The first of these two articles (Optician 18.01.2019) showed how all of the current indirect tonometry techniques have common limitations in that their measured values might be affected by the dynamic changes in IOP and the biomechanical properties of the eye. Both of these will be considered in detail in this second article.
Dynamic changes in IOP
The dynamic changes in intraocular pressure (IOP) due to the cardiac cycle and respiration are usually of the order of 1 to 2mmHg. Also, the IOP changes with the circadian rhythm; these diurnal variations are approximately from 1 to 5mmHg.1 These small changes suggest that the mechanism for maintaining constant IOP is very tightly regulated. Despite much research, it is still not clear why the IOP must be so regulated because it is not known with certainty what the function of the established baseline IOP is.
One argument could be that the eye is an optical apparatus and the features of this apparatus must be kept as constant as possible in order to avoid fluctuations in the imaging performance. However, a weak correlation between refractive error, the image quality of the eye and the eye pressure negate this hypothesis.2,3 It might be assumed that the eye pressure is controlled only secondarily, as a result of another important process. For example, it could be that the production of aqueous humour should be kept constant in order to nourish the lens and cornea accordingly and to dispose of the metabolic products. Currently, there is no clear evidence for this. The IOP is a dynamic value, which underlies a number of physiological and individual influencing factors such as diurnal variation, arterial pulse, eating and drinking (see figure 1).
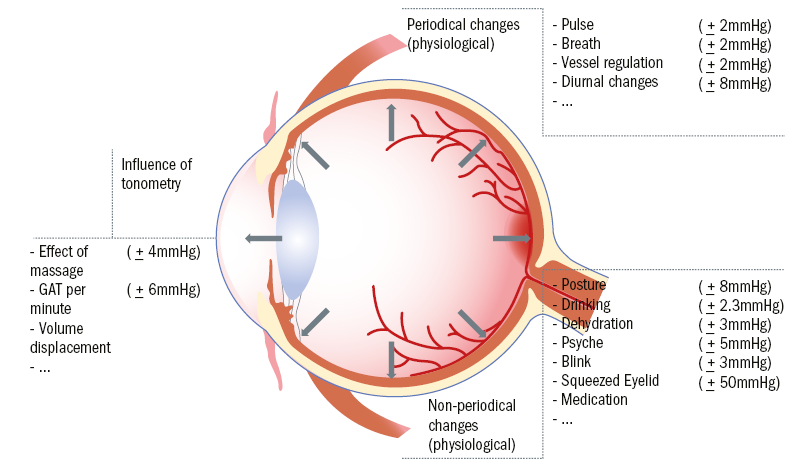
Figure 1: Intrinsic and extrinsic influences upon IOP (values from Draeger4)
Continuous 24-Hour IOP monitoring
Because of intrinsic corneal dynamic changes and the limitations of indirect tonometer methods, a continuous 24-hour IOP monitoring has long been required by eye care specialists. Continuous measurement of IOP may now provide new insights into the pathophysiology of glaucomatous damage and help to reassess the importance of known risk factors. Such new findings could considerably influence diagnostics, therapy recommendations and prophylaxis in glaucoma.
Currently, there are three types of sensors available to continuously record the IOP:
- Manometer-based sensor integrated into an implantable lens
- Capacitive sensor measuring IOP directly (an implanted sensor in the anterior chamber)
- Resistive sensor on a contact lens
Benefits
Continuous IOP measurement sensors are mainly used as self-monitoring home tonometers, to facilitate self-measurements as part of the patient’s daily routine. Barriers exist in the use of traditional self-monitoring devices that prevent their optimal use. Patients might not take the required numbers of readings, report selected findings or fail to follow the recommended measurement protocol.5
Continuous measurement prevents the main barriers for optimal use of self-monitoring devices. Novel sensors are able to display the IOP when the patient requests a reading while the IOP is continuously recorded. In general, self-monitoring devices show a significant improvement in the efficiency of treatment of chronic diseases.5-7 A review of the literature studies, assessing the records of patients admitted for 24-hour IOP monitoring, found that in 50 to 70% of patients the peak IOP occurred outside the office hours.8,9
Thus, the benefit of 24-hour continuous IOP monitoring is not limited to readings outside office hours as it also allows the eye care specialist to assess the effectiveness of any prescribed glaucoma treatment, allowing both the patient and the doctors to benefit from individually tailored therapy. It has been shown that 24-hour IOP monitoring results in an immediate treatment change in approximately 40% of the patients and a general clinical management change in about 80% of cases.9 Also, the nocturnal efficacy of medications that lower IOP can be significantly different from their efficiency during daytime.10-12
Limitations
Although 24-hour continuous IOP monitoring represents a major advance for those patients who are eligible for receiving these sensors, there is still one major drawback regarding the detected IOP. Considering the eye as a physical body, it contains two separate pressure-influenced areas:
- Anterior segment – production and drainage of aqueous humour
- Posterior segment – optic disc pressure damage
To date, it is still not clear how any increase in pressure in the anterior part of the eye is translated to the posterior segment. Based on the pressure-volume relation between two physical bodies, it cannot be assumed pressure translation from anterior to posterior segment is either linear or direct. It is instead understood to be significantly dependent on the biomechanical properties of the outer shells. Clinically speaking, even if a normal or suspicious IOP can be measured anteriorly, it is not evident that the same pressure level can be found in the posterior segment.
IOP monitoring – Current developments
Current developments try to address both significant limitations, that is continuous pressure measurement and the unknown pressure in the posterior segment.13 In order to measure the posterior segment pressure, compliant nanocomposite thin films have been fabricated, with a thickness of the order of 10 to 20µm. The films are fabricated with a piezoelectric polymer. Their development aims to produce an implantable continuous IOP measurement sensor for the posterior segment of the eye, so monitoring the IOP close to the optic nerve.
Biomechanics of the outer shell
As already discussed, the biomechanical characteristics of the outer shell of the globe, namely the cornea and the sclera, are the most significant influencing factors determining the pressure inside the globe. Due to technical limitations, early studies in ocular biomechanics were mostly ex vivo experiments with enucleated human or porcine eyes.14-17 With the introduction of new devices like the Ocular Response Analyzer and the Corvis ST, it is now possible to estimate in vivo corneal biomechanical properties in human eyes. The remainder of this article aims to provide an overview of the basic concepts of corneal biomechanics, both anatomical and physical.
Biomechanics is an interdisciplinary science that describes biological systems and their intrinsically and extrinsically influenced movement based on their anatomy and physiology, using the concepts, methods and laws of mechanics. Living structures have properties dependent on common biological components, including proteins, cells, tissues and organs; these are important in the regulation of their multiple functions. Therefore, to fully understand the pathophysiology of any disease, a thorough appreciation of tissue biomechanics is essential.
Mechanics can be divided into the sub-disciplines of dynamic and kinematic mechanics. Dynamic mechanics is the branch that is concerned with understanding the cause of movement under the action of forces; in other words, the study of the relationship between movement and its causative forces. When considering ocular biomechanics, only dynamic mechanics is of relevance and when evaluating the anterior segment in isolation, both static and kinetic mechanics need to be assessed to understand the physiological and pathological behaviour of ocular tissues.
Anatomical structure of the extracellular matrix
Connective tissue forms diverse structures in the body with very different functions and mechanical properties. These are largely determined by the extracellular matrix, which is composed of ground substance and different fibres. The extracellular matrix (ECM) is a 3D matrix consisting of macromolecules whose specific composition and structure vary between types of biological tissues and surrounds our cells to provide mechanical stability (see figure 2). The matrix components themselves are synthesised and released by cells embedded within the matrix. The ground substance is an amorphous, gel-like substance, which fills the empty space between the fibre and consists mainly of water (90%) and sugar-protein complexes, in which proteoglycans and proteins like collagen and elastin are embedded.18
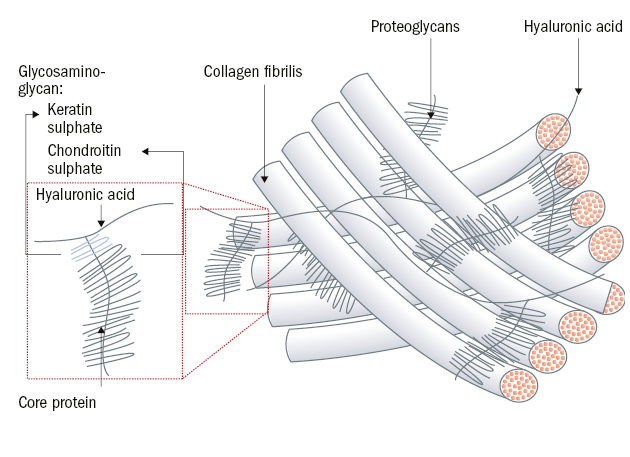
Figure 2: Microstructure of the extracellular matrix
The most important macromolecules found in the extracellular matrix are collagens, elastin and fibrillin, and non-collagenic glycoproteins, glycosaminoglycans and proteoglycans.19
Collagens are glycoproteins and the most common extracellular matrix proteins. They consist of a triple helix formed by three collagen α chains. Three procollagen molecules assemble into a triple helix which is released from the cell into the extracellular space and is formed by cleavage of peptides from the ends of tropocollagen. Subsequently, the tropocollagen molecules of fibrillar collagens are cross-linked to form fibrils which further assemble into collagen fibres or bundles.
Elastin contains much proline and glycine. These two amino acids are located mainly in the hydrophobic areas of the protein, which alternate with α-helical sections. Elastin biosynthesis is similar to that of collagen. Tropoelastin is released from the cell and is deposited on fibrillin microfibrils. The tropoelastin molecules are then cross-linked by lysine, creating elastin. Elastin and fibrillin microfibrils form a network of elastic fibres.
The non-collagenous glycoproteins, acting as anchor proteins, mediate the contact between the individual components of the extracellular matrix, between the cells and the matrix as well as between individual cells. Two important non-collagenous glycoproteins are fibronectin and laminin.
Glycosaminoglycans are the major components of the extracellular matrix. The four main groups are hyaluronate, which always occurs as a pure glycan, as well as chondroitin sulphates, heparan sulphate and keratan sulphate, which are exclusively components of proteoglycans.
Hyaluronate is synthesised directly into the extracellular matrix. The other glycosaminoglycans are transferred to protein nuclei in the Golgi apparatus by various specific glycosyltransferases where they are extended and modified.
Glycosaminoglycans form shock-absorbing gels, act as lubricants, and provide an aqueous environment for the diffusion of water-soluble substances. In addition, they have a function in wound healing and take over as components of proteoglycans’ further important tasks.
Proteoglycans are a heterogeneous group of proteins with covalently (glycosidic) linked glycosaminoglycans as carbohydrate side chains. Their diversity is due to the combination of diverse and variously sized proteins with the different glycosaminoglycans. The different proteoglycans are tissue-specific and involve the components of extracellular matrix interactions. Two examples are aggrecan, which forms large complexes with hyaluronate and is responsible for the shock absorber effect in cartilage, and pearlcan, which is contained in the basal lamina and has a filtering function.
A key feature of ECM is its structural support for cells, but it also acts as a physical barrier and filter of soluble molecules.20 The principle structure of the ECM dictates its shape, the elasticity and the water content of the tissue.21
The healthy cornea has three important requirements; namely to provide a smooth, refractive and transparent ocular surface. The orderly structure of the epithelium provides the cornea with a smooth surface, while the pre-corneal tear film and the precise arrangement of the collagen across the stroma afford the refraction and transparency, respectively. There are up to fifteen types of collagen within the human cornea.
The main component of the corneal stroma is the ECM. The ECM consists mainly of collagens, proteoglycans, non-collagenous glycoproteins and glycosaminoglycan. The most important component of the human ECM is the collagen fibrils. The fibrils are tightly packed as lamellae, and the adjacent lamellae are arranged in an orientation that is dictated by their corneal depth; this is critical for maintaining corneal transparency.22 Within the anterior human stroma, the lamellar orientation is more haphazard, whereas in the deeper stroma the collagen lamellae show a preferred inferior-superior and nasal-temporal orientation. Here, the collagen fibrils are part of a very dense interfibrillar ECM, which is responsible for increased fibre stability in the interfacial region.23,24
The nature of collagen fibril orientation in the ECM is a key determinant of the mechanical strength provided by the ocular connective tissues. The ECM defines the shape and function of ocular connective tissues. Ocular connective tissues act as both a mechanical and protective outer layer. Also, connective tissues determine the shape of the tissue and, in particular for corneal tissues, guarantee transparency. In corneal ECM, collagen is the principle structural element. The collagen fibre orientation differs across the cornea (figure 3).
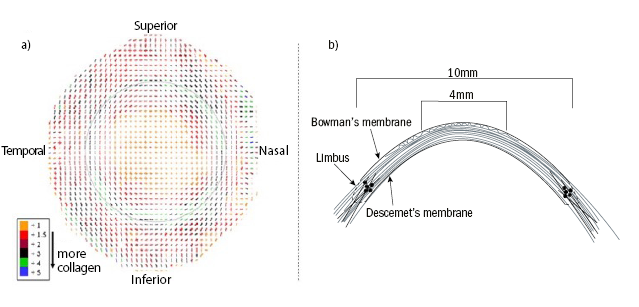
Figure 3: a) Preferential collagen lamellar orientation of a right human cornea. The outer dotted line represents the limbus. The amount of collagen is classified and displayed in colours. Reproduced from Meek and Boote28 with permission of the copyright holder. b) Schematic of the arrangement of collagen lamella. In the central cornea, the anterior lamellae are ordered in a relatively wide angle with Bowman’s membrane. This angle decreases in the deeper stroma and increases from the centre to the posterior. In the peripheral cornea, around 4 to 5mm from centre, the lamellae intertwine with each other. Reproduced from Abass et al.29 with permission of the copyright holder
The corneal stroma comprises approximately 90% of the cornea and is mainly comprised of water and collagen fibres. The collagen fibrils in the stroma have a uniform diameter of 30.8 (± 0.8)nm. The distance between the fibrils is constant at 1.6nm. With increasing age, three-dimensional growth of the collagen fibrils occurs as a result of an increase in collagen molecules and an expansion of the intermolecular space by cross-linking.25 The collagen fibres form approximately 240 lamellae within the stroma. The unique arrangement between corneal, limbal and stromal collagen is particularly important for the biomechanical properties of the cornea. Newton and Meek26 demonstrated that only 17% of the central collagen fibrils were oriented orthogonally, while 83% showed isotropic alignment. Moreover, with increasing distance from the central cornea, the collagen fibres show increased alignment along the superior-inferior axis (figure 3).26,27
However, the direction of the lamellae varies in the adjacent layers at an angle of 90° in temporal-nasal and superior-inferior orientation.27 This arrangement is found mainly in the posterior two-thirds of the stroma. Within the posterior layers of the central, peripheral and limbal cornea, the collagen fibres are highly orientated, and additionally within the posterior limbal region there is an annulus of highly aligned corneal fibres which surrounds the corneal tissue. There, fibres of the sclera can be interwoven and thus contribute to the structural strength of the limbal region.28
Basic concepts of plasticity, elasticity and viscoelasticity
A basic understanding of elasticity, elasticity and viscoelasticity is essential for understanding the biomechanical characteristics of the cornea. In general, if a force (stress) is applied to a body with a defined shape, the response of the body material (strain) can be either plastic, elastic or viscoelastic referring to the resultant change in shape. A deformation is considered plastic if, after removing the force from the body, the shape change caused by the applied force is permanent. In elastic deformations, the initial shape is regained directly after the impact of the force is removed and there is no constant shape change in the body. A mixture of both, plastic and elastic responses, is described as a viscoelastic response. A material can be considered as viscoelastic when the response of a force (and the response after removing the force) is time-dependent and so, directly after removing the force, the body remains for a certain time deformed and then over time regains its initial shape.
The cornea can be considered as a viscoelastic tissue.30 If pressure is applied to the cornea (such as through tonometry, eye rubbing, blinking or trauma), energy is distributed throughout the tissue and the cornea deforms. The corneal response depends on the duration and amount of force applied. After the pressure subsides, the cornea recovers only incompletely. This is corneal hysteresis (CH). It is assumed that collagen and elastin are responsible for the firmness and elasticity of the cornea, while the ground substance of the tissue is responsible for the viscoelastic properties.31 The biomechanical properties are influenced by the viscoelasticity of the ECM, intraocular pressure, corneal thickness and corneal topography.32
Correlation between stress, strain and deformation
The relationship between stress and strain is represented graphically in a stress-strain diagram (figure 4). The stress-strain response of viscoelastic materials is characterised by both a linear and viscous component. If the tissue is loaded continuously at a constant temperature, increasing deformation (creep) and decreasing tension (relaxation) will result. In such an event, the deformation is initially spontaneously reversible, however with increasing load and delayed reversibility permanent deformation occurs.
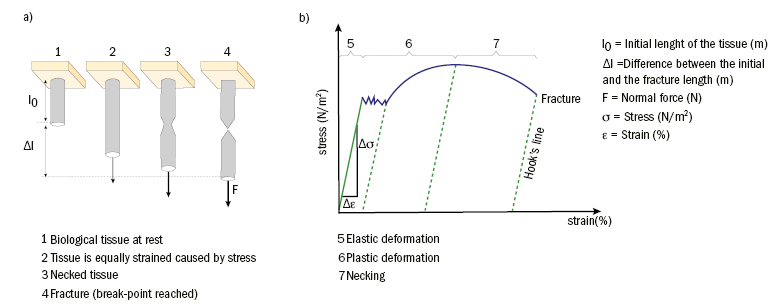
Figure 4: Experimental assessment of the stress-strain behaviour: a) Schematic of the different stages of biological tissue during the application of normal force. b) Stress-strain diagram of viscoelastic materials
A distinction is made between elastic, viscous and viscoelastic response. Furthermore, the response can be categorised quantitatively by characterising the relative stress to deformation and the time of the response.
Elastic response
The relationship between stress and strain for small and linear deformation can be described by the Hooke’s law. In the case of an elastic response, the body would return to its original shape once application of the force stops.
Viscous response
Application of stress to a viscous body leads to deformation that cannot be described by Hooke’s law but instead by the viscosity function of the Newton equation;
σ = η ∙ dε/dt
σ = Stress (m-1 • kg • s-2)
η = Viscosity (m-1 • kg)
dε/dt = Strain depending on time (% • s-1)
In this equation, the stress is not a direct function of strain but considers the derivative of strain over time. For example, a constant time-independent strain does not result in stress; only a changing stress leads to strain. Newton’s equation suggests that the stress-strain relationship can only be described if time is considered as a variable.
Viscoelastic response
Many biological tissues exhibit viscoelastic behaviour. Viscoelasticity describes a time-dependent stress-strain behaviour, which is affected by the initial state. These viscoelastic changes are reversible. The viscoelastic behaviour can be described by characteristics such as hysteresis, creep, relaxation and conditioning (figure 5).
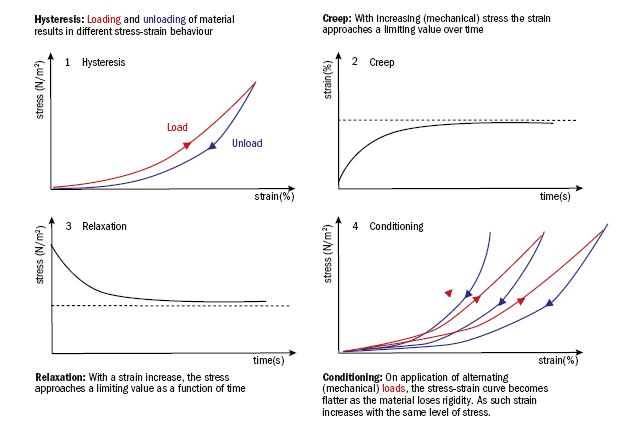
Figure 5: Mechanical behaviour of viscoelastic tissues under stress
Mechanical properties of collagen-based tissues
The ECM of connective tissues is mainly composed of water, collagen and elastin; hence, the mechanical properties of the ECM are defined by the structural proteins, collagen and elastin. Collagen is a stiff (ε=300 to 2500MPa), solid (σm=60MPa) and tough (1 to 10 kJ/m2) material,33 whereas elastin is a stretchable material, which is arranged in network structures (ε=0.1 to 0.4MPa, σm=0.6MPa).
Both collagen and elastin become stiffer with increasing load. However, in comparison to elastin, the stiffening of collagen is quicker and of a greater magnitude. Such mechanical attributes of collagen ensure that it is resilient to overstretching. Elastin is significantly more stretchable than collagen, but collagen has a much higher tear resistance (figure 6). Collagen offers the tissue strength, resistance to deformation and prevents overstretching and tearing; whereas elastin provides extensibility and elasticity.
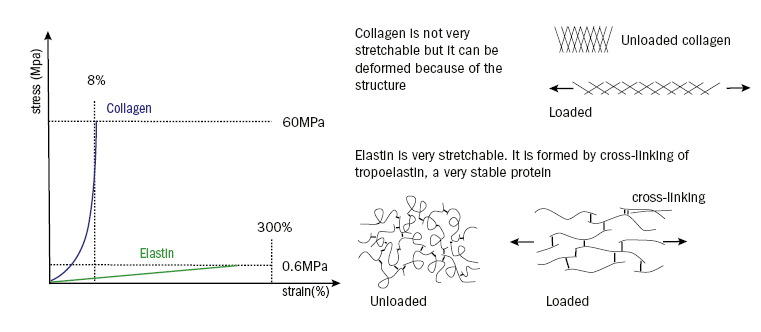
Figure 6: Stress-strain behaviour of collagen (blue) and elastin (green). The vertical dashed lines show the maximum strain, the horizontal lines the maximum load
Studies have shown corneal biomechanical behaviour is primarily influenced by the collagen fibril arrangement within the ECM.21,29,34
The regular structure of the cornea’s collagen provides the mechanical strength as well as the transparency of the cornea.
As discussed above, the mechanical strength of the cornea is determined by the density and orientation of the collagen fibres. Since the collagen architecture varies across the cornea, several studies have demonstrated significant regional variation in biomechanical strength.35 In general, the cornea appears to become stiffer from the apex to the limbus and also with increased IOP, but much of this evidence is based upon ex vivo studies.35
A review of the literature reveals little consensus on the exact nature of ocular biomechanics. Large variations in methodologies and data analysis have contributed to this. The average modulus ε of the cornea is approximately 4.8MPa. Wang et al36 suggested corneal swelling has a significant effect on corneal rigidity. The variability of results may be due to physiological and anatomical variations as well as different viscoelastic conditioning or different measurement times. A complete description of all measurement conditions is not described in any of the studies.
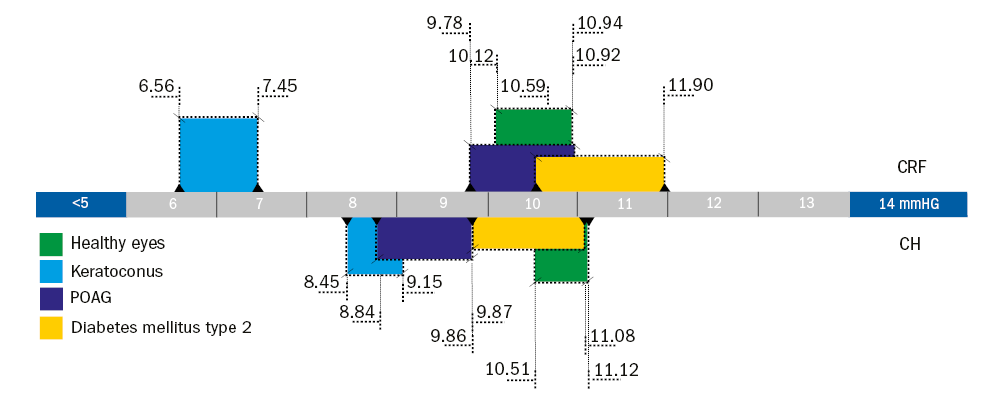
Figure 7: Graphical analysis of the ORA biomechanics; the diagram shows the corneal resistance factor (CRF, top) and the corneal hysteresis (CH, bottom) for different eye conditions. The 95% confidence interval of the average was calculated using the mean value and standard deviation published by the listed studies for each condition
In vivo measurement of corneal biomechanical properties
In the past, corneal biomechanics have been assessed by several different measurement principles. Of these, the in vivo biomechanical metrics derived during non-contact tonometry are the most studied. The Corvis ST (CST) and Ocular Response Analyzer (ORA) are both based on the principle of corneal applanation with a defined air puff (see part 1).
Recently, the number of studies evaluating the CST biomechanics in healthy eyes has grown. With regard to healthy eyes with a central corneal thickness (CCT) of approximately 540µm and an IOP of approximately 15.8mmHg, the corneal parameters are as follows:
- The 1st applanation (A1) – length 1.8mm, time 7.7ms, corneal velocity 0.16m/s
- The corneal hysteresis (CH) – curvature 11.1mm, time 17.3ms, amplitude 1.1mm
- The 2nd applanation (A2) – length 1.9mm, time 18.7ms, corneal velocity 0.34m/s
The CST provides the potential to evaluate gross and subtle corneal biomechanical variation that are likely to occur with surgical and pathological changes. Early reports of the application of the CST to pathological eyes are encouraging. In a study examining healthy and keratoconic eyes pre- and post-collagen crosslinking, Lanza et al.37 reported significant differences in the times for A1 and A2. Specifically, the investigators identified that the keratoconic and the post-crosslinking corneas reached A1 0.42ms earlier and A2 0.40ms later than the healthy eyes. Additionally, the deformation amplitude at the highest concavity was significantly larger for the keratoconic eyes at 1.12 (±0.16)mm compared to the healthy eyes at 1.02 (±0.10)mm.37
Wang et al36 compared corneal deformation responses in healthy and open-angle glaucoma patient’s eyes. The study reported significant differences in the time for A1 and A2 as well as the corneal velocity during A2. In primary open angle glaucoma, the time between the two applanation phases was 1.95ms shorter, A1 was reached 0.96ms later and A2 0.99ms earlier. The velocity of the cornea during the outward movement was significantly faster 0.40 (±0.02)ms-1 than in healthy eyes (0.28 ±0.02)ms-1. They also conducted a meta-analysis. The results they found were consistent with those of the study.36
Since the ORA has been commercially available for over 20 years, the available literature on healthy and pathological eyes is enormous. A literature review suggests normal values for corneal hysteresis (CH) are between 9.5 to 12.5mmHg, and corneal resistance factor (CRF) values are within the range of 9.2 to 11.9mmHg. It is unclear why such a variation exists between studies, but influences including the diversity of the subjects, particularly with regards to ethnicity, age, gender and refractive error.
The biomechanical properties of the cornea are known to change following refractive surgery and with post-surgical oedema. Furthermore, pathological changes associated with keratoconus and diabetes have also been found to alter corneal biomechanics. It can therefore be surmised that any changes that cause variation in corneal geometry or its microstructure will lead to alteration of corneal mechanical properties (figure 7).
Summary
Modern tonometric techniques are all influenced by corneal structure. An understanding of this structure is allowing a better understanding of these influences and helping to develop a better understanding of the relationship with intraocular pressure.
Dr Daniela Oehring is based at the Faculty of Health and Human Sciences, Plymouth University.
References
1 Augustin, AJ. 2007. Augenheilkunde, Springer.
2 Asejczyk-Widlicka, M & Pierscionek, BK. 2007. Fluctuations in intraocular pressure and the potential effect on aberrations of the eye. British Journal of Ophthalmology, 91, 1054-1058.
3 David, R, Zangwill, LM, Tessler, Z & Yassur, Y. 1985. The correlation between intraocular pressure and refractive status. Archives of Ophthalmology, 103, 1812.
4 Draeger, J. 2008. Tonometrie, Rethra-Verlag.
5 Qin, ZY, Yan, JH, Yang, DZ, Deng, HR, Yao, B, Weng, JP & Guangdong Type 1 Diabetes Mellitus Translational Study, G. 2017. Behavioral Analysis of Chinese Adult Patients with Type 1 Diabetes on Self-monitoring of Blood Glucose. Chin Med J (Engl), 130, 39-44.
6 Association, AD. 2017. Standards of Medical Care in Diabetes-2017: Summary of Revisions. Diabetes Care, 40, S4-S5.
7 Logan, AG, Irvine, MJ, Mcisaac, WJ, Tisler, A, Rossos, PG, Easty, A, Feig, DS & Cafazzo, JA. 2012. Effect of home blood pressure telemonitoring with self-care support on uncontrolled systolic hypertension in diabetics. Hypertension, 60, 51-7.
8 Barkana, Y, Anis, S, Liebmann, J, Tello, C & Ritch, R. 2006. Clinical utility of intraocular pressure monitoring outside of normal office hours in patients with glaucoma. Arch Ophthalmol, 124, 793-7.
9 Hughes, E, Spry, P & Diamond, J. 2003. 24-hour monitoring of intraocular pressure in glaucoma management: a retrospective review. J Glaucoma, 12, 232-6.
10 Bagga, H, Liu, JH & Weinreb, RN. 2009. Intraocular pressure measurements throughout the 24 h. Curr Opin Ophthalmol, 20, 79-83.
11 Liu, JH, Medeiros, FA, Slight, JR & Weinreb, RN. 2009. Comparing diurnal and nocturnal effects of brinzolamide and timolol on intraocular pressure in patients receiving latanoprost monotherapy. Ophthalmology, 116, 449-54.
12 Liu, JH, Medeiros, FA, Slight, JR & Weinreb, RN. 2010. Diurnal and nocturnal effects of brimonidine monotherapy on intraocular pressure. Ophthalmology, 117, 2075-9.
13 Jenkins, D, Bloor, J, Peddakotla, V & Oehring, D. 2016. Thin film nanocomposite sensors incorporating graphene for spatially mapping the air pressure pulse used in glaucoma testing instrumentation. 1st International Conference on Nanoscience and Nanotechnology (ICNAN ’16).
14 Friedenwald, JS. 1957. Tonometer calibration; an attempt to remove discrepancies found in the 1954 calibration scale for Schiotz tonometers. Trans Am Acad Ophthalmol Otolaryngol, 61, 108-22.
15 Kobayashi, AS, Staberg, LG & Schlegel, WA. 1973b. Viscoelastic properties of human cornea. Experimental Mechanics, 13, 497-503.
16 Schlegel, WA, Lawrence, C & Staberg, LG. 1972. Viscoelastic response in the enucleated human eye. Investigative Ophthalmology & Visual Science, 11, 593-599.
17 Ytteborg, J. 1960. The role of intraocular blood volume in rigidity measurements on human eyes. Acta Ophthalmol, 38.
18 Feigl, W. 1997. System theorie in der Medizin: theoretische Grundlagen für die Ganzheitsmedizin, Wien, Facultas.
19 Theocharis, AD, Skandalis, SS, Gialeli, C & Karamanos, NK. 2016. Extracellular matrix structure. Adv Drug Deliv Rev, 97, 4-27.
20 Adams, JC & Watt, FM. 1993. Regulation of development and differentiation by the extracellular matrix. Development, 117, 1183-98.
21 Müller, LJ, Pels, E, Schurmans, LR & Vrensen, GF. 2004. A new three-dimensional model of the organization of proteoglycans and collagen fibrils in the human corneal stroma. Exp Eye Res, 78, 493-501.
22 Maurice, DM. 1957. The structure and transparency of the cornea. J Physiol, 136, 263-86.
23 Aghamohammadzadeh, H, Newton, RH & Meek, KM. 2004. X-ray scattering used to map the preferred collagen orientation in the human cornea and limbus. Structure, 12, 249-56.
24 Meek, KM & Newton, RH. 1999. Organization of collagen fibrils in the corneal stroma in relation to mechanical properties and surgical practice. J Refract Surg, 15, 695-9.
25 Daxer, A. & Fratzl, P. 1997. Collagen fibril orientation in the human corneal stroma and its implication in keratoconus. Invest Ophthalmol Vis Sci, 38, 121-9.
26 Newton, R. H. & Meek, K. M. 1998. The integration of the corneal and limbal fibrils in the human eye. Biophys J, 75, 2508-12.
27 Boote, C., Dennis, S., Newton, R. H., Puri, H. & Meek, K. M. 2003. Collagen fibrils appear more closely packed in the prepupillary cornea: optical and biomechanical implications. Invest Ophthalmol Vis Sci, 44, 2941-8.
28 Meek, K. M. & Boote, C. 2009. The use of X-ray scattering techniques to quantify the orientation and distribution of collagen in the corneal stroma. Prog Retin Eye Res, 28, 369-92.
29 Abass, A., Hayes, S., White, N., Sorensen, T. & Meek, K. M. 2015. Transverse depth-dependent changes in corneal collagen lamellar orientation and distribution. J R Soc Interface, 12, 20140717.
30 Kobayashi, A. S., Staberg, L. G. & Schlegel, W. A. 1973a. Viscoelastic properties of human cornea. Experimental Mechanics.
31 Luce, D. A. 2005. Determining in vivo biomechanical properties of the cornea with an Ocular Response Analyzer. J Cataract Refract Surg, 31, 156-62.
32 Liu, J. & Roberts, C. J. 2005. Influence of corneal biomechanical properties on intraocular pressure measurement: quantitative analysis. J Cataract Refract Surg, 31, 146-55.
33 Fratzl, P., Misof, K., Zizak, I., Rapp, G., Amenitsch, H. & Bernstorff, S. 1998. Fibrillar structure and mechanical properties of collagen. J Struct Biol, 122, 119-22.
34 Wenger, M. P., Bozec, L., Horton, M. A. & Mesquida, P. 2007. Mechanical properties of collagen fibrils. Biophys J, 93, 1255-63.
35 Hjortdal, J. O. 1996. Regional elastic performance of the human cornea. J Biomech, 29, 931-42.
36 Wang, H., Prendiville, P. L., Mcdonnell, P. J. & Chang, W. V. 1996. An ultrasonic technique for the measurement of the elastic moduli of human cornea. J Biomech, 29, 1633-6.
37 Lanza M, Cennamo M, Iaccarino S, Romano V, Bifani M, Irregolare C, Lanza A. Evaluation of corneal deformation analyzed with a Scheimpflug based device. Cont Lens Anterior Eye. 2015 Apr;38(2):89-93.
