, b, c) should prompt urgent referral to a tertiary paediatric ophthalmic centre for appropriate assessment and, where indicated, lens surgery.5 Best care is multidisciplinary team management involving a paediatric ophthalmologist in combination with a paediatric optometrist and an orthoptist.6
The presence of dense cataracts (figures 1a, b, c) should prompt urgent referral to a tertiary paediatric ophthalmic centre for appropriate assessment and, where indicated, lens surgery.5 Best care is multidisciplinary team management involving a paediatric ophthalmologist in combination with a paediatric optometrist and an orthoptist.6
Visual development, amblyopia and timing of surgical intervention
The immature visual system is significantly affected by the visual deprivation caused by a media opacity such as congenital cataract. Animal studies have shown that unilateral deprivation produces specific deleterious neurophysiological changes in the lateral geniculate nuclei and striate cortex. A different pattern of change is induced by bilateral deprivation.4 If prolonged these changes become irreversible and lead to poor visual outcomes.
Unilateral cataract surgery performed by six weeks of (corrected gestational) age can result in good visual results but surgery carried out later than this ‘critical period’, even with optimal refractive and amblyopia therapy (occlusion) compliance, usually results in progressively poorer outcomes.7
The equivalent critical period for optimal intervention in bilateral visual deprivation, is more difficult to quantify, and is probably up to eight weeks.8-11,13,18 Visual functions other than acuity may have different critical periods and may thus be more easily disrupted. For eye alignment and fixation stability this could be as short as three weeks.12
However, the developing visual system retains some plasticity and can thus be modified before full visual maturation. This modification can be beneficial, where occlusion and optical correction are used to optimise and balance visual development, or detrimental, where amblyopia is induced by strabismus or media opacities. The period of plasticity in humans is approximately seven to eight years. Regular monitoring of acuity and fixation behaviour of each eye enables amblyopia treatment to be titrated.6
Infants treated for unilateral congenital cataract usually need long term occlusion regimes to obtain useful vision in the affected eye.6,9,14,15 Amblyopia therapy is onerous and needs to be worn for several hours a day for seven to nine years. This can have adverse effects on the child and their family.
Dense bilateral cataracts typically cause strabismus, even after optimal surgery16 and thus affected children are at risk of strabismic amblyopia. This is usually managed by part time occlusion. In one study, approximately 60% of cases of unilateral congenital cataract (who had optimal management) achieved ‘useful’ vision in the treated eye (VA better than 6/60)18 while in another study, 32% of unilateral aphakic eyes developed an acuity of equal to or better than 0.6 logMAR18(i).
Assessment and Investigations
A careful history is important and can prevent unnecessary investigations. Co-existent illness may indicate the presence of one of the syndromes or systemic illnesses associated with cataract. If suspected, referral to a paediatrician is essential. Age of onset of the cataract may be difficult to establish in children presenting outside early infancy. Clues include the presence of strabismus and/or nystagmus, visual acuity (in comparison to any available prior acuity), history of trauma or steroid use, and most importantly any family history of childhood cataract. Both parents should be examined to look for signs of lens opacity. Dysmorphic features, neuro-developmental or systemic problems in the child should instigate referral to a paediatrician and geneticist.
An example of this type of case which can present initially to the paediatric ophthalmology is Lowe (oculocerebrorenal) syndrome. This condition is caused by mutations in the OCRL1 gene and inherited in an X-linked manner. Affected boys have dense nuclear cataracts, are floppy, often have a prominent forehead (‘frontal bossing’) and go on to show learning difficulties and renal problems. Female carriers of the gene are systemically well, but exhibit specific lens changes with, in particular, multiple bilateral peripheral cortical flecks and sometimes other lenticular opacities such as posterior lenticonus.
Infants with Down syndrome (trisomy 21) may also occasionally present with congenital cataract to the ophthalmologist prior to establishment of a diagnosis by the paediatrician. The cataracts in affected children are often dense, white nuclear opacities associated with posterior lenticonus defects in the posterior lens capsule.
There are many other rare syndromes associated with congenital cataract. Traditional investigations to establish diagnosis include TORCH (TOxoplasmosis, Rubella, Cytomegalovirus, Herpes simplex) titres, urinalysis, blood glucose, full blood count, urea and electrolytes, and metabolic investigations particularly looking for abnormalities in sugar metabolism (to detect galactosaemia and galactokinase deficiency). The clinical utility of this raft of tests, when used in a blanket manner, is very poor19 and their use should thus be guided by clinical indications.
Congenital cataract can be caused by chromosomal abnormalities such as trisomies (in particular Down syndrome), deletions and duplications. Mitochondrial disorders may also be associated with later onset cataract. The majority of inherited cataracts arise from single nucleotide variants within a gene which in turn leads to alteration of an amino acid sequence and thus disrupts the function of the protein encoded by the gene. Mutations in over 115 genes have been associated with congenital and childhood cataract. Non-syndromic (isolated) cataract is probably caused by only around 25 genes but this group makes up 70% of all inherited cataract. Mutations in genes that code for crystallin proteins account for up to half of these isolated cases with connexin genes and transcription factors (such as PAX6 and FOXE3) the next most frequent causes.
Cataract morphology can vary hugely and there is often tremendous variability (phenotypic heterogeneity) within family members with the same gene mutation. It is thus important to examine other family members as the phenotype can be very mild in some cases, particularly in autosomal dominant disease. The traditional use of the type of cataract to determine the genetic aetiology is thus often unhelpful. However, some conditions do produce a specific abnormality such as the osmotic ‘oil droplet’ appearance caused by the accumulation of galactitol in the lens of the infant with galactosaemia. A diagnostic algorithm incorporating genetic testing has now been shown to be vastly more efficient in reaching a precise diagnosis in paediatric bilateral cataract and is now readily available as an NHS investigation.20
When to operate
Quantitative measurement of visual acuity in infants can be challenging although preferential looking grating acuity (Keeler or Teller cards) is widely used.6 Behavioural assessments, such as fixation preference and objection to occlusion, are good indicators of poor vision in unilateral cataract. Roving eye movements and poor visual behaviour suggest visually significant cataracts in bilateral cases. Electrophysiology can be used as a useful adjunct to objectively assess visual function.
Cataract morphology also informs likely visual significance21 and aids the decision of when to operate. Lens opacities of 3mm or more in the central visual axis are usually visually significant. Other associated ocular abnormalities may include microphthalmia, anterior segment dysgenesis, and persistent foetal vasculature (particularly in unilateral cases).
Figure 2 An IOL in the capsular bag of a two-year-old child following lensectomy with primary posterior capsulorrhexis and anterior vitrectomy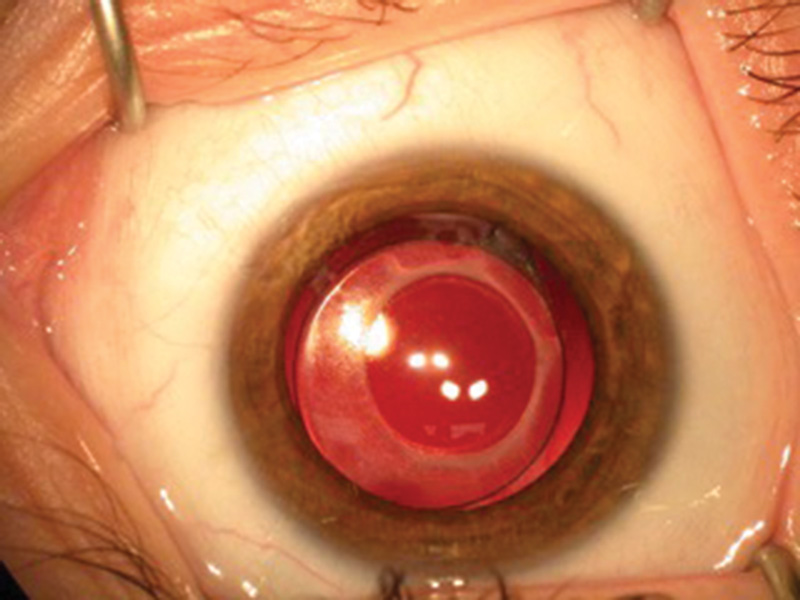 A B-scan ultrasound is mandatory where the cataract prevents an adequate fundal view in order to rule out posterior segment abnormalities including intraocular tumours and other significant retinal pathology. Further important investigations include measurement of intraocular pressure, corneal diameter and axial length.
A B-scan ultrasound is mandatory where the cataract prevents an adequate fundal view in order to rule out posterior segment abnormalities including intraocular tumours and other significant retinal pathology. Further important investigations include measurement of intraocular pressure, corneal diameter and axial length.
Surgery
For older children, primary intraocular lens (IOL) implantation is now accepted routine practice.24,29 Advocacy of this technique has fallen in the under two age group because of findings from recent randomised and population-based cohort studies.30,31 Long term refractive planning for pseudophakic infants is complex, the infant eye is easily inflamed by an IOL, the surgery is technically demanding, and the eye is frequently too small for implantation. In addition, the existent robust evidence, from a randomised controlled trial and a national cohort study, suggests that for congenital or infantile cataract, there is no visual benefit of IOL implantation over contact lenses fitted for aphakia.30,31 There is also a significantly higher risk of secondary surgery within the first post-operative year.30,31
In addition, repeated exposure to anaesthesia during early childhood may lead to poorer neurodevelopmental outcomes.32 Thus, secondary IOL implantation may be safer if delayed until later childhood. The threshold of five years of age fits with available evidence32 and is the age at which many parents would choose for their child’s IOL implantation, due in part to the start of formal schooling.33
In cases where aphakic contact lens use is difficult because of environmental or family factors, primary or early secondary IOL implantation may remain the preferred clinical choice.
Majority surgical practice is currently a foldable hydrophobic one-piece acrylic lens implanted within the capsular bag (figure 2), or a three-piece IOL if sulcus fixated24,30,31,33,35 with limbus or pars plana/plicata approach posterior capsulotomy.24,31 Frequent topical steroids are required post-operatively.31 Innovative approaches have shown promising early results. One such example is the ‘Bag-In-the-Lens’ IOL fixation, where the edges of the capsulorrhexis (the continuous circular tear made in the anterior capsule) are secured within an inter-haptic groove. Follow up data is still awaited.36 Whatever the technique, pro-inflammatory trauma is almost inevitable in the youngest children, in whom an IOL with a 5-6mm optic diameter is inserted through a pupil which even at maximal dilation is less than 7.5mm in diameter.40
Pupil size is not the only barrier: while the adult capsule measures 10.5mm, the mean neonate capsule diameter is considerably smaller at 7mm, although it grows to 9mm by two years of age and 10mm by five years.34 As such, most IOLs designed for use in adults are too large for the infant eye. However, IOL implants in post mortem infant eyes show ‘acceptable’ levels of stretching with smaller IOLs (haptic diameters of 12mm and optic diameters 5-6mm across).41 It may be thus unsafe to implant an IOL in an eye which is significantly small for age, particularly those with horizontal corneal diameters of less than 10mm.42
Refractive correction and optical rehabilitation
Appropriate correction of the aphakia is key to the child achieving their full visual potential following cataract surgery. Relatively short periods of uncorrected aphakia causing form deprivation during the first years of life can result in severe or dense amblyopia often in conjunction with nystagmus. Aphakic contact lenses enable improved and constant visual rehabilitation. In comparison to aphakic glasses, they may provide a better central vision and field of vision22,23 although vision can develop well with aphakic spectacles especially in bilateral aphakes23(i) and the measured acuity may, in fact, be better as the spectacle image size is magnified as compared to contact lenses.
Younger children form the majority of those undergoing cataract surgery24 so their contact lenses need to be cleaned, inserted and replaced by their parents. Parents typically describe initial difficulty with insertion and removal although most quickly become adept.25-27 However, up to a third of families struggle with contact lenses, due to child intolerance, lens loss or difficulties with insertion and removal.25,28 In theory intraocular lens (IOL) implantation offers permanent full or partial refractive correction but the challenge lies in determining whether, when and how to undertake this in children.
Refractive changes
The first two years of life is a time of rapid development of ocular anatomy and physiology. The myopic shift in paediatric pseudophakic eyes is larger than that seen in aphakic eyes, due in part to the optical effect of a fixed lens power in a growing eye. As the eye becomes larger, the IOL shifts further away from the retina, inducing myopia to a degree relative to the power of the lens. However axial elongation following paediatric cataract surgery has also been reported to be more variable in pseudophakic eyes than in aphakic eyes43-45 indicating a possible alternative impact of IOL implantation on ocular growth.
It is important to be aware of a myopic shift that is greater than expected or asymmetrical in bilateral aphakia as this is likely to be caused by raised intraocular pressure.
The most appropriate post-operative refractive outcome to aim for is not fully established. For example, the suggested refractive goals for children aged under six months range from +8.00DS to +4.00DS.31,45-47 Biometry in adults is a fast, simple, automated, non-contact procedure but young children cannot comply with this in the same way. Paediatric biometry requires ocular contact either directly or through a globe immersion device. Direct contact methods may have reduced accuracy when compared to immersion techniques, possibly due to inadvertent flattening of the cornea and thus shortening of the measured axial length.50 There is not yet consensus regarding the best method of choosing a power calculation formula, and although the SRK-T, Hoffer Q and Holladay 1 are the most commonly used and appear to be the most reliable,31,38 most children still end up around a dioptre away from their planned refractive outcome.38,39,48
Figure 3 Marked visual axis opacification in a pseudophakic eye following infantile lensectomy and IOL implantation, despite posterior capsulotomy and anterior vitrectomy
Overall, most investigators recommend leaving infants aphakic, while primary IOL implantation has become routine practice for children over two years old. The key remaining challenges for clinicians are in improving support for aphakic children and their families, and providing an evidence base for best practice with regards paediatric pseudophakia (refractive planning and peri-operative control of inflammation).
Complications
Management of amblyopia and associated refractive error is key to a good outcome (see Paediatic eye care part 3 – amblyopia in Optician 09.03.18) but the avoidance of complications is also vital5.
Glaucoma is a common problem following cataract surgery in infants and children (see Paediatic eye care part 4 – glaucoma in Optician 20.04.18). It may occur in the early postoperative period or as late as decades after surgery.30,31,49-51 Glaucoma or ocular hypertension was found to be as high as 59% in one prospective observational study of 63 patients over a 10-year period following paediatric cataract surgery. Other groups have demonstrated rates of around 20% following paediatric lensectomy.49-51 A relationship with early surgery has been demonstrated by several studies.30,31,52 Microphthalmia has also been found to be a risk factor.53
Two types of aphakic glaucoma are typically observed. An early onset type associated with angle closure and a (usually) later type associated with an open drainage angle.
It has been suggested that primary lens implantation may protect against glaucoma.54 However, this hypothesis is not borne out by findings from either the IATS and IOLu2 studies.30,31 The tendency to reserve implantation for older children reduced the observed incidence of glaucoma in implanted children but it remains a significant risk for infants undergoing primary implantation.
Measured intraocular pressure is affected by central corneal thickness (CCT). A study of CCT in aphakic/pseudophakic children compared with normal controls, showed that mean CCT was 626 microns and 556 microns respectively.55 This increased corneal thickness falsely elevates measured intraocular pressure. Concurrent assessment of optic nerve and visual field changes (where possible) are thus essential to coming to a precise diagnosis.
Treatment of glaucoma following congenital cataract surgery is usually initially medical but surgery is frequently required. Cyclodestructive procedures are useful but have a short-lived effect.57,58 Greater long-term success in controlling intraocular pressure has been achieved with the implantation of drainage tube devices such as the Ahmed Valve59 or Baerveldt implant.60
Visual axis opacification (VAO) due to development of posterior capsule opacification (PCO) or other fibro-cellular opacity within the visual axis is the most common reason for further surgical intervention in young children after IOL implantation.61 Capsular management at the time of lens extraction is age dependant. In children younger than six years primary posterior capsulotomy is appropriate because subsequent and often rapid opacification of residual capsule is highly amblyogenic.61 Hydrophobic acrylic IOLs lens implants appear to be more biocompatible and incite less inflammation than PMMA or silicone IOLs.34,62
A primary posterior capsulotomy should be supplemented by anterior vitrectomy to adequately prevent VAO which can still develop as a result of proliferation of lens epithelial cells across the anterior hyaloid face (figure 3). A surgical technique known as posterior optic capture has been advocated to minimise this. In this manoeuvre the optic of the intraocular lens is posteriorly displaced (‘captured’) through the posterior opening in the capsular bag. However, this technique requires the use of a posteriorly vaulted three-piece intraocular lens with separate polypropylene haptics – particularly when the lens is placed in the ciliary sulcus rather than in the capsular bag. In this situation the larger, relatively sharp edged haptics of a one piece acrylic lens prevent adequate capture of the optic in the capsular opening and tend to rub against the posterior iris causing recurrent inflammation.63
Retinal detachment is a rare sight threatening complication following lensectomy. The incidence may be increased in conditions such as persistent foetal vasculature (PFV) where traction on the retina may be exacerbated by surgical disturbance of an abnormally thick anterior vitreous face.64
Strabismus is also common after both bilateral and unilateral cataract surgery and was found in 85% of patients with unilateral aphakia.18(i) Strabismus frequently requires surgery to improve the cosmesis. This is likely to be carried out at a later date, often when the child is about to start school at around five years old.
The Future
Improvements in the management of childhood cataract are likely to be driven by collaborative clinical research in this rare but important condition. Carefully planned multi-centre prospective studies should lead to identification of the predictors of good (and poor) visual outcomes. Genomic medicine may initiate treatment for the developing embryo or provide early precise diagnosis to enable implementation of effective personalised treatment regimes particularly for those children with multi-system and metabolic disorders associated with cataract.
Kelly Weston is consultant ophthalmologist at Surrey and Sussex Healthcare NHS Trust.
Marina Rayside is consultant ophthalmologist, Perth Children’s Hospital, Fremantle Hospital, Perth, Australia. Ameenat Lola Solebo is an NIHR academic clinical lecturer based at the Moorfields and GOS Institute of Child Health NIHR Biomedical Research Centres.
Professor IC Lloyd is a consultant ophthalmologist at Great Ormond Street Hospital, senior lecturer at University College, London and honorary consultant ophthalmologist at Manchester Royal Eye Hospital.
References
- 1 Foster A, Gilbert C, Rahi J. Epidemiology of cataract in childhood: a global perspective. J Cataract Refract Surg.1997;23:601-604
2 World Health Organisation. Global initiative for the elimination of avoidable blindness. Geneva, WHO;1977, publication no.PBL/97.61
3 Rahi JS, Dezateux C. Measuring and interpreting the incidence of congenital ocular anomalies: lessons from a national study of congenital cataract in the UK. Invest Ophthalmol Vis Sci 2001;42:1444-1448.
4 Dawe NW. Visual Development. 2nd Edition 2006. Springer New York.
5 Taylor D. Congenital cataract: the history, the nature and the practice: The Doyne Lecture. Eye 1998;12:9-36
6 Lloyd IC, Dowler J, Kriss A, Speedwell L, Thompson D, Russell-Eggitt I, Taylor D. Modulation of amblyopia therapy following early surgery for unilateral congenital cataracts. Br J Ophthalmol 1995; 79: 802-6
7 Birch EE, Stager DR. The critical period for surgical treatment of dense congenital unilateral cataract. Invest Ophthalmol Vis Sci 1996; 37:1532-8.
8 Lambert SR, Lynn MJ, Reeves R, Plager DA, Buckley EG, Wilson ME. Is there a latent period for the treatment of children with dense bilateral congenital cataracts? JAAPOS 2006; 10:30-36.
9 Gelbart SS, Hoyt CS, Jastrebski G, Marg E. Long term visual results in bilateral congenital cataracts. Am J Ophthalmol 1982; 93:615-621.
10 Maurer D, Lewis T. Visual acuity and spatial contrast sensitivity: Normal development and underlying mechanisms. In: The handbook of developmental cognitive neuroscience (eds Nelson C, Luciana M), MIT Press. 2001; pp. 237-251.
11 Rogers GL, Tischler CL, Tsou BH, Hertle RW, Fellows RR. Visual acuities in infants with congenital cataracts operated on prior to 6 months of age. Arch Ophthalmol. 1981; 99:999-1003.
12 Abadi RV, Forster JE, Lloyd IC. Ocular Motor Outcomes after bilateral and unilateral infantile cataracts. Vision Research 2006;46: 940-52.
13 Duration of form deprivation and visual outcome in infants with bilateral congenital cataracts. Jain S, Ashworth J, Biswas S, Lloyd IC. J AAPOS 14(1):31-34(2010)
14 Lambert SR. Management of monocular congenital cataracts. Eye; 1999 13: 474-479.
15 Beller R, Hoyt CS, Marg E, Odom JV. Good visual function after neonatal surgery for congenital monocular cataracts. Am J Ophthalmol 1981; 91:559-65
16 France TD and Frank JW. The association of strabismus and aphakia in children. J Ped Ophthalmol Strab 1984;21(6):223-6
17 Lambert SR. Treatment of congenital cataract. Br J Ophthalmol 2004; 88:854-5.
18 Lambert SR, Drack AV. Infantile Cataracts. Survey of Ophthalmology 1996;40:427-458.
18(i) Allen R, Speedwell L, Russell-Eggitt I. (2010) Long-term visual outcome after extraction of unilateral congenital cataracts. Eye 24 (7): 1263-7
19 Diagnosing the cause of bilateral paediatric cataracts: comparison of standard testing with a Next Generation Sequencing approach. Ashworth JL, Musleh M, Hall G, Lloyd IC, Gillespie R, Waller S, Douzgou S, Clayton-Smith J, Kehdi E, Black GCM. Eye (2016) 15-1069
20 Personalised diagnosis and management of congenital cataract by next generation sequencing. Gillespie R, O’Sullivan J, Ashworth JL, Bhaskar S, Williams S, Biswas S, Kehdi E, Ramsden S, Clayton-Smith J, Black G, Lloyd IC. Ophthalmol (2014) 121 (11) 2124-2137
21 Forster JE, Abadi RV, Muldoon M, Lloyd IC. Grading infantile cataracts. Ophthal Physiol Opt 2006; 26:372-379.
22 Hing S, Speedwell L, Taylor D. Lens surgery in infancy and childhood. Br J Ophthalmol 1990; 74(2):73-77
23 Hiles DA. The need for intraocular lens implantation in children. Ophthalmic Surg 1977; 8(3):162-169
23(i) Kim, D-H, Kim, J.H, Kim, S-J, Yu, Y.S. 2012; Long-term results of bilateral congenital cataract treated with early cataract surgery, aphakic glasses and secondary IOL implantation. Acta Ophthalmol. 90:231-236
24 Repka MX, Dean TW, Lazar EL, Yen KG, Lenhart PL, Freedman SF, Hug D, Rahmani B, Wang SX, Kraker RT, Wallace DK, for the Pediatric Eye Disease Investigator Group. Cataract Surgery in Children from Birth to Less than 13 Years of Age, Baseline Characteristics of the Cohort. Ophthalmology 2016;123:2462-2473
25 Ma JJ, Morad Y, Mau E, Brent HP, Barclay R, Levin AV. Contact lenses for the treatment of pediatric cataracts. Ophthalmology 2003; 110(2):299-305
26 Saltarelli DP. Hyper oxygen-permeable rigid contact lenses as an alternative for the treatment of pediatric aphakia. Eye Contact Lens 2008; 34(2):84-93
27 Russell B1, DuBois L, Lynn M, Ward MA, Lambert SR; Infant Aphakia Treatment Study Group. The Infant Aphakia Treatment Study Contact Lens Experience to Age of 5 Years. Eye Contact Lens. 2016
28 Amaya LG, Speedwell L, Taylor D. Contact lenses for infant aphakia. Br J Ophthalmol 1990; 74(3):150-154
29 Solebo AL, Russell-Eggitt I, Nischal KK, Moore AT, Cumberland P, Rahi JS; British Isles Congenital Cataract Interest Group. Cataract surgery and primary intraocular lens implantation in children < or = 2 years old in the UK and Ireland: finding of national surveys. Br J Ophthalmol. 2009 Nov;93(11):1495-8
30 Infant Aphakia Treatment Study Group, Lambert SR, Lynn MJ, Hartmann EE, DuBois L, Drews-Botsch C, Freedman SF, Plager DA, Buckley EG, Wilson ME. Comparison of contact lens and intraocular lens correction of monocular aphakia during infancy: a randomized clinical trial of HOTV optotype acuity at age 4.5 years and clinical findings at age 5 years. JAMA Ophthalmol. 2014 Jun;132(6):676-82
31 Solebo AL, Russell-Eggitt I, Cumberland PM, Rahi JS; British Isles Congenital Cataract Interest Group. Risks and outcomes associated with primary intraocular lens implantation in children under 2 years of age: the IoLunder2 cohort study. Br J Ophthalmol. 2015 Nov;99(11):1471-6
32 Ing, CH, DiMaggio, CJ, Malacova, E et al. Comparative analysis of outcome measures used in examining neurodevelopmental effects of early childhood anesthesia exposure. Anesthesiology. 2014; 120: 1319–1332
33 Wood KS, Tadros D, Trivedi RH, Wilson ME. Secondary intraocular lens implantation following infantile cataract surgery: intraoperative indications, postoperative outcomes. Eye (2016) 30, 1182–1186
34 Wilson ME, Apple DJ, Bluestein EC, Wang XH. Intraocular lenses for pediatric implantation: biomaterials, designs, and sizing. J Cataract Refract Surg 1994; 20(6):584-591
35 Lin AA, Buckley EG. Update on pediatric cataract surgery and intraocular lens implantation. Curr Opin Ophthalmol 2010; 21:55–59
36 Van Looveren J, Ni Dhubhghaill S, Godts D, et al. Pediatric bag-in-the-lens intraocular lens implantation: long-term follow-up. J Cataract Refract Surg 2015; 41:1685–1692
37 Trivedi RH, Wilson ME. Axial Length Measurements by Contact and Immersion Techniques in Pediatric Eyes with Cataract. Ophthalmology 2010.
38 Vanderveen DK, Trivedi RH, Nizam A, et al., Infant Aphakia Treatment Study Group. Predictability of intraocular lens power calculation formulae in infantile eyes with unilateral congenital cataract: results from the Infant Aphakia Treatment Study. Am J Ophthalmol 2013; 156:1252–1260
39 Moore DB, Ben Z, I, Neely DE, Plager DA, Ofner S, Sprunger DT et al. Accuracy of biometry in pediatric cataract extraction with primary intraocular lens implantation. J Cataract Refract Surg 2008; 34(11):1940-1947
40 Wheatcroft S, Sharma A, McAllister J. Reduction in mydriatic drop size in premature infants. Br J Ophthalmol 1993; 77(6):364.
41 Pandey SK, Werner L, Wilson ME, Jr., Izak AM, Apple DJ. Capsulorhexis ovaling and capsular bag stretch after rigid and foldable intraocular lens implantation: experimental study in pediatric human eyes. J Cataract Refract Surg 2004; 30(10):2183-2191
42 Vasavada VA, Dixit NV, Ravat FA, Praveen MR, Shah SK, Vasavada V et al. Intraoperative performance and postoperative outcomes of cataract surgery in infant eyes with microphthalmos. J Cataract Refract Surg 2009; 35(3):519-528
43 Flitcroft DI, Knight-Nanan D, Bowell R, Lanigan B, O'Keefe M. Intraocular lenses in children: changes in axial length, corneal curvature, and refraction. Br J Ophthalmol 1999; 83(3):265-269.
44 Griener ED, Dahan E, Lambert SR. Effect of age at time of cataract surgery on subsequent axial length growth in infant eyes. J Cataract Refract Surg 1999; 25(9):1209-1213.
45 Fan DS, Rao SK, Yu CB, Wong CY, Lam DS. Changes in refraction and ocular dimensions after cataract surgery and primary intraocular lens implantation in infants. J Cataract Refract Surg 2006; 32(7):1104-1108
46 Lloyd IC, Ashworth J, Biswas S, Abadi RV. Advances in the management of congenital and infantile cataract. Eye 2007; 21(10):1301-1309
47 Salcone EM, Kazlas M. Pediatric Intraocular Lens Implantation: Historic Perspective and Current Practices. Int Ophthalmol Clin 2010; 50(1):71
48 C Tromans, PM Haigh, S Biswas, IC Lloyd. Accuracy of intraocular lens power calculation in paediatric cataract surgery. Brit J Ophthalmol 2001;85:939-941.
49 Egbert JE, Christiansen SP, Wright MM, Young TL, Summers CG. The natural history of glaucoma and ocular hypertension after pediatric cataract surgery. J AAPOS.2006 ;10: 54-7.
50 Magnusson G, Abrahamsson M, Sjostrand J. Glaucoma following congenital cataract surgery: an 18-year longitudinal follow-up. Acta Ophthalmol Scand. 2000 ;78:65-70.
51 Chen TC, Bhatia LS, Walton DS Complications of pediatric lensectomy in 193 eyes. Ophthalmic Surg Lasers Imaging. 2005;36: 6-13.
52 Vishwanath M , Cheong-Leen R, Taylor D, Russell-Eggitt I, Rahi J. Is early surgery a risk factor for glaucoma? Br J Ophthalmol 2004;88:905-910
53 Trivedi RH, Wilson ME Jr, Golub RL. Incidence and risk factors for glaucoma after pediatric cataract surgery with and without intraocular lens implantation.J AAPOS. 2006;10:117-23.
54 Asrani S, Freedman S, Hasselblad V, Buckley EG, Egbert J, Dahan E, Gimbel H, Johnson D, McClatchey S, Parks M, Plager D, Maselli E. Does primary intraocular lens implantation prevent ‘aphakic’ glaucoma in children? J AAPOS. 2000 ;4:33-9.
55 Simsek T, Mutluay AH, Elgin U, Gursel R, Batman A. Glaucoma and increased central corneal thickness in aphakic and pseudophakic patients after congenital cataract surgery. Br J Ophthalmol. 2006;90:1103-6.
56. Simon JW, O’Malley MR, Gandham SB, Ghaiy R, Zobal-Ratner J, Simmons ST. Central corneal thickness and glaucoma in aphakic and pseudophakic children. J AAPOS 2005; 9:326-9
57 Kirwan JF, Shah P, Khaw PT. Diode laser cyclophotocoagulation: role in the management of refractory pediatric glaucomas. Ophthalmology 2002;109:316-23.
58 Autrata R, Rehurek J. Long-term results of transscleral cyclophotocoagulation in refractory pediatric glaucoma patients.Ophthalmologica. 2003 ;217:393-400
59 Kirwan C, O'Keefe M, Lanigan B, Mahmood U. Ahmed valve drainage implant surgery in the management of paediatric aphakic glaucoma. Br J Ophthalmol. 2005 ;89:855-8.
60 van Overdam KA, de Faber JT, Lemij HG, de Waard PW. Baerveldt glaucoma implant in paediatric patients.Br J Ophthalmol. 2006;90:328-32
61 Jensen AA, Basti S, Greenwald MJ, Mets MB. When may the posterior capsule be preserved in pediatric intraocular lens surgery? Ophthalmology. 2002;109:324-7
62 Wilson ME, Elliott L, Johnson B, Peterseim MM, Rah S, Werner L, Pandey SK. AcrySof acrylic intraocular lens implantation in children: clinical indications of biocompatibility J AAPOS. 2001;5:377-80
63 Vasavada AR, Trivedi RH: Role of optic capture in congenital cataract and intraocular lens surgery in children. J Cataract Refract Surg 2000;26: 824–31
64 Rabiah PK, Du H, Hahn EA. Frequency and predictors of retinal detachment after pediatric cataract surgery without primary intraocular lens implantation. J AAPOS. 2005; 9:152-9
