Vitreous floaters are common in the general population, presenting with a spectrum of symptoms that can vary from mild awareness to anxiety and significant visual dysfunction in a small proportion of patients. Acute vitreous floaters are indicative of potentially blinding retinal pathologies, but this article concentrates primarily on the patient with more chronic symptoms in the presence of a normal retinal examination. Most optometrists will have witnessed the distress occasionally caused by vitreous opacity and the frustrations of the patient seeking a cure.
The traditional attitude among many ophthalmic professionals has been the encouragement of tolerance or the frank denial that any treatment is possible. This has prevailed despite the advent of micro-incisional, sutureless vitrectomy surgery and improving laser techniques.
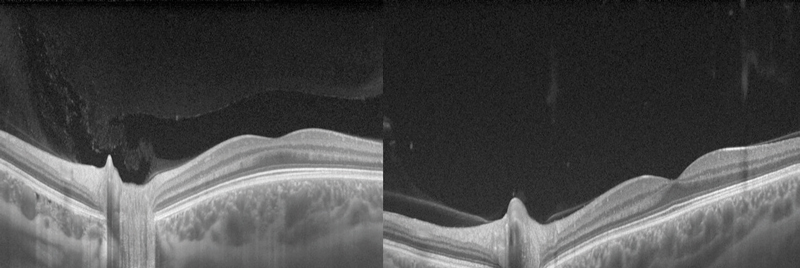
Figure 1 Swept-source OCT showing vitreous changes between an 18-year-old (left) and a 42-year-old (right)
This article provides a brief overview of the nature of vitreous, the pathophysiology of vitreous opacity, assessment of the patient with floaters, indications for intervention, vitrectomy surgery and laser vitreolysis. Our aim has been to produce a practical guide for the optometrist faced with a symptomatic patient. Readers seeking a more in-depth scientific analysis are directed towards two excellent review articles. Milston et al1 correlated the scientific and surgical work of Dr Jerry Sebag, an authority on the pathophysiology of the vitreous. Ivanova et al2 published a comprehensive evidence-based review and coined the term ‘symptomatic vitreous opacity’ or SVO.
Our hope is that with an improved understanding of the disease process and the available treatments, optometrists will be better positioned to provide reassurance and, where appropriate, to guide patients towards vitreoretinal surgeons with a sympathetic view of the significant visual morbidity resulting from SVO.
Nature of floaters and normal vitreous degeneration
Floaters arise following changes in the structure of the vitreous body and this is most commonly associated with the ageing process. Structural changes within the vitreous can also results from inflammation, vitreoretinal dystrophies, myopia and diabetes. The vitreous is a gel-like structure, mainly comprised of water, with a few cells, ‘hyalocytes’, in the peripheral vitreous cortex. The gel is maintained by a network of long thin collagen fibrils organised into interconnected small bundles. The concentration of collagen fibrils increases in the cortical layer of the vitreous which attaches to the inner surface of the retina. Between the collagen fibrils is a network of hyaluronan and this glycosaminoglycan attracts water and swells the clear gel.1
At birth the vitreous body is a colloidal gel. During the ageing process, the collagen fibrils aggregate causing variation within the consistency of the gel and pockets of liquid ‘lacunae’ form within it. This is likely to be due to dissociation of hyaluronan from its association with collagen fibrils which redistribute to become aggregated, and when sufficiently large, these aggregated fibrils can be seen as vitreous opacities. These changes are expedited in myopic individuals.1
Liquefaction of the vitreous and age-related weakening of post-basal vitreoretinal adhesion can lead to posterior vitreous detachment (PVD) which is present in around two-thirds of eyes by the eighth decade (figure 1).3 Risk factors for earlier PVD include myopia and collagen disorders such as Stickler syndrome. PVD in younger age groups is more likely to induce retinal tears and detachment due to the firm vitreoretinal adhesion. The strength of attachment of the vitreous to the retina is greatest at the vitreous base due to the relatively higher concentration of collagen compared to hyaluronan. The vitreoretinal adhesion is stronger at the margin of the optic disc, over blood vessels and surrounding the fovea.1 A ring of fibrous tissue, a Weiss ring (figure 2), typically becomes detached from the optic nerve, and is often seen on the posterior hyaloid membrane. It can be seen during ophthalmoscopy as an annular structure anterior and adjacent to the optic disc. It can cast a shadow on the retina which patients often describe as semicircular/circular. Over time as the gel collapses further, the posterior hyaloid membrane moves anteriorly and inferiorly, and the floater becomes less noticeable.
Figure 2 A Weiss ring
 For the majority of those affected, the symptoms of floaters are not overly troublesome. However, in a small proportion of patients they can have a significant impact on quality of life, particularly when vitreous liquefaction starts earlier in life and continues to progress with age.
For the majority of those affected, the symptoms of floaters are not overly troublesome. However, in a small proportion of patients they can have a significant impact on quality of life, particularly when vitreous liquefaction starts earlier in life and continues to progress with age.
Vitreous floaters can arise from structures endogenous to the vitreous body as well as from other sources such as amyloid, asteroid bodies, macrophages, lymphocytes, blood, intravitreal implants, foreign bodies and parasites. Secondary floaters are opacities in the vitreous body that originate from outside the vitreous such as vitreous haemorrhage which induces sudden onset floaters and hazy vision.1 Acute PVD can, with inherent traction on the retinal blood vessels, lead to vitreous haemorrhage and, in some instances, retinal tears. Tears can also be associated with the release of pigment epithelial cells into the vitreous body producing symptomatic floaters and subsequent retinal detachment causes the characteristic curtain-like progressive visual loss and subsequent retinal detachment causes the characteristic curtain-like progressive visual loss.
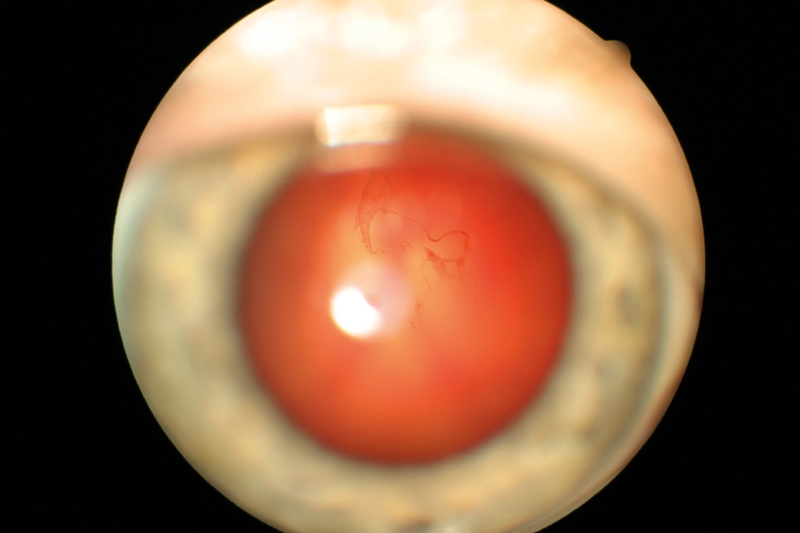
Figure 3 Asteroid hyalosis
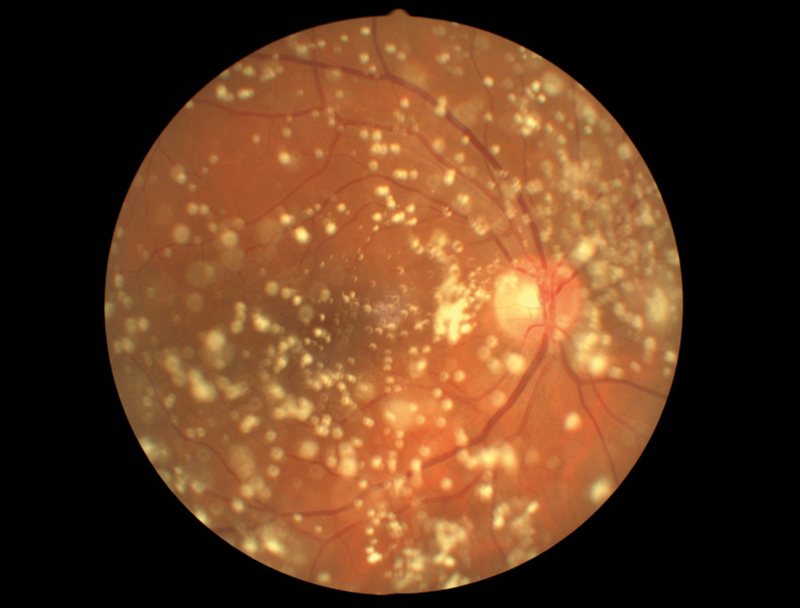
Asteroid hyalosis is an accumulation of calcium pyrophosphate spheres within the vitreous that typically causes minimal visual disturbance (figure 3). Secondary vitreous floaters can also present following inflammatory conditions that lead to increased cellular content in the vitreous and visual symptoms. Secondary floaters can also arise iatrogenically, for example following intravitreal anti-VEGF injections, but usually disappear within days.
Symptoms of SVO
The decision to undertake surgical treatment for SVO depends solely upon the patient’s symptoms. For the clinician this demands an understanding of precisely which symptoms can be attributed to vitreous opacity and why those symptoms arise. Functional loss from SVO relates mostly to factors tending not to affect high-contrast visual acuity testing and introduces concepts less familiar to clinicians in everyday practice. An understanding of these terms and the basic physics underlying various forms of visual disturbance helps us to comprehend both the patient’s symptoms and the results of physical examination.
Figure 4 No clear photographic image possible in a case of asteroid hyalosis (left) but fluorescein angiogram remains unaffected (right)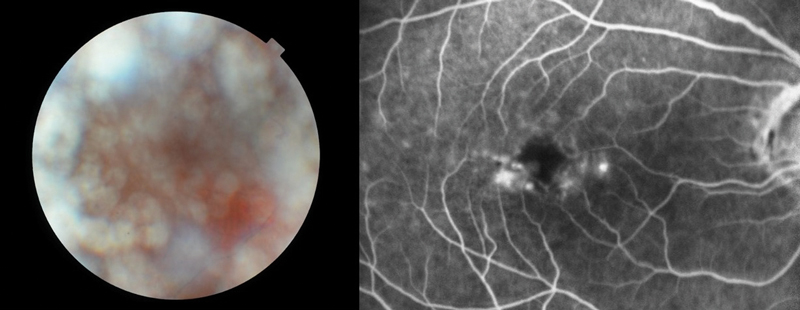 Patients do not ‘see’ floaters; they perceive the shadow (or visual phenomena around a central shadow) cast by media opacity and they witness the effects of light scatter. Defined opacity close to the retina, for example a Weiss ring in the early stages of PVD, tends to produce a precise shadow that may be described in great detail by some patients. As vitreous gel collapses and the detached posterior vitreous cortex moves forward, a well-defined opacity produces a less well-defined shadow on the retina.
Patients do not ‘see’ floaters; they perceive the shadow (or visual phenomena around a central shadow) cast by media opacity and they witness the effects of light scatter. Defined opacity close to the retina, for example a Weiss ring in the early stages of PVD, tends to produce a precise shadow that may be described in great detail by some patients. As vitreous gel collapses and the detached posterior vitreous cortex moves forward, a well-defined opacity produces a less well-defined shadow on the retina.
More nebulous opacity in the anterior vitreous produces an ill-defined haze. In general, the key to differentiating SVO from other media opacity is the typical drifting phenomenon, which continues when gaze re-fixates after ocular movement.
Most SVO is accentuated when viewing against lighter backgrounds such as a blue sky or white wall, but this is not invariable with some patients complaining of a generalised loss of contrast, haziness, or glare from point light sources at night.
While most of us have learned that visual acuity is a poor marker for SVO we have been less successful in adopting a truly useful objective test. The work of Dr Tom van den Berg on the concept of straylight has begun to fill this gap in our comprehension.4 Straylight describes a visual defect caused by light scattering from a media disturbance resulting in a veiling of the retinal image perceived as haziness, loss of contrast, increase glare and occasionally colour vision anomalies. Standard clinical examination at the slit lamp cannot truly evaluate straylight as the subject and the observer witness different optical phenomena. The observer sees light reflected back from media opacity, defined as ‘back-scatter’ while the subject is troubled by the imperfect transmission of light and the resulting ‘forward-scatter’.
The difference between back-scatter and forward-scatter explains (at least in part) why a subject and an observer may have very different perceptions of the severity of media opacity, for example posterior capsular opacity or asteroid hyalosis. The functional deficit from forward-scatter and straylight can now be quantified using the C-Quant Straylight Meter (Oculus, Germany), although this has not yet been adopted widely into everyday clinical practice.
Contrast sensitivity (CS) testing is more widely available and, in some cases, may provide the reassurance of measurable pathology in the presence of normal visual acuity. CS has been shown to fall in the presence of SVO and improve following vitrectomy, but again is rarely considered part of the standard evaluation of a patient with SVO. CS can be regarded as an intermediary between visual acuity (determined by light deflection in the central area of <0.1°) and straylight (caused by light scattering over angles up to 90°). Van den Berg has stated that a fivefold increase in light scattering would lower CS by only 20%, limiting the application of the latter as a diagnostic tool.
It has been suggested that a more useful modification of CS testing is the simultaneous application of glare (for example from side illumination) with glare testing equipment now commercially available. At a practical level it is worth noting that a particular form of symptomatic glare is common with SVO, becoming manifest as physical discomfort. Such symptoms were traditionally regarded as ‘psychological’ and viewed with suspicion by clinicians but it is now widely accepted that ‘discomfort glare’ is a very real (but unmeasurable) phenomenon associated with SVO.
Gaining a true understanding of the impact of SVO has been a challenge for clinicians, but research has highlighted the severity of symptoms experienced by some patients. One study used ‘utility values’ to assess the subjective impact of SVO. This revealed patients with floaters being willing to trade off an average 1.1 years out of every 10 years of their remaining life for a cure. Furthermore, these patients were willing to take, on average, an 11% risk of death and a 7% risk of blindness to eliminate the symptoms of SVO.5
Symptoms of vitreous opacity – summary points
- The severity of symptoms is paramount in the decision to consider surgery.
- Symptoms vary from well-defined floaters to nebulous mistiness.
- Vitreous opacity demonstrates characteristic drifting following ocular movement.
- Patients are troubled primarily by forward-scatter of light, which is not routinely evaluated with standard clinical examination.
- Symptoms can be more severe in brighter photopic conditions.
- Clinicians typically underestimate and downplay the effect of SVO.
Signs of SVO
Examination of the patient with vitreous opacity
A full assessment of vitreous opacity necessitates pupillary dilation. This, of course, is particularly important in individuals with an acute onset of floaters in whom acute posterior vitreous detachment is suspected and a full examination of the retinal periphery is mandatory.
The immediate retrolental gel is best examined with a bright slit beam and a comparison between the two eyes. Asking the patient to look up and then re-fixate on the horizontal swirls the vitreous gel increasing the chance of visualising blood or pigment, as well as giving a better impression of vitreous syneresis. As discussed above we are likely to be impressed by back-scatter of light whilst remaining oblivious to the patient’s symptomatic forward-scatter.
The degree of vitreous collapse in some patients post-PVD can sometimes be brought home to the examiner by the visualisation of a Weiss ring in the anterior vitreous gel. Once the posterior limit of examination has been reached with the slit beam alone, a 90 D lens or similar can be used to assess the more posterior gel and look for thickened cortical vitreous. A better appreciation of the opacity can sometimes be obtained by offsetting the slit been slightly from coaxial, but it is only by regular and repeated practice on patients of differing ages, that the observer can acquire a feel for what is normal.
An alternative means of assessing gel opacity is through an appreciation of the quality of the retinal image obtained from slit lamp biomicroscopy, retinal photography or OCT retinal scanning. It is tempting to think that ‘our view’ of the central retina (in the absence of other causes of media opacity) may reflect the patient’s quality of vision. To more fully appreciate why this approach may sometimes fail it is worth considering the specific entity of asteroid hyalosis in which there is a notorious disparity between subjective vision and the quality of retinal imaging.
The asteroid hyalosis paradox
Many of us will have been amazed that a patient with asteroid hyalosis can be asymptomatic when we are confronted by a dense mass of asteroid bodies and no useful view of the central retina. One common explanation for this phenomenon is that asteroid bodies are spherical and regularly interspersed, with a minimum of light scattering, casting less dramatic shadowing and thus less degradation of the retinal image. Vitreous syneresis results in a more irregular clustering of collagen fibrils, which scatter light causing a greater penumbra (partial shadow). It is interesting that some patients with asteroid become markedly more symptomatic following posterior vitreous detachment (with gel collapsing, moving anteriorly and losing uniformity of structure) and in the pseudophakic eye where, presumably, forward movement of the gel towards the more anteriorly positioned posterior surface of the implant lens adversely affects the optics of the visual system.
A second explanation of our overestimation of the severity of asteroid relates to the double-pass phenomenon. The quality of the retinal image depends upon a single transmission of light through the vitreous whereas we see (and image) light that has, in addition, been reflected from the retina and traversed a second pass through media opacity. This is beautifully illustrated when fluorescein angiography is performed on an eye with asteroid: the colour photograph and red-free image may be virtually unintelligible whilst the fluorescein angiogram is clear (figure 4). The explanation for this paradox is that only emitted light from the fluorescein is visualised and this makes a single pass through the vitreous gel (from retina to camera) with backscatter from the exciting wavelength eliminated by the filter.
A final point to bear in mind is the effect of the wavefront as a whole moving through the vitreous in negating the effects of small opacities. Light from an object being transmitted to the fovea, for example, traverses all of the gel as limited by the size of the pupillary aperture, not just the thin central beam that we tend to use with a 90 D lens at the slip lamp. We may have the impression that a defined opacity should cast a precise shadow on the retina when in reality there are multiple rays traversing the media from an object to the retina, the vast majority of which will not be impeded. This explains the phenomenon by which a solid opacity is perceived by the patient as a dark central shadow (the umbra) with a grey ring (the penumbra) around it. As the opacity moves further from the retina the umbra becomes less defined and ultimately the entire shadow may disappear.
Diagnosis of posterior vitreous detachment
Acute posterior vitreous detachment (PVD) is the commonest cause of new SVO and something all optometrists will face as a routine part of clinical practice. Diagnosis of PVD gives the optometrist both an acceptable cause of SVO and a requirement to eliminate the possibility of significant vitreoretinal pathology. Most vitreoretinal surgeons will have faced the situation where a confident diagnosis of PVD in our patients is then followed by the unexpected presence of attached gel partway through a vitrectomy procedure. Surgical intervention (and ultrasound scanning) has also taught us that a PVD may not be complete: the presence of a superior retinal flap tear does not guarantee that gel will have separated cleanly from the macula or optic disc; conversely, a clearly visible Weiss ring does not mean that gel has separated to its limit (the posterior vitreous base) throughout 360°.
For the optometrist, however, the situation is more pragmatic; the priority being the identification of a Weiss ring and the visualisation of either red blood cells or pigment in the anterior vitreous. For practical purposes the presence of a Weiss ring can be taken to indicate the presence of a PVD. Blood in the anterior vitreous indicates a strong possibility of a retinal tear whereas pigment in the anterior vitreous (the Shafer sign) implies a virtual inevitability.6
The nature of the gel also changes as it collapses anteriorly often being described as more ‘fibrillary’ or showing thickening of the mobile veils. These are relatively soft signs necessitating bilateral pupillary dilation, which will not always be possible for the optometrist in high-street practice. For the surgeon these can be important signs lending weight to surgical decision-making.
Vitreous and retinal imaging
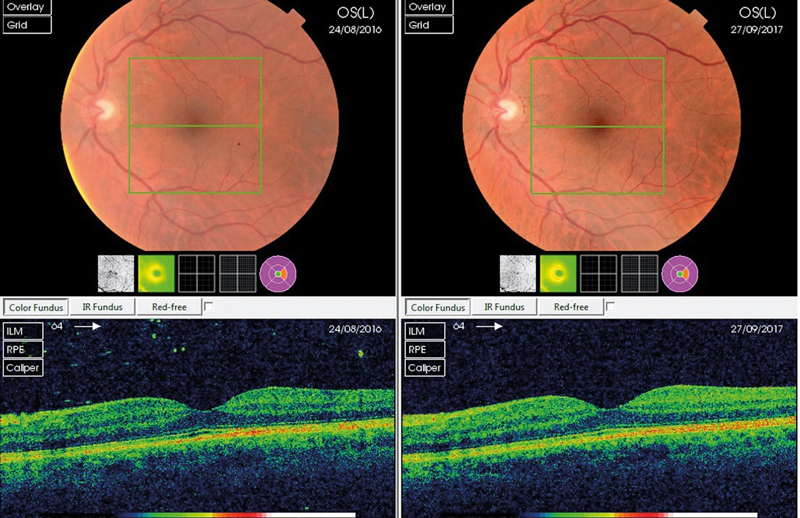
The irregular structure of degenerative vitreous can have a profound effect on degradation of retinal image quality. This allows us to make some assessment of severity on the basis of a loss of detail when viewing either a retinal photograph or OCT. Sometimes it is only in retrospect, comparing pre- and post-vitrectomy images, that optical degradation becomes apparent (figures 5 and 6).
Figure 5 Improved colour image quality post vitrectomy for symptomatic vitreous opacity and loss of shadowing from condensed gel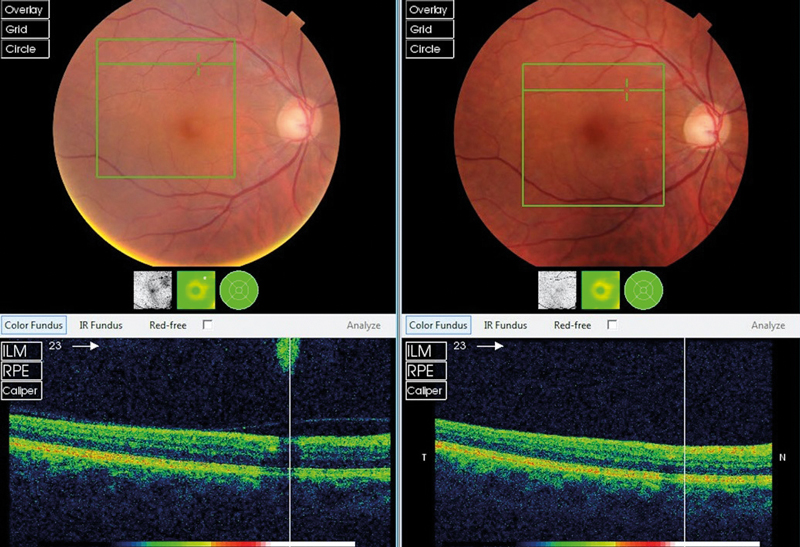
Figure 6 Improved colour image quality post vitrectomy for symptomatic vitreous opacity following PVD
Opaque vitreous may be identified on a retinal image either as a diffuse loss of quality or a specific shadow. In general, our experience is that the more anterior forms of gel opacity have the more diffuse effect. In patients with asymmetric vitreous opacity (as is commonly the case) there may be an obvious comparative difference between the two eyes (figure 7).
Assessment of optical image quality is not diagnostic. As with symptoms, patients with what appears to be an obvious objective finding on retinal imaging may be completely asymptomatic. Imaging is only of use in providing reassurance that a diagnosis of SVO is correct and adding weight to surgical decision-making.
Figure 7 Right symptomatic vitreous opacity showing asymmetry and diffuse loss of colour on the right fundus image and macular OCT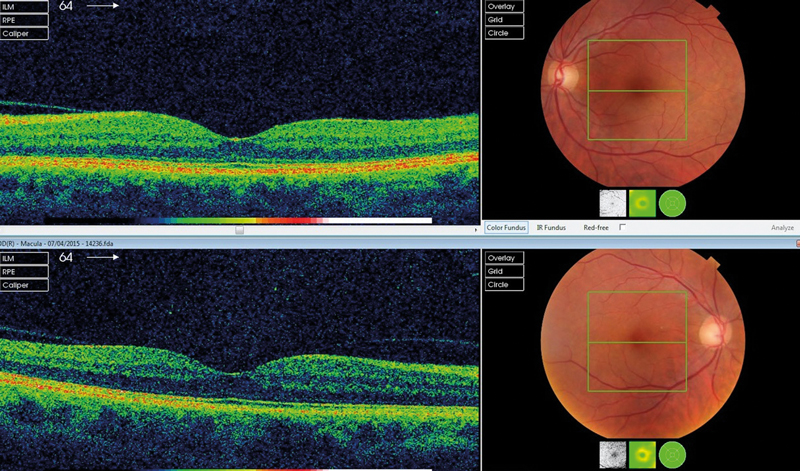 Signs of vitreous opacity – summary points
Signs of vitreous opacity – summary points
- The benign optical effect of asteroid hyalosis encourages the traditional underestimation of visual loss from opaque vitreous gel.
- Shadowing or diffuse loss of detail on a retinal image may indicate SVO.
- Asymmetric SVO can manifest as asymmetric image quality.
- Acute SVO necessitates a full examination for the presence of PVD and possible associated peripheral retina pathology.
Vitrectomy Surgery for Symptomatic Vitreous Opacity
Contemporary vitreoretinal surgery is almost unrecognisable from the pioneering procedures first attempted almost 50 years ago. Evolution, however, appears to be approaching a current plateau with a 3-port, trans-conjunctival sutureless approach. The gauge size of vitrectomy instruments has decreased with most surgeons choosing between 23g, 25g and 27g. The smaller gauge has the advantage of improved self-sealing characteristics offset against decreased flow rates and less rigid instrumentation.
As confidence in vitrectomy techniques has increased, the indications for surgery have expanded and the threshold for intervention has lowered. For many pathologies pars plana vitrectomy is simply the initial step prior to the primary purpose of intervention, for example membrane peeling or macular hole repair. In the case of surgery for SVO, vitrectomy alone is the sole purpose of intervention and it might appear, therefore, that floater vitrectomy could be regarded by surgeons as more minimal and low risk. In fact, the opposite is the case with many surgeons being extremely apprehensive about operating on eyes with good high contrast visual acuity. In recent years, however, there has been a gradual shift in attitude as we understand the importance of placing greater emphasis on both the patient’s symptoms and other measurable parameters of visual function (figures 8 and 9).
Figure 8 Pseudophake with opaque anterior gel showing MTF and simulated Snellen E improvement post-vitrectomy (acuity maintained at 6/15)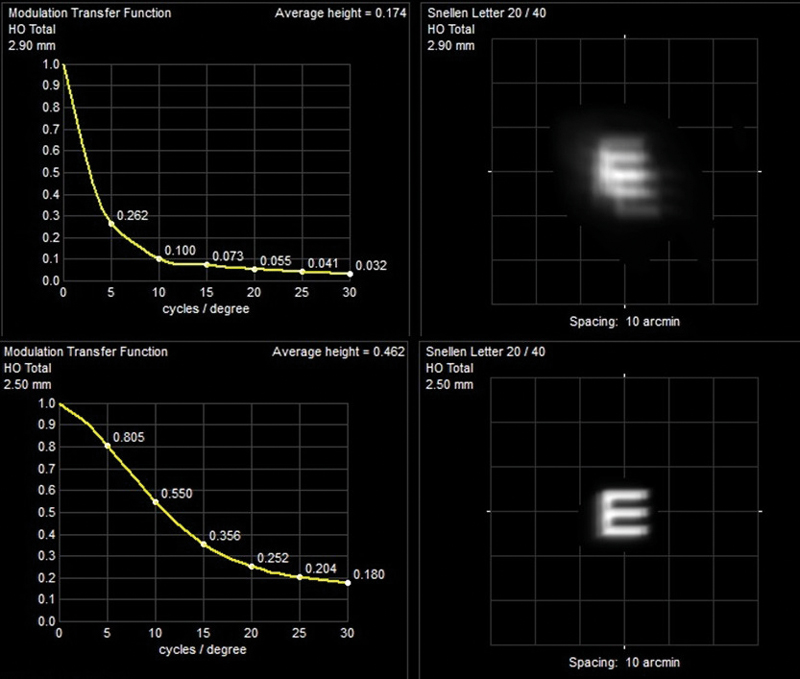 Alternatives to a 3-port vitrectomy have been described specifically for SVO. These include ‘deep anterior vitrectomy’ carried out at the time of cataract surgery and 1-port vitrectomy inserting only a vitreous cutter, viewing with an indirect ophthalmoscope and simply attempting to remove a single opacity without the infusion usually required to maintain intraocular pressure. Neither of these has gained popularity.1,2
Alternatives to a 3-port vitrectomy have been described specifically for SVO. These include ‘deep anterior vitrectomy’ carried out at the time of cataract surgery and 1-port vitrectomy inserting only a vitreous cutter, viewing with an indirect ophthalmoscope and simply attempting to remove a single opacity without the infusion usually required to maintain intraocular pressure. Neither of these has gained popularity.1,2
Figure 9 Gel syneresis with high myopia and maculopathy showing improved post-operative MTF and simulated Snellen E (acuity increase from 6/30 to 6/15)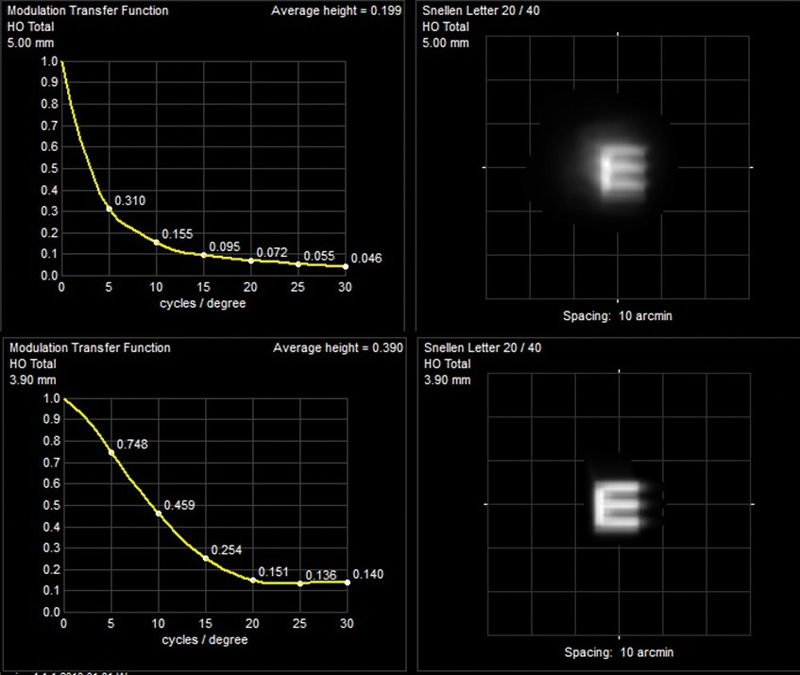 The timing of intervention generally depends on a combination of factors driven by patient dissatisfaction and surgical enthusiasm. Following acute PVD we know that many patients will settle with the combination of forward movement of gel, the shift of opacity away from the visual axis and neuroadaptation. Most surgeons will be reluctant to consider vitrectomy for at least three months, during which time there is ample opportunity for patient education. For those with chronic or more gradually evolving vitreous change, debilitating SVO requires careful and, usually, repeated discussion. Many patients will have been told that SVO is untreatable, so their natural initial enthusiasm for intervention on hearing that a surgical option exists has to be tempered.
The timing of intervention generally depends on a combination of factors driven by patient dissatisfaction and surgical enthusiasm. Following acute PVD we know that many patients will settle with the combination of forward movement of gel, the shift of opacity away from the visual axis and neuroadaptation. Most surgeons will be reluctant to consider vitrectomy for at least three months, during which time there is ample opportunity for patient education. For those with chronic or more gradually evolving vitreous change, debilitating SVO requires careful and, usually, repeated discussion. Many patients will have been told that SVO is untreatable, so their natural initial enthusiasm for intervention on hearing that a surgical option exists has to be tempered.
Vitrectomy surgery is routinely carried out under local anaesthesia with sedation (oral or intravenous) as required, patients generally spending no more than half a day in hospital with few post-operative symptoms and rapid visual rehabilitation. Occasionally air tamponade will be required if retinal breaks have been encountered or there is a suggestion that the scleral wounds may fail to self-seal. This is more common in myopic eyes where the sclera is thin, air within the vitreous cavity tending to remain as a single bubble (as a consequence of the laws of surface tension) and thus preventing leakage. An air bubble will clear naturally from within the vitreous cavity in about one week as aqueous is produced with air dissolving in the fluid and leaving the eye via aqueous outflow. For patients undergoing simultaneous phacoemulsification there may be a need for refractive correction as with any lens surgery and dependent upon the refractive plan for that particular patient.
Several studies have analysed the success of floater vitrectomy surgery with generally positive outcomes and a happy patient population. These studies are summarised in the two review papers previously referenced.1,2 Patient satisfaction will inevitably depend upon appropriate pre-operative counselling and realistic patient expectation, with the latter being a challenge in some cases in spite of lengthy consultation, the provision of written information and rigorous consenting. For patients with apparently minimal vitreous opacity but significant psychological overlay the persistence of even a fraction of the number of original floaters can be devastating. These patients are also prone to an apparent exaggeration of post-operative features generally accepted as commonplace and transient by most individuals such as mild dry eye, minor accommodative changes in the phakic eye, and an awareness of a peripheral vitreous frill. Peripheral vitreous cannot be completely removed from the retina as gel is intimately bound to the eye wall at the ‘vitreous base’, an area straddling the ora serrata extending a couple of millimetres or so posteriorly onto peripheral retina and anteriorly onto the pars plana of the ciliary body. Unfortunately, some younger patients will already have read passionate reports on websites describing apparently disproportionate symptoms, visual phenomena that the vast majority of post-vitrectomy patients do not see.
All vitrectomy surgery carries a risk of retinal detachment with the perception of this risk being higher in eyes requiring induction of PVD. Whether PVD occurs spontaneously or is induced surgically this is the point at which peripheral retinal tears are most likely to occur and, thus, the time of maximum risk for retinal detachment. Reported rates of retinal detachment following vitrectomy surgery for SVO vary so widely that no useful consensus figure can be gained from the literature. It is generally accepted that a rate of less than 1% can be quoted; with prompt diagnosis and treatment, only a tiny percentage of this group will be unfortunate enough to lose vision in the longer term.
The higher incidence of SVO in myopic eyes is a potential confounding variable. It has long been recognised that myopia predisposes both to early PVD and an association with greater vitreous syneresis resulting in a higher chance of SVO. Following PVD in a myopic eye, the chance of progressing to retinal detachment is higher because of the increased incidence of lattice degeneration or other forms of peripheral vitreoretinal weakness likely to result in a retinal tear. Thus, it is inevitable that a cohort of myopic patients presenting with SVO belongs to a group with a life-long increased risk of retinal detachment, irrespective of any secondary surgical effect.
All patients must be warned that pre-existing cataract will be accelerated and younger patients with clear lenses will develop cataract prematurely. The actual mechanism is unknown but the use of intraocular air or gas tamponade perioperatively constitutes an additional risk. Otherwise, attempts at changing the many parameters of the procedure (for example the type of infusion fluid or the wavelength of light used in the operating microscope) has made little difference.
Other complications following vitrectomy such as glaucoma, epiretinal membrane or macular hole are rare. The most potentially devastating complication of any intraocular surgery is endophthalmitis with a reported rate following vitrectomy surgery of around 1:2000.7 However, the move towards smaller gauge surgery (with a lower risk of leakage from scleral wounds) along with the more confident use of on-table antibiotic therapy (both intra-cameral post-phacoemulsification and sub-conjunctival over the scleral wounds) has almost certainly led to a radical decrease in this number.
A recent suggestion for SVO patients who have not yet undergone spontaneous PVD is the floaters-only-vitrectomy (FOV). There are solid scientific grounds supporting this approach for reducing the risk of retinal tears, slowing the speed of onset of cataract and negating the presence of vitreous frill. Avoiding induction of PVD should, in theory, dramatically reduce the risk of retinal tears as this is the moment during surgery when tears are most likely to form. Leaving a barrier of anterior cortical vitreous attached to the posterior surface of the crystalline lens might, in theory, slow the development of cataract by protecting the posterior surface from the direct effect of infusion fluid. In the absence of PVD there is no anterior vitreous frill present so patients can be reassured that this much-debated phenomenon cannot occur. Of course, it has to be explained to patients that PVD remains likely over the course of a lifetime and that it is not yet known if this is accelerated by FOV. There must be some concern that those individuals symptomatic from vitreous opacity pre-PVD have an increased chance of becoming symptomatic post-PVD. As yet there are no long-term data available, but the concept will be appealing to surgeons apprehensive about the possible sequelae of PVD induction in the younger eye.
Summary
- Small-gauge sutureless vitrectomy is an increasingly safe surgical technique.
- Surgery under local anaesthesia with optional sedation necessitates no more than a half-day in hospital.
- The development or acceleration of cataract is inevitable.
- The small risk of post-vitrectomy retinal detachment can be minimised, but never completely eliminated.
- Some post-vitrectomy visual symptoms may be amplified in a subgroup of patients psychologically sensitive to SVO.
- The floaters-only-vitrectomy (FOV) is an increasingly popular option for the younger, pre-presbyopic patient with attached vitreous gel.
Combined Phacoemulsification-vitrectomy
While the overall surgical risk of vitrectomy is low, acceleration of the development of cataract is an inevitability. The speed of onset post-vitrectomy is age-dependent so younger patients (those in their 20s or 30s) may go decades before significant lens opacity becomes an issue. However, this is widely variable and generally out of the control of the operating surgeon. It is claimed that floaters-only-vitrectomy with sparing of the post-lenticular gel may delay the onset of lens opacity, but this is unproven in the longer term.
Accepting that vitrectomy speeds the onset of lens change has done little to promote meaningful discussion on the pros and cons of a combined phacoemulsification-vitrectomy procedure. The pervasive influence of North American surgical practice, in terms of both surgical numbers and journal publication, has a powerful potential effect. North American ophthalmic training differs from the UK in terms of sub-specialisation: the vast majority of vitrectomy surgeons in North America do not undertake phacoemulsification and a combined procedure is, therefore, something of a logistic challenge in coordinating two separate surgical teams, sometimes from two separate units.
The situation in the UK (and much of Europe) is different, as many vitrectomy surgeons will be comfortable with phacoemulsification and some equally at home with refractive lens strategies. The view that concomitant phacoemulsification should only be offered with vitrectomy when a significant level of cataract is present has become something of an anachronism when clear lens extraction is growing in popularity. Ultimately, the decision will come down to the experience and attitude of the operating surgeon as well as the refractive wishes of an individual patient.
In determining the pros and cons of a combined phacoemulsification-vitrectomy procedure there are limited published data and thus an inevitability that we rely on more anecdotal reports. Adding phacoemulsification to vitrectomy makes virtually no difference to the risk of retinal detachment or endophthalmitis and the increased surgical time is minimal.
Advantages of combined phacoemulsification-vitrectomy
Phacoemulsification in the presence of vitreous gel (rather than a vitrectomised eye) is, in general, a more predictable procedure, particularly in myopic eyes with deep anterior chambers and the potential for extreme mobility of the lens-iris diaphragm. Cataract surgeons unfamiliar with vitrectomised eyes may be tempted to wait for symptoms to reach a more significant level, extending the time (post-vitrectomy) a patient suffers visual dysfunction.
Vitrectomy in the pseudophakic eye allows more complete removal of vitreous gel, the fashioning of a primary central surgical capsulectomy and a thorough inspection of the peripheral retina at completion. Scleral indentation can be performed either in the presence of a new implant lens or the aphakic eye (immediately prior to intraocular lens implantation) giving a clear view of the peripheral retina out to the ora serrata and the confident application of retinopexy as required.
The sole aim of surgery is visual rehabilitation: patients developing lens opacity in vitrectomised eyes tend to live with a period of increasing index myopia, this being a characteristic feature of post-vitrectomy cataract, along with a loss of quality. A second procedure at a later date inevitably increases both the overall cost and the cumulative complication rate. This can be particularly frustrating when there is an unexpectedly short period of time between floater vitrectomy and the onset of lens opacity.
Combined phacoemulsification-vitrectomy: decision-making
Whatever the view of an individual surgeon, it is vital patients are made aware of the surgical options, the potential complications and the longer-term sequelae. Broadly speaking, we can divide our patients into three groups taking into consideration factors including age, refraction, lens opacity, presbyopic status and vitreous attachment.
The SVO patient with cataract
A combined procedure is usually offered, with cataract surgery to the fellow eye for refractive balance and the best overall binocular function. Any refractive manipulation, for example monovision or implantation of a multifocal intraocular lens, can be considered as in standard cataract surgery. Most patients in this group will either have developed PVD or be of an age where a PVD can be induced easily by the surgeon with a minimal risk of complications.
The SVO patient with no cataract – presbyopic
This group will include emmetropic subjects for whom readers are a new phenomenon, hyperopes with increasing spectacle dependence for all distances and myopes with more long-standing refractive needs. Those psychological aspects of personality that usher patients towards floater vitrectomy surgery may also be a stimulus to the search for a lasting refractive solution. Again, decision-making is dependent upon the maintenance of binocular balance and the enthusiasm of the patient to consider second eye-surgery for purely refractive indications. The ever-increasing array of ‘premium’ intraocular lenses increases the chance of a patient finding an implant lens to meet their refractive goals, with new extended-depth-of-focus technology adding to the more traditional bifocal and trifocal platforms.
The SVO patient with no cataract – pre-presbyopic
This group inevitably includes the youngest subjects with SVO for whom avoidance of post-vitrectomy cataract takes priority. The floaters-only-vitrectomy is an increasingly popular solution, acknowledging that PVD may be accelerated by surgery.
Summary
- Cataract is an inevitable development following vitrectomy surgery.
- For some patients a combined phacoemulsification-vitrectomy procedure offers distinct advantages beyond the obvious benefit of a single procedure.
- The combined procedure offers both an attractive safety profile and cost benefit.
- The ever-expanding choice of intraocular lenses will appeal to some patients tempted by the prospect of a degree of spectacle independence.
- The floaters-only-vitrectomy option is likely to increase in popularity amongst the younger pre-presbyopic group with attached vitreous gel.
The younger patient with symptomatic physiological vitreous opacity
Vitreous floaters are common in younger people, frequently being present from the third decade of life. In the vast majority of cases symptoms can be ignored, rationalised or adapted to over a period of time. A very small minority of younger patients with apparently ‘normal’ vitreous gel complain vehemently, with symptoms apparently disproportionate to clinical findings.
Some in this group will be reassured by the absence of any significant underlying vitreoretinal pathology and a thorough explanation of the process of vitreous syneresis. A proportion of subjects in this set will volunteer aspects of their medical history suggesting a tendency to introspection and anxiety including the need for psychological counselling and medication. It is not uncommon for the younger patient with apparently physiological vitreous degeneration to arrive with an encyclopaedic knowledge of floater vitrectomy websites and the blogosphere.
As ever, it is vital that a full ophthalmic examination is carried out and the patient’s concerns respected, in spite of the lack of overt treatable disease. As discussed above it is imperative to remember that the patient does not experience what we see at the slit lamp with an unremarkable examination being difficult (for the observer) to reconcile with reportedly life-changing symptoms. Some in this group will have given up work, stopped driving or even ended relationships because of their preoccupation with SVO.
The attitude of the vitreoretinal surgical community to this group of patients varies widely. Some surgeons regard psychological profiling or even a psychiatric assessment as mandatory before considering intervention. Others simply believe that it is in the patient’s best interest to be told that nothing can be done, acceptance and adaptation being the correct advice.
Some highly symptomatic patients can be reassured and gently coaxed towards acceptance, but not all. There will remain a very small cohort for whom normal life has been destroyed by their symptoms and, over time, surgery may be the only viable option. In addition to the standard list of complications needing discussion it is worth emphasising this particular group suffer a variety of additional post-vitrectomy features, some of which can be almost as devastating to the patient as their original floaters. These include nebulous visual symptoms, accommodative spasm, ocular discomfort, a persisting awareness of minimal vitreous floaters, photopsia, an intrusive peripheral frill of anterior vitreous cortex and an increased awareness of gel in the fellow eye. If surgery is undertaken then the floaters-only-vitrectomy is now the treatment of choice.
YAG Laser floater vitreolysis
The concept of YAG laser disruption of vitreous opacity has been with us for many years but failed to gain broad popularity. Negativity has been centred on many factors including uncertainty of outcome; fear of potential complications; and the constraints of visualising the deeper vitreous when using traditional YAG laser machines. It is primarily through addressing the last concern that we have seen a recent resurgence in enthusiasm for YAG vitreolysis.
The traditional YAG laser delivery system, used commonly for posterior capsulotomy, will only function with the slit beam off-set as is normally the case for viewing anterior segment structures. Of course, visualisation of the posterior segment, with a 90 D lens or similar, is usually best performed with coaxial (or just off-axis) viewing. On traditional YAG machines coaxial viewing is not possible and visualisation of posterior structures has been potentially challenging. The recently introduced True Coaxial Illumination (TCI) YAG laser technology (Ellex Medical Lasers, Australia) allows simultaneous coaxial viewing and laser delivery, which claims to permit potentially safer delivery of laser energy and a minimised risk of complications, for example inadvertent damage to the retina or lens.
Laser vitreolysis has been associated with a number of complications including raised IOP and glaucoma, cataract, retinal tears and detachment, retinal haemorrhage and an increase in subjective floaters.8 A recent Cochrane database review designed to compare the safety and efficacy of YAG laser vitreolysis to pars plana vitrectomy simply concluded that no studies met the inclusion criteria of the review.9 What is not yet known is whether these complications related significantly to poor visualisation and orientation with non-coaxial viewing systems and thus whether we can expect a much higher safety profile from the TCI YAG.
A recent clinical trial with TCI showed laser vitreolysis treatment to be superior to sham with an excellent safety profile. However, this study involved only those with symptoms from a defined Weiss ring post-PVD and as yet there are limited anecdotal reports on treating either floaters in the absence of a PVD or gel opacity distinct from a defined Weiss ring.10
Summary
- Laser vitreolysis has been practised for many years without gaining widespread popularity.
- The advent of True Coaxial Illumination allowing the simultaneous coaxial viewing and delivery of laser to the deeper vitreous is an exciting development.
- There are few published data but anecdotal reports suggest the best candidates have small, well-defined floaters in the presence of PVD.
Conclusion
Vitreous floaters that arise in myopic individuals, as a result of the natural ageing process or due to secondary causes such as vitreous haemorrhage or uveitis, can be symptomatic and, in rare cases, can severely impact vision and quality of life. Current management options for symptomatic visual opacities include patient education and observation, pars plana vitrectomy and Nd:YAG laser vitreolysis. Contemporary small gauge pars plana vitrectomy surgery has good success rates, although it is not without potential risks. Referral for assessment and possible treatment may be an appropriate option to consider for a small sub-set of symptomatic individuals with clinically significant, persistent floaters that are adversely impacting on quality of life. Further prospective studies are required in order to better understand the natural history of floaters and to compare the longer-term safety and efficacy of the available treatment options. Meanwhile the optometrist as the trusted eye healthcare provider is uniquely placed to help patients with symptomatic vitreous opacity. Professional and sympathetic guidance is the important first step towards our patient making an informed decision on the potential risks and benefits of intervention.
Mr Andrew Luff is a Consultant Ophthalmic Surgeon at Optegra Hampshire and Optegra Surrey Eye Hospitals and Founder of Sapphire Eyecare Újluff@me.com
Dr Clare O'Donnell is Head of Eye Sciences at Optegra Eye Health Care
References
- Milston R, Madigan MC, Sebag J. Vitreous floaters: etiology, diagnostics and management. Surv Ophthalmol. 2016 61 211-27
- Ivanova T, Jalil A, Antoniou Y, Bishop PN, Vallejo-Garcia JL and Patton N. Vitrectomy for primary symptomatic vitreous opacities: an evidence-based review. Eye. 2016 30 646-55
- Foos RM, Wheeler NC. Vitreoretinal juncture. Synchysis senilis and posterior vitreous detachment. Ophthalmology. 1982 89 (12) 1502-12
- Van den Berg TJ, Franssen L, Coppens JE. Straylight in the human eye: testing objectivity and optical character of the psychophysical measurement. Ophthal Physiol Opt. 2009 29 3 345-50
- Wagle AM, Lim W-Y, Yap T-P, Neelam K, Au Eong K-G. Utility values associated with vitreous floaters. Am J Ophthalmol. 2011 132 60-5
- Chignell AH, Harle D, Tanner V, Williamson TH. Acute posterior vitreous detachment. Optom Practice 2000. 1 97-102
- Chen G, Tzekov R, LI W, Jiang F, Mao S, Tong Y. Incidence of endophthalmitis after vitrectomy: A systematic review and meta-analysis. Retina 2018 doi 10.1097/iAE.00000000002055
- Hahn P, Schneider EW, Tabandeh H, Wong RW. Emerson GG. Reported complications following laser vitreolysis. JAMA Ophthalmology. 2017 135 973-76
- Kokavec J, Wu Z, Sherwin JC, Ang AJS, Ang GS. Nd:RAG laser vitreolysis versus pars plana vitrectomy for vitreous floaters Review. Cochrane Database of Systematic Reviews 2017 Issue 6. Art No CD0111676 DOI: 10.1002/14651858. CD011676.pub2
- Shah CP, Heier JS. YAG Laser vitreolysis vs sham YAG vitreolysis for symptomatic vitreous floaters. A randomized clinical trial. JAMA Ophthalmology. 2017 135 918-23
