
Maintaining a healthy ocular surface requires a healthy pre-ocular tear film. The tear film is composed of multifaceted layers of lipids, aqueous and mucin components,1 which work together to protect the cornea and conjunctiva. A breakdown of any of these layers can contribute to the development of dry eye disease (DED).1 Dry eye disease (DED) affects 5-50% of the population and negatively influences quality of life for those suffering from it.2,3 Current techniques for diagnosing DED include symptom questionnaires, tear production measurements, ocular surface staining and tear osmolarity, to name a few, but these methodologies require tests in combination to provide a definitive DED diagnosis.4 There are several tear and ocular surface biomarkers that have been found in DED, but detecting these various factors has largely been limited to the laboratory setting.5-7 With advancing technologies now able to create lab-on-a-chip devices, such as handheld osmolarity measurement,8 testing for DED biomarkers could soon make the leap from the laboratory to the clinic.7 However, the complexity of DED continues to make both diagnosis and management difficult for practitioners and patients alike. While researchers are continuously striving to determine a single test or marker that can diagnose DED confidently, the variability that is intrinsic to DED makes this task difficult.9-12 One test that shows some promise — and can be performed in a clinical setting — is that of tear ferning.13,14
History of fluid ferning for tear film and ocular surface analysis
Fluid ferning is a technique that has evolved from the knowledge that drying bodily fluid samples on a glass microscope slide produces a unique crystallisation pattern.15 This technique was first reported in 1791 using tears,16 but was not evaluated further for other bodily fluids until 1946, when Papanicolaou used the technique to study cervical mucous.17 Vaginal and cervical mucous ferning patterns change throughout the menstrual cycle and those changes can be used to track ovulation and fertility.18-20 Saliva ferning patterns have also been investigated as a means to pinpoint ovulation.21 Saliva ferning patterns have been investigated as a means to aid in the diagnosis of Sjögren’s syndrome,22,23 though to-date this has not been tested sufficiently to become common practice.
In 1982, Tabbara and Okumoto used the ferning patterns of conjunctival scrapings to differentiate cicatrising (ie Stevens-Johnson syndrome) vs non-cicatrising (ie viral, bacterial, allergic) conjunctivitis.24 In 1984, Rolando elaborated on the findings of Tabbara and Okumoto and developed a grading system using the ferning patterns of tear samples.25 Tear ferning has also been used to evaluate keratoconjunctivitis sicca in various animals. The tear ferning patterns of dogs and horses correlate well with Schirmer tear volume testing.26,27 Interestingly, when comparing tear ferning patterns of camels to those of humans, the camel tear film produces much higher quality ferning patterns.27,28
What is tear ferning and how is it performed?
Creating optimal tear ferning patterns requires fairly precise conditions. A 1µl to 10µl sample of collected tears is placed on a microscope slide and allowed to dry under normal room temperature (20° to 26°C) and normal room humidity (up to 50%).13,26,29,30 Lower surface tension of the fluid will allow the sample to spread on the slide quicker, promoting crystallisation.31 Evaporation of the fluid starts at the edges of the sample and moves inward and, as water evaporates, the solute in the sample is concentrated. When a region becomes supersaturated (excess concentration compared to equilibrium), nuclei begin to form.15 Nuclei are composed of a number of regularly arranged ions, which continue to have more ions added to them, increasing the crystal unit, eventually taking on the form of a cubic nucleus.15 Normal crystals can form if the sample continues to have areas of lower solute concentration to diffuse into, which requires slow growth rate, low solution viscosity and low impurity levels for solute diffusion.15 If these conditions are not present, dendritic crystalline growth occurs as needles begin to form off the cube and continue to lengthen and branch, forming the classic ferning pattern.15,31 If a cover slip is placed over the microscope slide, this slows the growth rate and ferning would not occur.31
Several factors contribute to favourable ferning patterns. The type of ferning pattern achieved relies on the balance between salt and macromolecule concentration (ie proteins, mucous). In a solution with high protein, ferning does not occur; similarly in high salt concentration solutions, ferning does not occur.31 In the setting of dry eye, the ferning pattern of a sample provides a gross biochemical overview of the tear composition.
To perform the tear ferning test, a tear sample is collected. This can be undertaken in several ways, but the ideal method is via a capillary tube, wicking tears from the lower tear meniscus. The tear sample is pipetted onto a glass microscope slide and allowed to dry under normal room temperature and humidity. The sample should then be observed under phase contrast light microscopy within 10 minutes of collection.31 The ferning pattern obtained can subsequently be subjectively graded using one of several grading scales, to obtain insight into the particular pattern of that sample.
Grading scales
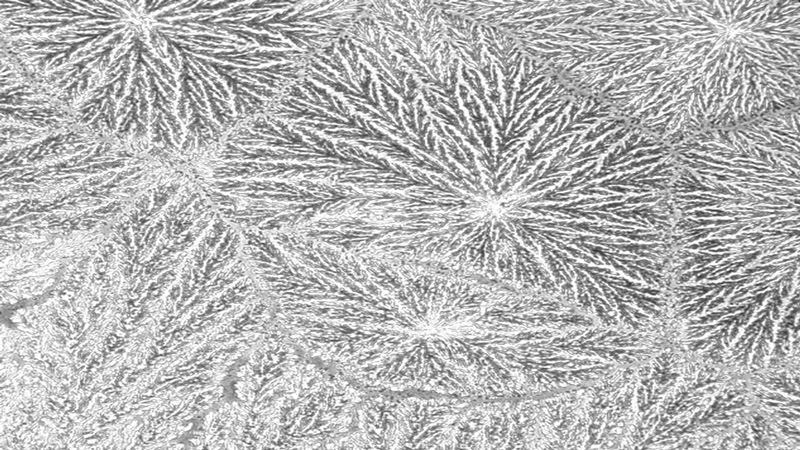
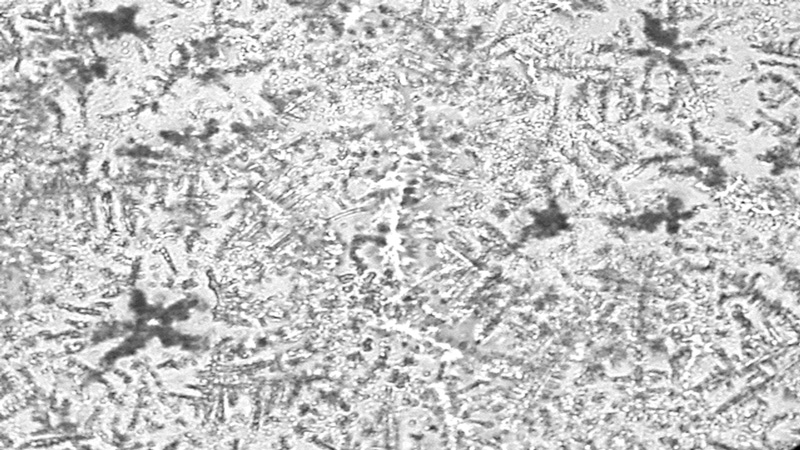
The most frequently used scale for grading tear ferning patterns is that described by Rolando in 1984,25 which subjectively categorises ferning patterns into one of four discrete types (Types I-IV). Type I refers to a pattern with extensive branching and no spaces between ferns. Type II ferns are smaller than Type I with more space between ferns. Type III ferns are incomplete and have minimal branching. Type IV shows no ferning at all (figure 1).13,25,32
Figure 1 Tear ferning patterns according to Rolando’s grading scale;13 (a) Type I, (b) Type II; (c) Type III, (d) Type IV
a
 b
b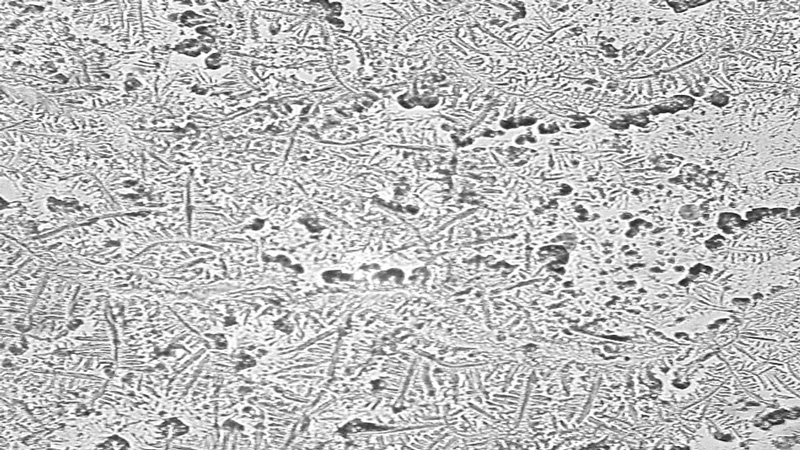 c
c
 d
d While Rolando’s scale continues to be the most frequently used for tear ferning, it has several shortcomings, including no defined protocol for sampling and only four discrete types, decreasing the sensitivity of the grading system. Despite this, the Rolando scale remains a simple scale to use and shows excellent intra-observer (85.41%) and inter-observer (80.62%) agreement.33
While Rolando’s scale continues to be the most frequently used for tear ferning, it has several shortcomings, including no defined protocol for sampling and only four discrete types, decreasing the sensitivity of the grading system. Despite this, the Rolando scale remains a simple scale to use and shows excellent intra-observer (85.41%) and inter-observer (80.62%) agreement.33
Norn proposed a scale that evaluated the angle of the fern branches, categorising them as right-angle ferning and acute-angle ferning.34 In dry eye tear samples, acute-angle ferning was found to be the most predominant branching pattern, whereas non-dry eye tear samples displayed more right-angle fern branches.34
In 2014, Masmali developed a more sensitive five-grade scale to evaluate tear ferning patterns.35 Optometrists experienced in clinical grading evaluated numerous sample ferning images, which led to the development of a five-point scale with a linear relationship between groups.35 This five-point scale also gave better reproducibility between sessions when compared to Rolando’s scale.35
Clinical Utility of Tear Ferning
Tear ferning patterns have been successfully used to differentiate dry eyes from non-dry eyes.23,36,37 These ferning patterns can also be used to differentiate Sjögren’s syndrome patients from normal patients.37,38 Tear samples from Sjögren’s patients are mainly Type III and IV ferning patterns, whereas normal patient tear samples are mainly Type I and II (Rolando grading system).23,38 Tear ferning patterns have also been used to predict contact lens (CL) tolerance; tear ferning showed 78.95% sensitivity and 78.35% specificity in predicting future CL intolerance.39 Further studies have not been able to demonstrate this same level of sensitivity and specificity in predicting ocular surface comfort in CL wearers, but CLs wearers consistently produce higher grades (Rolando types III-IV) of tear ferning.40 Therefore, a tear sample from a CL wearer would show less branching and ferning than that of a non-CL wearer. There is also poor correlation of tear ferning with traditional dry eye testing (ie tear break-up time, Schirmer scores) in the setting of ocular comfort in CL and non-CL wearers.40 The tear ferning patterns of smokers show less branching and ferning when compared to patterns of non-smokers, showing the negative impact of cigarette smoke on tear film quality.41
As ferning patterns result from the precise balance of salt and macromolecules in a tear sample, it would seem reasonable that ferning patterns could be linked to osmolarity measurements, yet a strong correlation between tear ferning patterns and tear osmolarity has not been found.40,42,43 In patients with pterygia, there is less branching and ferning of the tears,44 but surgical excision of the pterygium can improve this, with 90% of patients showing normal ferning patterns one month after excision.45 Tear ferning patterns do not show diurnal variation, which adds to their utility in a clinical setting.14 DED is an intricate disease and the tear ferning test only confirms its complexity, given its weak correlation with tear osmolarity yet strong association with other dry eye characteristics.38,42,46
Tear ferning has been investigated as a means to evaluate various systemic diseases, particularly those that result in biochemical changes of the various bodily fluids. It has been found that higher grades of tear ferning patterns are found in patients with uncontrolled diabetes, which may point to uncontrolled diabetes as a risk factor for DED.47 Early uses of tear ferning included using ferning patterns as a diagnostic test for cystic fibrosis, but more recent studies have found that the ferning patterns are more useful in determining the clinical status of the cystic fibrosis patient, rather than being of value for diagnosis.48, 49
Conclusion
Knowledge surrounding the ferning patterns of various bodily fluids holds great utility in the health care field, as it is a straightforward technique that gives insight into the gross biochemical properties of a sample. As technologies continue to advance, techniques that have previously been confined to the laboratory will become more clinically accessible. Tear ferning is currently not widely used because of certain limitations, including access to the required equipment and a controlled environment and time to obtain results, but studies have tested its potential. These limitations must be overcome for this test to be used in the clinic setting for the assessment of tear film factors.
Dr Marian Elder, Professor Sruthi Srinivasan and Professor Lyndon Jones are based at the Centre for Ocular Research & Education (CORE), School of Optometry & Vision Science, University of Waterloo.
REFERENCES
- Bron, AJ, et al, TFOS DEWS II pathophysiology report. Ocul Surf, 2017. 15(3): p.438-510.
- Stapleton, F, et al, TFOS DEWS II Epidemiology Report. Ocul Surf, 2017. 15(3): p.334-65.
- Baudouin, C, et al, Severe impairment of health-related quality of life in patients suffering from ocular surface diseases. J Fr Ophtalmol, 2008. 31(4): p.369-78.
- Wolffsohn, JS, et al, TFOS DEWS II Diagnostic Methodology report. Ocul Surf, 2017. 15(3): p. 539-74.
- Pflugfelder, SC and CS de Paiva, The Pathophysiology of Dry Eye Disease: What We Know and Future Directions for Research. Ophthalmology, 2017. 124(11S): p.S4-S13.
- Hagan, S, Biomarkers of ocular surface disease using impression cytology. Biomark Med, 2017. 11(12): p.1135-47.
- von Thun Und Hohenstein-Blaul, N, S Funke, and FH Grus, Tears as a source of biomarkers for ocular and systemic diseases. Exp Eye Res, 2013. 117: p.126-37.
- Versura, P and EC Campos, TearLab(R) Osmolarity System for diagnosing dry eye. Expert Rev Mol Diagn, 2013. 13(2): p.119-29.
- Baenninger, PB, et al, Variability of Tear Osmolarity Measurements With a Point-of-Care System in Healthy Subjects-Systematic Review. Cornea, 2018. doi: 10.1097/ICO.0000000000001562 [Epub ahead of print).
- Benito, MJ, et al, Intra- and inter-day variation of cytokines and chemokines in tears of healthy subjects. Exp Eye Res, 2014. 120: p.43-9.
- Markoulli, M, et al, The diurnal variation of matrix metalloproteinase-9 and its associated factors in human tears. Invest Ophthalmol Vis Sci, 2012. 53(3): p.1479-84.
- Sullivan, B, Challenges in using signs and symptoms to evaluate new biomarkers of dry eye disease. Ocul Surf, 2014. 12(1): p. 2-9.
- Masmali, AM, C Purslow, and PJ Murphy, The tear ferning test: a simple clinical technique to evaluate the ocular tear film. Clin Exp Optom, 2014. 97(5): p.399-406.
- Masmali, AM, et al, Repeatability and Diurnal Variation of Tear Ferning Test. Eye Contact Lens, 2015. 41(5): p.262-7.
- Buckley, HE, Crystal growth. 1951, London: Chapman & Hall Ltd.
- Murube, J, Tear crystallization test: two centuries of history. Ocul Surf, 2004. 2(1): p. 7-9.
- Papanicolaou, GN, A general survey of the vaginal smear and its use in research and diagnosis. Am J Obstet Gynecol, 1946. 51: p.316-28.
- Abou-Shabanah, EH and EJ Plotz, A biochemical study of the cervical and nasal mucus fern phenomenon. Am J Obstet Gynecol, 1957. 74(3): p.559-68.
- Pal, T and AK Bhattacharyya, Structural changes in human cervical mucus. Indian J Med Res, 1989. 90: p.44-50.
- Said, S, ED Johansson, and C Gemzel, Return of ovulation during the postpartum period. Acta Obstet Gynecol Scand, 1974. 53(1): p.63-7.
- Barbato, M, A Pandolfi, and M Guida, A new diagnostic aid for natural family planning. Adv Contracept, 1993. 9(4): p.335-40.
- el-Miedany, YM, S.M el-Hady, and MA el-Baddin, Validity of the saliva ferning test for the diagnosis of dry mouth in Sjogren's syndrome. Rev Rhum Engl Ed, 1999. 66(2): p.73-8.
- Maragou, M, et al, Tear and saliva ferning tests in Sjogren's syndrome (SS). Clin Rheumatol, 1996. 15(2): p.125-32.
- Tabbara, KF and M Okumoto, Ocular ferning test. A qualitative test for mucus deficiency. Ophthalmology, 1982. 89(6): p.712-4.
- Rolando, M, Tear mucus ferning test in normal and keratoconjunctivitis sicca eyes. Chibret Int J Ophthalmol, 1984. 2: p.32-41.
- Williams, D and H Hewitt, Tear ferning in normal dogs and dogs with keratoconjunctivitis sicca. Open Vet J, 2017. 7(3): p. 268-72.
- Silva, LR, et al, Tear ferning test in horses and its correlation with ocular surface evaluation. Vet Ophthalmol, 2016. 19(2): p.117-23.
- Am, M, et al, Structure and microanalysis of tear film ferning of camel tears, human tears, and Refresh Plus. Mol Vis, 2018. 24: p.305-14.
- Masmali, AM, et al, Application of a new grading scale for tear ferning in non-dry eye and dry eye subjects. Cont Lens Anterior Eye, 2015. 38(1): p.39-43.
- Horwath, J, et al, Ocular Ferning test - effect of temperature and humidity on tear Ferning patterns. Ophthalmologica, 2001. 215(2): p.102-7.
- Golding, TR, Brennan, NA, The basis of tear ferning. Clinical & Experimental Optometry, 1989. 72(4): p.102-12.
- Rolando, M, F Baldi, and M Zingirian, The effect of hyperosmolarity on tear mucus ferning. Fortschr Ophthalmol, 1986. 83(6): p.644-6.
- Pensyl, CD and SM Dillehay, The repeatability of tear mucus ferning grading. Optom Vis Sci, 1998. 75(8): p. 600-4.
- Norn, M, Ferning in conjunctival-cytologic preparations. Crystallisation in stained semiquantitative pipette samples of conjunctival fluid. Acta Ophthalmol (Copenh), 1987. 65(1): p. 118-22.
- Masmali, AM, PJ Murphy, and C Purslow, Development of a new grading scale for tear ferning. Cont Lens Anterior Eye, 2014. 37(3): p.178-84.
- Norn, M, Quantitative tear ferning. Clinical investigations. Acta Ophthalmol (Copenh), 1994. 72(3): p.369-72.
- Vaikoussis, E, P Georgiou, and D Nomicarios, Tear mucus ferning in patients with Sjogren's syndrome. Doc Ophthalmol, 1994. 87(2): p.145-51.
- Felberg, S, et al, [Reproducibility of the classification of ocular ferning patterns in Sjogren's syndrome patients]. Arq Bras Oftalmol, 2008. 71(2): p. 228-33.
- Ravazzoni, L, et al, Forecasting of hydrophilic contact lens tolerance by means of tear ferning test. Graefes Arch Clin Exp Ophthalmol, 1998. 236(5): p.354-8.
- Evans, KS, RV North, and C Purslow, Tear ferning in contact lens wearers. Ophthalmic Physiol Opt, 2009. 29(2): p.199-204.
- Masmali, AM, et al, Assessment of Tear Film Quality among Smokers Using Tear Ferning Patterns. J Ophthalmol, 2016. 2016: p.8154315.
- Srinivasan, S, E Joyce, and LW Jones, Tear osmolality and ferning patterns in postmenopausal women. Optom Vis Sci, 2007. 84(7): p.588-92.
- Versura, P, V Profazio, and EC Campos, Performance of tear osmolarity compared to previous diagnostic tests for dry eye diseases. Curr Eye Res, 2010. 35(7): p.553-64.
- Julio, G, et al, Tear osmolarity and ocular changes in pterygium. Cornea, 2012. 31(12): p.1417-21.
- Li, M, et al, Tear function and goblet cell density after pterygium excision. Eye (Lond), 2007. 21(2): p.224-8.
- Ye, F, et al, Evaluation of meibomian gland and tear film changes in patients with pterygium. Indian J Ophthalmol, 2017. 65(3): p.233-7.
- Masmali, AM, et al, Investigation of Ocular Tear Ferning in Controlled and Uncontrolled Diabetic Subjects. Eye Contact Lens, 2017. doi: 10.1097/ICL.0000000000000419. [Epub ahead of print].
- Rolando, M, F Baldi, and G Calabria, Tear mucus crystallization in children with cystic fibrosis. Ophthalmologica, 1988. 197(4): p.202-6.
- Kalayci, D, et al, Clinical status, ocular surface changes and tear ferning in patients with cystic fibrosis. Acta Ophthalmol Scand, 1996. 74(6): p.563-5.
