Glaucoma is a leading cause of irreversible blindness worldwide1 and its incidence is predicted to increase over the next three decades, from the 64.3 million affected in 2013, to 76 million in 2020, and to 111.8 million by 2040.2 Glaucoma is defined as a multifactorial optic neuropathy characterised by the accelerated loss of retinal ganglion cells resulting in peripheral visual field loss.3 Age and raised intraocular pressure (IOP) are considered the main risk factors for glaucoma. Reduction in IOP, either by medical or surgical intervention, is currently the only proven treatment option.4,5
It is estimated that over 30 to 50% of glaucoma patients have normal IOP upon diagnosis6,7 suggesting that other factors, including tissue biomechanics, may have a role in glaucoma risk and optic nerve damage progression.4,5
Corneal Hysteresis
Recent studies suggest that low corneal hysteresis (CH) is not only a strong risk factor for glaucoma progression, but that it appears to be a more appropriate parameter for considering intraocular pressure and glaucoma risk than central corneal thickness (CCT) because it describes corneal physical and biomechanical properties better.4,8,9 Whereas corneal thickness is a static physical property, corneal biomechanics refer to the dynamic, or viscoelastic, behaviour of the cornea,10 meaning that it contains characteristics of both elastic and viscous materials.5
Elasticity can be thought of as the ability of a body to change its shape when a force is applied and to return into its original shape in a linear manner when the influence of the force is removed.11 Conversely, a viscous material does not follow the exact path of deformation to regain its original shape upon removal of the stress.10
The cornea exhibits both elastic and viscous properties that can be explained by the two constituents of its stroma: collagen fibres and the ground substance.12 Collagen fibres are the main load-bearing substance and provide elasticity whereas the ground substance, composed of glycosaminoglycans (GAGs) and proteoglycans (PGs), offers viscosity under friction. Given its viscoelastic nature, the cornea absorbs and dissipates energy as heat during a stress-strain cycle. This energy loss is termed hysteresis (figure 1).10
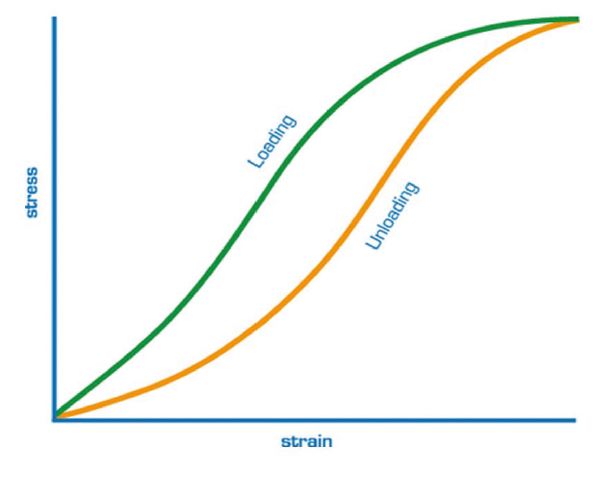
Figure 1: Hysteresis is the difference in behaviour of the material between loading and unloading. Corneal hysteresis is the difference between the pressure at which the cornea bends inwards and the pressure at which the cornea bends outwards 11
Goldmann applanation tonometry (GAT) is regarded as the reference standard by which to measure IOP. GAT is known to be influenced by factors related to the corneal properties, such as corneal curvature and central corneal thickness.14,15,16 It has been suggested that factors other than CCT, including corneal hydration, connective tissue composition, and bioelasticity, contribute to the response of the cornea during the measurement of IOP.17,18
The Ocular Response Analyzer (ORA) utilises a dynamic, bidirectional applanation process for measuring IOP (see figure 2).18

Figure 2: Reichert Ocular Response Analyzer (a) and an example of output data (b)
A precisely metered, collimated air pulse (seen as the green symmetric curve on figure 3) applies force to the central cornea causing it to move inwards.
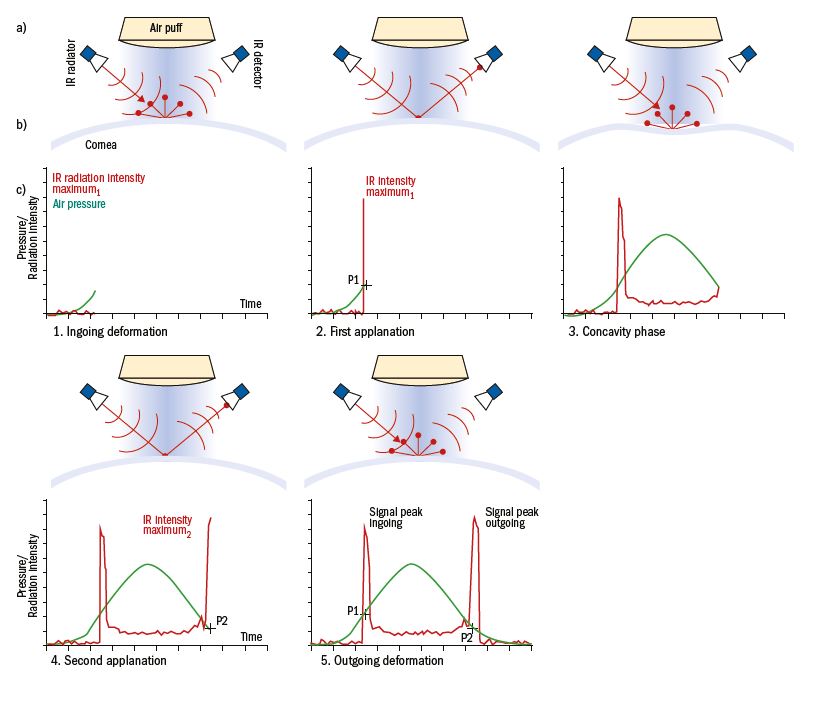
Figure 3: Measurement principle of the ORA; a) The top scheme shows the IR light radiator and the optical detector that is right angled to the beam, as well as the air puff generator and the air puff. b) Corneal profile images during the measurement. c) ORA waveform diagram shows the changes of air pressure (green) and detected IR intensity (red) during the five phases of measurement over time
The deformation produced is monitored using an advanced electro-optical system and registers the first applanation (P1) similar to the air pulse system usually found in conventional non-contact tonometers. Using the ORA, the air impulse continues to deform the cornea past applanation into a slight concave shape. Then, the air pump shuts off and, as the pressure decreases, the cornea begins to return to its normal configuration. During this process, it once again passes through an applanated state (seen as the second peak (P2) on the asymmetric red curve) before returning to its normal curvature. The applanation pressure is determined by drawing a line down from the peak of each applanation spike to the intersection of the green pressure curve. The entire process takes only 20 milliseconds, a time sufficiently short to ensure that ocular pulse effects or eye position do not change during the measurement process.18,19
Due to the dynamic nature of the air pulse, energy absorption during the stress-strain cycle,20 also known as viscous damping,21 causes delays in the inward and outward corneal applanation events, resulting in two different pressure values. Corneal hysteresis is the difference between pressures P1 and P2, a numerical value denoting viscoelastic corneal tissue response to a dynamic deformation (figure 4). A greater difference generates a higher CH, suggestive of a stiffer cornea and greater viscous dampening, effectively acting as a buffer against potentially harmful IOP fluctuations.19

Figure 4: Corneal hysteresis
Clinical Data
Using this bidirectional applanation measurement, the ORA is able to present the following four different parameters (figure 2b)18:
- Goldmann-correlated IOP (IOPg) – This is the average of the inward (P1) and outward (P2) applanation pressures. This parameter is closely correlated with GAT-IOP.
- Corneal-compensated IOP (IOPcc) – Derived from both IOP and corneal biomechanical data.
- Corneal Hysteresis (CH) – During the ORA measurement process, the cornea absorbs some energy from the initial air pulse, which causes the second applanation pressure measurement to be lower than the initial measurement. The difference between the two pressures (P1-P2) is CH. This ORA parameter is thought to represent the viscoelastic nature of the cornea, or its ‘viscous-damping’ capacity.
- Corneal Resistance Factor (CRF) – The CRF is derived from the formula (P1-kP2) where k is the constant determined from an empirical analysis of the relationship between both P1 and P2 and CCT. CRF offers a measurement of corneal resistance.19, 22
The ORA provides IOP measurements taking into consideration these biomechanical properties of the cornea. IOPcc and IOPg have shown good correlation with GAT IOP measurements.21 GAT IOP measurements are significantly associated with CCT, whereas IOPcc measurements are not associated with any of the variables, namely CCT, corneal curvature, axial length, and age.23,24 The difference between GAT IOP and IOPcc measurements is significantly influenced by corneal thickness.17 Patients with thicker corneas tend to have higher GAT IOP measurements compared with IOPcc, whereas in patients with thin corneas, GAT IOP measurements tend to be lower than IOPcc.19
Corneal Hysteresis in Normal Eyes
CH varies from individual to individual,5 with an average range being quoted from 10.24 to 10.725,26 and does not vary diurnally.5,27,28 Age affects the composition of the structures responsible for CH changes with a steady decline in CH of 0.24 to 0.7mmHg per decade.28,29,30 The effect of ethnicity on CH is variable,31 some findings pointing to African-Americans having lower CH than Caucasians.32 Gender, axial length, spherical equivalent, and myopia have no significant effects on ORA measurements.33,34
Impact of Corneal Thickness and Intraocular Pressure on Corneal Hysteresis
The influence of central corneal thickness (CCT) upon intraocular pressure (IOP) measurements using Goldmann applanation tonometry (GAT) has been well-recognised15,18,35 and should be considered when assessing corneal hysteresis. In healthy patients where there is a thicker cornea, a higher viscous damping ability is expected due to a healthy stroma and more viscous substance.
Corneal properties appear to differ between healthy and glaucoma patients.36 Corneal hysteresis was significantly lower in eyes with treated POAG than in non-glaucomatous eyes.37 The corneal biomechanical response was strongly associated with CCT in non-glaucoma subjects, but only moderately so in glaucoma patients. It can be assumed that diverse structural factors, in addition to thickness, determine the differences in the corneal biomechanical profile between non-glaucomatous and glaucomatous eyes. IOP is negatively correlated with CH such that the higher the IOP, the lower the CH. As IOP increases, the cornea becomes stiffer and its damping ability reduces.38 It is therefore important to account for CCT and IOP to isolate the effect of CH. It appears that combining CH and CCT may result in more precise risk assessment compared with using either factor in isolation.39
Corneal Hysteresis in Primary Open Angle Glaucoma
It has been shown that corneal hysteresis (CH) is lower in eyes with primary open angle glaucoma (POAG) compared to normal eyes, with mean CH values in POAG ranging from 8 to 10mmHg16,40 Similarly, CH has also been shown to be lower in normal tension glaucoma (NTG) when compared to normal eyes. It has also been reported that CH values can discriminate between normal, POAG, and ocular hypertensive/glaucoma suspect eyes.37 In addition, CH was recently shown to decline over time at a faster rate in POAG compared to normal eyes.30
CH and Structural Changes
The cornea, optic nerve and sclera do not have a common embryological origin. However, the extracellular matrix of each those structures is considered histologically equivalent.10 Various investigators have found associations between corneal hysteresis and optic nerve head (ONH) morphology.5 A low CH could be related to a stiffening of the peripapillary sclera, resulting in reduced capability of displacement and dampening effect would increase the strain in the lamina cribrosa (LC) and therefore more prone to glaucomatous damage.41,42 Studies with Spectralis OCT have allowed examination of the lamina cribrosa and observed that CH is related to LC displacement. The greater the CH, the greater the anterior displacement and thickening of the Lamina Cribrosa after IOP reduction.43
Low corneal hysteresis was associated with greater mean cup depth5,41,44 and a larger cup-to-disc ratio, independent of IOP and disc size. Low CCT was only associated with mean cup depth.44 Corneal hysteresis is further associated with structural markers of glaucoma, as measured by Heidelberg Retinal Tomograph (HRT) and GDx VCC. Specifically, there is a positive association between rim area (as measured by HRT) and retinal nerve fibre layer (RNFL) average thickness as measured by GDx, and a negative association with HRT linear cup-to-disc ratio. Baseline CCT was not associated with ONH parameters changes.45 Eyes with higher corneal hysteresis experienced more ONH deformation with IOP elevation, a process that may allow the eye to dissipate mechanical forces and better protect the retinal nerve fibres than an eye with lower corneal hysteresis.10
Corneal hysteresis is lower in patients with normal tension glaucoma (NTG) compared with normal patients.40,46 A recent study suggests CH as an independent risk factor and recommends incorporating its measurement as a screening tool for the diagnosis and risk of progression of NTG.13
CH and Glaucoma Progression
Corneal hysteresis can be used in monitoring and predicting glaucoma progression.5 Low corneal hysteresis is associated with progressive glaucomatous optic neuropathy,47 faster rates of progressive RNFL loss detected by SD-OCT48 and faster rates of visual field loss.8 It has been stated for every 1mmHg lower CH was associated with a 0.25% per year faster visual field index decline.9 Many studies support a role for CH as a risk factor for progression in glaucoma.48
CH and IOP Therapies
An inverse relationship between corneal hysteresis and IOP exists,49,50 whereby as IOP decreases, corneal hysteresis increases, and vice versa. Studies have shown IOP reduction through topical therapy is associated with increased CH.51 This inverse relationship holds true for glaucoma surgical techniques.50,52
CH and Glaucoma Therapy – Predicting IOP Reduction
Establishing a baseline CH can help in predicting the magnitude of the IOP reduction from PGA therapy.5 A recent study found the lower the initial CH prior to therapy, the greater the IOP reduction predicted.53 Specifically, a 29% IOP reduction in the lowest CH group, to a 7.6% IOP reduction in the group with initial high CH. The use of CH as a predictor of IOP reduction following SLT has also been seen.54
The clinical use of CH is becoming more widespread with its introduction in some NHS glaucoma settings, although a precise clinical protocol is yet to be defined.55 It has been proposed that CH could be considered as a surrogate marker for the biomechanical properties of tissues in the back of the eye. Some clinicians consider CH a new factor for glaucoma progression, stating a 21% increased risk of developing glaucoma for every 1mmHg reduction in CH measured.56
Conclusion
Corneal hysteresis (CH) is a dynamic biomechanical property of the cornea that measures the ability of the cornea to resist deformation, describing the cornea’s physical and biomechanical properties better than CCT.8,9 CH is lower in eyes with glaucoma when compared to normal eyes. Low CH has also been associated with structural and functional progression of glaucoma, and it is associated with better IOP reduction following hypotensive treatment.4,5,16 Eyes with a low CH would probably need to be monitored more often or receive early treatment.57
There is a significant body of evidence to say that CH is a valuable tool to consider including in the risk assessment of glaucoma patients, helping in both the identification and management of higher risk patients.4
Clinical Case Study

History:
- 36 year old male, healthy and on no medications
- Had been under the hospital eye service some 4 years ago for raised intraocular pressures
- There was no family history of glaucoma
- Plan – discharged after initial visit
Presentation today:
- GAT right eye; 24 mmHg
- CCT right eye; 535 µm
- Vertical C/D ratio of 0.5
- Full threshold fields; Full
- Plan: review in 2 years
Would you agree with this approach?
Find out how corneal hysteresis measurement influenced the management next week.
Dr Rohit Narayan is a therapeutic optometrist based in the Midlands.
References
- Murphy ML, Pokrovskaya, O, Galligan M and O’Brien C. Corneal hysteresis in patients with glaucoma-like optic discs, ocular hypertension and glaucoma. BMC Ophthalmology. 2017;17:1.
- Tham YC, Li X, Quigley HA, Aung T, Cheng CY. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121:2081–90
- Paul Harasymowycz, Catherine Birt, Patrick Gooi, et al., “Medical Management of Glaucoma in the 21st Century from a Canadian Perspective,” Journal of Ophthalmology, vol. 2016, Article ID 6509809, 22 pages, 2016. https://doi.org/10.1155/2016/6509809..
- Radcliffe NM, Alvarez-Ascencio D. “Update on risk factors for glaucoma progression.” Ophthalmology Management (9) 2018. https://www.ophthalmologymanagement.com/supplements/2018/september-2018/september-2018-glaucoma-physician/update-on-risk-factors-for-glaucoma-progression
- Deol M, Taylor DA, Radcliffe NM. Corneal hysteresis and its relevance to glaucoma. Curr Opin Ophthalmol. 2015;26(2):96-102.
- Klein B, Klein R, Sponsel WE, Franke T, Cantor LB, Martone J, et al. Prevalence of glaucoma. The Beaver Dam Eye Study. Ophthalmology. 1992;99:1499–504.
- Sommer A. Epidemiology as it relates to screening for glaucoma. Surv Ophthalmol. 1989;33:49–50.
- De Moraes CV, Hill V, Tello C, Liebmann JM, Ritch R. Lower corneal hysteresis is associated with more rapid glaucomatous visual field progression. J Glaucoma. 2012;21(4):209-213
- Medeiros, Felipe A et al. “Corneal hysteresis as a risk factor for glaucoma progression: a prospective longitudinal study” Ophthalmology vol. 120, 8 (2013): 1533-40.
- Hussnain SA (2017). The Role of the cirnea in Glaucoma Management. http://eyewiki.aao.org/The_Role_of_Cornea_in_Glaucoma_Management%3A_Central_Corneal_Thickness_and_Corneal_Hysteresis
- Oculus website: https://www.corneal-biomechanics.com/en/biomechanics/
- Terai, N., Raiskup, F., Haustein, M., Pillunat, L. E., & Spoerl, E. (2012). Identification of biomechanical properties of the cornea: the ocular response analyzer. Current eye research, 37(7), 553-562.
- Chen, Ming et al. “The role of corneal hysteresis during the evaluation of patients with possible normal-tension glaucoma” Clinical ophthalmology (Auckland, N.Z.) vol. 12 555-559. 21 Mar. 2018, doi:10.2147/OPTH.S161675
- Doughty MJ, Zaman ML. Human corneal thickness and its impact on intraocular pressure measures: A review and metaanalysis approach. Surv Ophthalmol 2000 Mar-Apr;44(5):367-408.
- Liu J, Roberts CJ. Influence of corneal biomechanical properties on intraocular pressure measurement: Quantitative analysis. J Cataract Refract Surg 2005 Jan;31(1):146-155.
- Kaushik S, Pandav SS, Banger A, etal. Relationship between corneal biomechanical properties, central corneal thickness, and intraocular pressure across the spectrum of glaucoma. Am J Ophthalmol 2012; 153:840 – 849.
- Congdon NG, et al. Central Corneal Thickness and Corneal Hysteresis Associated With Glaucoma Damage. Am J Ophthalmol 2006;141:868-75.
- Devi S “The ocular response analyzer.” Journal of Current Glaucoma Practice 3.1 (2009): 24-27.
- Kaushik, Sushmita and Surinder Singh Pandav. “Ocular Response Analyzer” Journal of current glaucoma practice vol. 6,1 (2012): 17-19.
- Broman AT, Congdon NG, Bandeen-Roche K, Quigley HA. Influence of corneal structure, corneal responsiveness, and other ocular parameters on tonometric measurement of intraocular pressure. J Glaucoma 2007;16:581–8.
- Touboul D, et al. Correlations between corneal hysteresis, intraocular pressure, and corneal central Pachymetry. J Cataract Refract Surg 2008;34:616-22.
- Al-Arfaj, Khalid & Yassin, Sanaa & Al-Dairi, Walaa & Al-Shamlan, Fatimah & Al-Jindan, Mohanna. (2016). Corneal Biomechanics in Normal Saudi Individuals. Saudi Journal of Ophthalmology. 30. 10.1016/j.sjopt.2016.05.001.
- Medeiros FA, Weinreb RN. Evaluation of the Influence of Corneal Biomechanical Properties on Intraocular Pressure Measurements Using the Ocular Response Analyzer. J Glaucoma 2006;15:364-70
- Pepose JS, et al. How Should We Measure IOP Following LASIK? Cataract and Refractive Surgery Today 2005;2-4.
- Shah S, Laiquzzaman M, Bhojwani R, Mantry S, Cunliffe I. Assessment of the biomechanical properties of the cornea with the ocular response analyzer in normal and keratoconic eyes. Invest Ophthalmol Vis Sci 2007;48:3026–31.
- CarbonaroF,AndrewT,MackeyDA,etal.Theheritabilityofcornealhysteresis and ocular pulse amplitude: a twin study. Ophthalmology 2008; 115:1545– 1549.
- Laiquzzaman M, Bhojwani R, Cunliffe I, Shah S. Diurnal variation of ocular hysteresis in normal subjects: relevance in clinical context. Clin Experiment Ophthalmol 2006; 34:114 – 118.
- Kida T, Liu JH, Weinreb RN. Effects of aging on corneal biomechanical properties and their impact on 24-hour measurement of intraocular pressure. Am J Ophthalmol 2008; 146:567–572.
- Kamiya K, Shimizu K, Ohmoto F. Effect of aging on corneal biomechanical parameters using the ocular response analyzer. J Refract Surg. 2009;25:888–893.
- Hussnain SA, Alsberge JB, Ehrlich JR, et al. Change in corneal hysteresis over time in normal, glaucomatous and diabetic eyes. Acta Ophthalmol. 2015 Apr 28.
- Leite MT, Alencar LM, Gore C, et al. Comparison of corneal biomechanical properties between healthy Blacks and Whites using the ocular response analyzer. Am J Ophthalmol 2010; 150:163 – 168.
- Haseltine SJ, Pae J, Ehrlich JR, et al. Variation in corneal hysteresis and central corneal thickness among Black, Hispanic and White subjects. Acta Ophthalmol 2012; 90:e626 – e631.
- Kamiya K, Hagishima M, Fujimura F, et al. Factors affecting corneal hysteresis in normal eyes. Graefes Arch Clin Exp Ophthalmol. 2008;246:1491–1494.
- David VP, Stead RE, Vernon SA. Repeatability of ocular response analyzer & metrics: a gender-based study. Optom Vis Sci 2013; 90:691 – 699.
- Whitacre MM, Stein RA, Hassanein K. The effect of corneal thickness on applanation tonometry. Am J Ophthalmol 1993;115:59-596.
- Gaspar, Ricardo, Pinto, Luís Abegão, & Sousa, David Cordeiro. (2017). Corneal properties and glaucoma: a review of the literature and meta-analysis. Arquivos Brasileiros de Oftalmologia, 80(3), 202-206. https://dx.doi.org/10.5935/0004-2749.20170050
- Mangouritsas G, Morphis G, Mourtzoukos S, et al. Association between corneal hysteresis and central corneal thickness in glaucomatous and nonglaucomatous eyes. Acta Ophthalmol. 2009; 87:901–905.
- Orssenjo GJ, Pye DC: Determination of the true intraocular pressure and modulus of elasticity of the human cornea in vivo. Bull Math Biol. 1999; 61:551—72.
- Pensyl, D et al. “Combining corneal hysteresis with central corneal thickness and intraocular pressure for glaucoma risk assessment” Eye (London, England) vol. 26,10 (2012): 1349-56.
- Grise-Dulac A, Saad A, Abitbol O, etal. Assessment of corneal biomechanical properties in normal tension glaucoma and comparison with open-angle glaucoma, ocular hypertension, and normal eyes. J Glaucoma 2012; 21:486 – 489.
- Wells AP, Garway-Heath DF, Poostchi A, Wong T, Chan KC, Sachdev N. Corneal hysteresis but not corneal thickness correlates with optic nerve surface compliance in glaucoma patients. Invest Ophthalmol Vis Sci. 2008;49(8):3262-3268.
- Burgoyne CF. A biomechanical paradigm for axonal insult within the optic nerve head in aging and glaucoma. Exp Eye Res. 2011;93(2):120-132.
- Lanzagorta-Aresti A, Perez-Lopez M, Palacios-Pozo E, et al. Relationship between corneal hysteresis and lamina cribrosa displacement after medical reduction of intraocular pressure. British Journal of Ophthalmology 2017;101:290-294.
- Prata TS, Lima VC, Guedes LM, et al.Association between corneal biomechanical properties and optic nerve head morphology in newly diagnosed glaucoma patients. Clin Experiment Ophthalmol 2012; 40:682 – 688
- Khawaja AP, Chan MPY, Broadway DC,et al. Corneal biomechanical properties and glaucoma-related quantitative traits in the EPIC-Norfolk eye. Invest Ophthalmol Visual Sci 2014; 55:117 – 124.
- Park, K., Shin, J., & Lee, J. (2018). Relationship between corneal biomechanical properties and structural biomarkers in patients with normal-tension glaucoma: a retrospective study. BMC ophthalmology, 18(1), 7.
- Chee RI, Silva FQ, Ehrlich JR, Radcliffe NM. Agreement of flicker chronoscopy for structural glaucomatous progression detection and factors associated with progression. Am J Ophthalmol 2013; 155:983 – 990.
- Zhang, Chunwei et al. “Corneal Hysteresis and Progressive Retinal Nerve Fiber Layer Loss in Glaucoma” American journal of ophthalmology vol. 166 (2016): 29-36.
- Ang GS, Bochmann F, Townend J, Azuara-Blanco A. Corneal biomechanical properties in primary open angle glaucoma and normal tension glaucoma. J Glaucoma 2008; 17:259 – 262.
- Sun L, Shen M, Wang J,etal. Recovery of corneal hysteresis after reduction of intraocular pressure in chronic primary angle-closure glaucoma. Am J Ophthalmol 2009; 147:1061 – 1066.
- Tsikripis P, Papaconstantinou D, Koutsandrea C, et al. The effect of prostaglandin analogs on the biomechanical properties and central thickness of the cornea of patients with open-angle glaucoma: a 3-year study on 108 eyes. Drug Des Devel Ther 2013; 7:1149 – 1156.
- Pakravan M, Afroozifar M, Yazdani S. Corneal biomechanical changes following trabeculectomy, phaco-trabeculectomy, Ahmed glaucoma valve implantation and phacoemulsification. J Ophthalmic Vis Res 2014; 9:7 – 13
- Agarwal DR, Ehrlich JR, Shimmyo M, Radcliffe NM. The relationship between corneal hysteresis and the magnitude of intraocular pressure reduction with topical prostaglandin therapy. Br J Ophthalmol 2012; 96:254 – 257.
- Hirneiss C, Sekura K, Brandlhuber U,et al. Corneal biomechanics predict the outcome of selective laser trabeculoplasty in medically uncontrolled glaucoma. Graefes Arch Clin Exp Ophthalmol 2013; 251:2383 – 2388.
- NICE guidelines (2018). “ORA G3 to measure conreal hysteresis”. https://www.nice.org.uk/advice/mib150/resources/ora-g3-to-measure-corneal-hysteresis-pdf-2285963509912261
- Susanna, Carolina N., et al. “A Prospective Longitudinal Study to Investigate Corneal Hysteresis as a Risk Factor for Predicting Development of Glaucoma.” American journal of ophthalmology 187 (2018): 148-152.
- Karmel (2018): Corneal Hysteresis: New Risk Factor for Glaucoma. American Academy of Ophthalmology, EyeNet Magazine March 2018 https://www.aao.org/eyenet/article/new-risk-factor-for-glaucoma
