Stereopsis is the ultimate goal of binocular vision, and in order for this to occur, flat fusion must occur, which in turn is dependent upon simultaneous perception of both retinal images, ie the hierarchy of sensory fusion must demonstrate all three stages. Typically, this requires both foveae to be directed towards the fixation target, so motor fusion must occur (the special case of ARC, anomalous retinal correspondence, will be considered in a future article).
The presence of sensory and motor fusion are the corner stones of normal, bifoveal binocular vision. Motor fusion is maintained by the vergence mechanism, but with increasing degrees of heterophoria there is increasing demand upon it. Subsequently it struggles to maintain fusion to the point where it will ultimately break down with the (latent) heterophoria becoming a (manifest) heterotropia with symptomatic diplopia. The interaction between the size of the underlying deviation and the ability of the vergence mechanism to cope with it leads to a spectrum of clinical situations ranging from the heterophoria being fully or well compensated (where the vergence mechanism can easily cope with the demands placed upon it), through decompensating (where motor fusion is maintained, but is under stress) to decompensated (where motor fusion has failed and heterotropia results). Figure 1 expands these categories further by adding subdivisions.
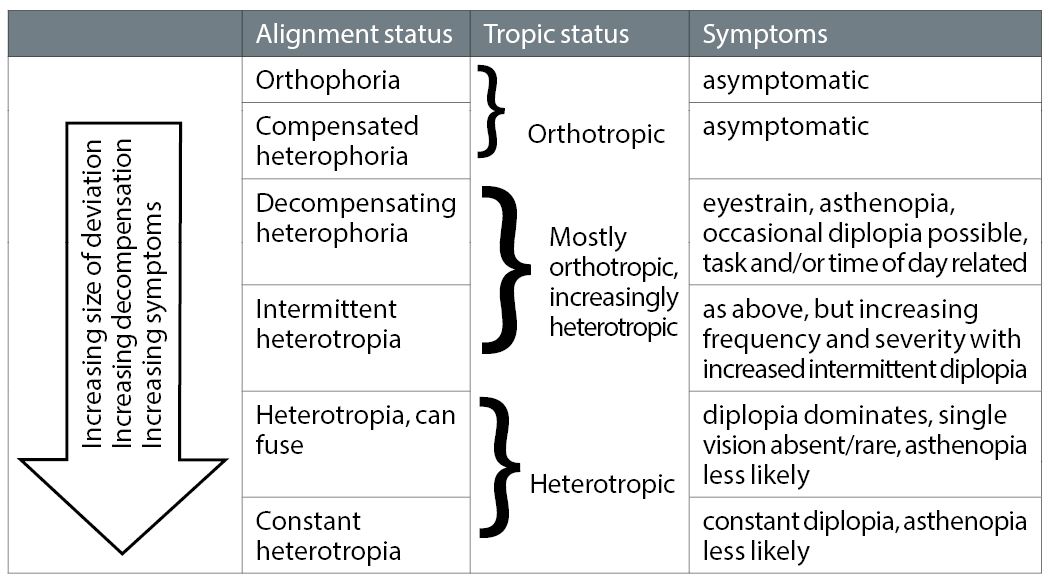
Figure 1: Increasing degree of heterophoria is likely to increase the chances of decompensation, leading to changes in severity, frequency and type of symptoms. However, it is not a simple linear relationship
The exact terminology is not important, nor is the number of subdivisions or the tipping point from one subdivision to the next: in reality there is variability and overlap between them. What is important is the recognition that orthophoria, heterophoria and heterotropia form a continual spectrum of increasing risk and/or levels of decompensation, accompanied by a changing pattern of symptoms which increase in frequency and/or severity. In order to address these problems in the clinical environment we must first understand the vergence mechanism and its relationship to misalignments, enough to allow us to understand the tests available to investigate this relationship, with the ultimate aim of using the relevant test results in developing a management plan that will alleviate the patient’s symptoms.
The vergence mechanism
The vergence mechanism itself is complex, with multiple interacting inputs. Tonic vergence, the underlying resting state, dictates the alignment of the visual axes in distance viewing when binocularity is suspended, for example with dissociation on Maddox Rod or with the eyes closed or in the dark. Ideally, this should leave the visual axes parallel, but this is clearly not the case when a distance phoria or tropia is present. From the tonic starting point, proximal vergence drives the eyes inwards when viewing or considering a near target. At the same time, accommodation (which has its own resting state and range of inputs) increases for a near target and drives accommodative convergence which again drives the eyes inwards, providing the convergence required for near objects. If all these inputs combine perfectly, orthophoria results at near.
Any inaccuracy in the combination of tonic, proximal and accommodative vergence, however, will result in heterophoria or heterotropia at near. Typically, the total input is inadequate, resulting in inadequate convergence at near with a resulting exo-deviation. Any such misalignment needs to be eliminated by the final vergence component, fusional vergence, if single vision is to be maintained. If successful, orthotropia (meaning alignment under normal binocular viewing when the eyes are associated, not dissociated as on the cover test or Maddox Rod or Wing) has been achieved, and if this has been done with ease and in the absence of any symptoms we have a compensated heterophoria. The deviation is still there, but is latent.
If fusional vergence fails completely, the misalignment remains manifest and we have heterotropia with accompanying diplopia. If successful, but not with ease, the patient may struggle to maintain orthotropia, and we have a problematic decompensating phoria. As stated previously, this can be subdivided further as detailed in figure 1. This interaction between fusional vergence and the underlying deviation leads to some basic underlying questions which need to be investigated clinically:
- Is fusional vergence adequate to overcome the deviation in a sustained manner?
- When overcoming the deviation, how robust is the orthotropia that has been achieved?
- While motor fusion is being maintained, how good is the sensory fusion?
As stated above, the vergence mechanism is complex. Also, the demands on the vergence mechanism are variable, but are increasing as our dependency on digital technology increases in everyday life and work life. Therefore, it is unrealistic to expect that a single simple clinical measure or formula will provide the perfect answer for all patients in all situations: this is the holy grail that practitioners and students alike yearn for, but it has not been achieved by any currently available test, and is unlikely to ever be achieved.
Instead, we need to consider multiple sources of information, from which we can develop a management plan for the patient. This sounds much more complicated than it actually is: we are simply trying to identify by how much we need to reduce the misalignment (typically via prescribing relieving prism, though manipulation of the spherical prescription may give a similar result) or improve the vergence mechanism (via exercises) so that the demands placed upon the vergence mechanism can be met.
First, we must consider the individual sources of information and how they relate to the questions posed above. As we are considering the impact of poor motor fusion maintenance on sensory fusion, there will be motor and sensory sources. We shall consider this in the order that the information might be expected to become available to us in a routine exam, with a few added tests as suggested by the suspicion of a decompensating phoria. The impact of the refractive error should always be considered first, as spherical changes in the prescription can alter the horizontal vergence position, and correction of any refractive error may help to clarify the retinal images and improve sensory fusion and hence the state of compensation. We will work on the assumption that accurate refractive error correction cannot fully resolve decompensation issues.
History and symptoms
The classic presentation for a decompensated phoria/tropia is diplopia. If the tropia is constant the diplopia is constant, and the degree may change from distance to near, but should not change with different directions of gaze. If it does, then identification of a potentially pathological condition underlying this incomitant deviation should be sought. Headaches are possible, especially if the patient resorts to closing one eye to eliminate the diplopia, but are not always present. If recent onset, the diplopia will be very troublesome to the patient, but may become less so with time. If breakdown occurs as a child, then suppression (and/or other adaptations) may develop and the patient will be generally asymptomatic, but will lack the benefits of stereopsis.
Orthophores or fully compensated heterophores are asymptomatic. But in between we have the decompensating heterophores, who typically have asthenopia and headaches as the first and main presenting symptoms, but as decompensation increases the almost-giveaway symptom of intermittent diplopia starts to occur. They may report a habit of closing one eye to alleviate these symptoms which typically increase throughout the day and possibly throughout the working week, especially if they are associated with decompensation at near (the most common presentation in adults).
Decompensation at distance is likely to result in strain or intermittent diplopia while driving or watching television, especially at night due to a combination of fatigue and reduced ambient lighting. Typically the symptoms are relieved by avoidance of the problematic task (if possible) and rest or sleep. As decompensation increases, the symptoms occur more frequently and earlier in the day, and may even persist upon waking the next day. A greater frequency or severity of symptoms increases the likelihood of needing to provide help and the amount of help needed, but a specific remedy (eg relieving prism) cannot be prescribed based on symptoms alone.
Stereopsis
Stereopsis occurs when objects more or less remote than the fixation target generate retinal disparity (see figure 2).
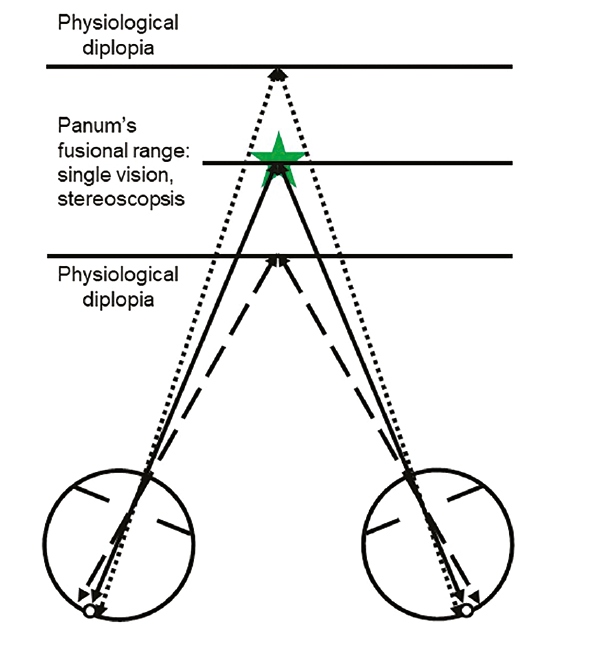
Figure 2: Single vision is maintained within a limited depth range either side of the fixation target. Nearer objects stimulate temporal retina, and more distant ones stimulate nasal retina. These retinal disparities are the basis of stereoscopic vison, which only occurs within Panum’s fusional range, which is always centred on the point where the visual axes cross. Beyond this, physiological diplopia occurs
It only occurs within Panum’s fusional range which is quite narrow, and hence it is not surprising that stereopsis may be compromised if decompensation starts to occur, especially if fixation disparity is present (see figure 3).
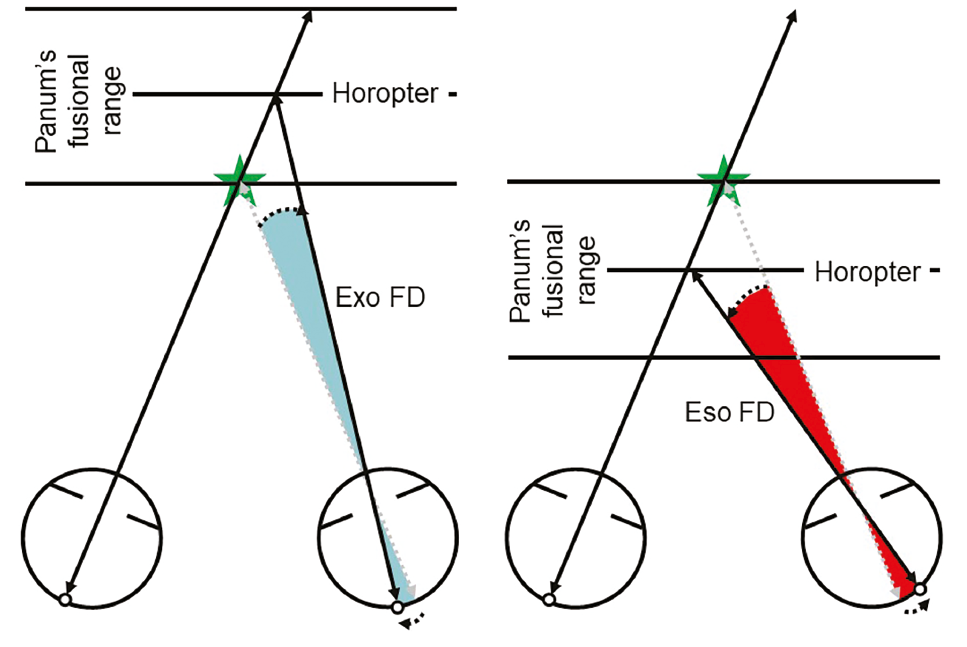
Figure 3: Panum’s fusional range allows for a slight misalignment of the visual axes under normal binocular viewing conditions whilst maintaining single vision – fixation disparity (FD). This can be eso or exo FD, and is potential evidence of a decompensating eso or exophoria respectively. Despite the single vision, stereopsis may be compromised. If the misalignment were to increase further, the target would fall outside of Panum’s fusional range and diplopia would occur
Indeed, it will automatically be eradicated as and when diplopia occurs, the diplopia demonstrating that there is simultaneous perception but no flat fusion (the heterophoria has currently decompensated to a heterotropia) and therefore no possibility of stereopsis. While motor fusion is maintained stereopsis should be possible and may well be normal, or it may be compromised. Note that demonstration of good stereopsis does not conclude that the phoria is fully compensated, just that good sensory fusion is possible during times when good bifoveality is achieved.
Due to the fatiguing nature of decompensation, stereopsis may be good in the morning but may decline throughout the day. If relieving prism improves stereo, then this proves the stereo was initially compromised and hence definitively proves the phoria is decompensating. If possible, the minimum prism that gives the maximum stereopsis should be recorded as a potential aid to prescribing, although this value can be difficult to pin down accurately.
Cover test recovery
For orthophoria, there will be no movement seen at any point of the cover test. However, the same is also true of small deviations, typically less than 2 to 4 Δ. Such small deviations, if eso or vertical, can be problematic to the point of decompensating, hence no movement on cover test is not a guarantee of good binocularity. If decompensation is complete and a tropia is present, rapid version movements will be seen (if they are large enough) as and when the unoccluded eye takes up fixation. These must be distinguished from the recovery movement seen on removal of the occluder in heterophoria, which is a direct observation of the fusional vergence in action.
The speed and smoothness of recovery holds important clues to the state of compensation. If the recovery is fast and precise, good compensation is likely. If, however, it is sluggish and inaccurate poor compensation is suspected as fusional vergence appears to be struggling to restore bifoveality. Repeating with increasing relieving prism until recovery is acceptable can give an objective estimate of the prism that should be prescribed, but this endpoint can be difficult to judge.
Fixation disparity
Fixation disparity can be defined as a slight misalignment of the visual axes under binocular viewing conditions. It typically occurs in the same direction as, but is much smaller than, the underlying phoria (ie eso FD with esophoria) and is considered to be evidence of the vergence mechanism struggling to maintain bifoveality; in other words, the phoria is decompensating (see figure 3).
Detection of fixation disparity requires the identification of the slight discrepancy in perceived directions by the two eyes under normal binocular viewing conditions. This requires partial dissociation, ie dichoptic stimuli (stimuli that are seen exclusively by one or other eye only) against a fusible binocular background (seen by both eyes). This is typically achieved in optometric
practice by the use of polarised filters in conjunction with the Mallett unit. Any misalignment of the bars perceived by the patient can be neutralised by the introduction of a suitably oriented prism (or prism bar) which can be increased in magnitude until the bars are seen as aligned and stable. The minimum amount of prism required to achieve this is recorded and considered for prescribing.
This does not measure the angular size of the fixation disparity, rather it measures the amount of relieving prism required to eliminate it, a more clinically relevant value termed the associated phoria. (Unfortunately and erroneously, this value of prism is typically referred to as fixation disparity in optometric practice, but the term associated phoria will be adhered to in this paper.) If one bar is not seen, then there is suppression of the corresponding eye (assuming the visual acuity under monocular conditions is good enough to see the bar). Adding relieving prism (based on cover test results) will reduce the size of the deviation, which in turn may eliminate the need for suppression, at which point the prism can be adjusted until alignment is achieved and prescribing can be considered.
Fusional reserves
No biological mechanism can work at full capacity for sustained periods, including fusional vergence. There must be some reserve capacity, in this case a fusional reserve. Fusional reserves are measured by gradually increasing prism until fusion breaks down and diplopia results (the break point). Some subjects will recruit their accommodation to drive the vergence further but while this maintains single vision, it will be blurred (hence the reporting of a blur point), before further increase of the prism delivers the break point. Once fusion has broken, the prism is gradually reduced until single vision is restored (the recovery point). The triplet of blur (if reported) break and recovery are recorded and used in prescribing prism. Prism can be gradually increased and decreased via a prism bar or a Risley prism as found in phoropters: it cannot be done easily with loose prisms.
It is the fusional reserve that opposes the phoria that is most important. For example, convergence is required to overcome an exophoria, therefore the total ability to converge (the positive fusional reserve measured by increasing base-out prism, as opposed to negative fusional reserve for divergence using base-in prism) is the most relevant. However, it is good practice to measure the reserves in both directions (ie base-in and -out, or base-up and -down for vertical reserves), as it is important for the total fusional range to be balanced (typically one reserve should not be more than twice the magnitude of its opposite reserve).
Therefore, the questions become: how much is there in reserve, and is there enough to maintain comfortable bifoveality throughout waking hours, and are the reserves balanced? In other words, are the reserves good enough to compensate the phoria, and if not, how much relieving prism is required to achieve compensation? These questions can be answered by choosing between three established criteria that calculate the required prism prescription (see table 1).
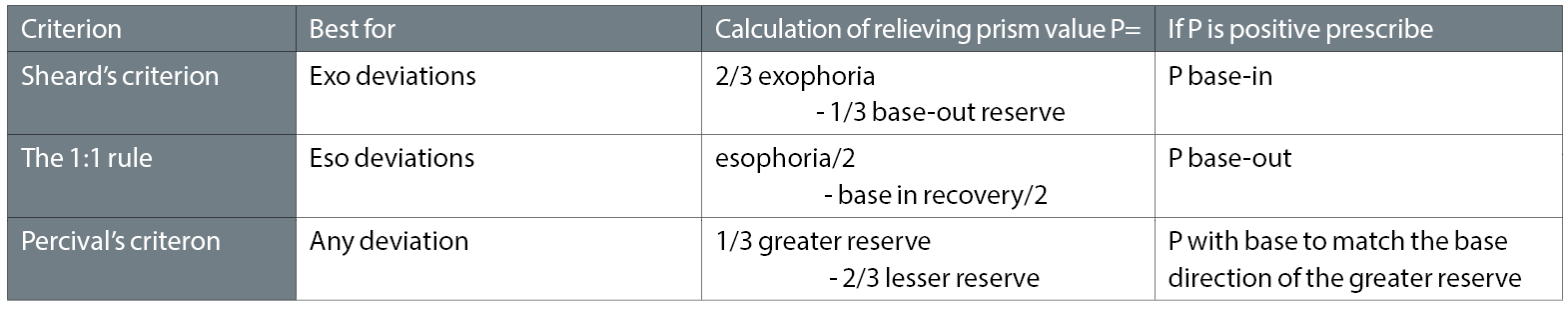
Table 1: Options available for calculation of prescribed prism using fusional reserves. Other sources of evidence of decompensation need to be considered before a final decision is made
Sheard’s criterion is best for exophorias and the 1:1 rule for esophorias. Note the interaction between the deviation and the opposing fusional reserves in these cases. Percival’s criterion provides supporting evidence for both, and can also be used for vertical. Note that Percival’s ignores the size of the deviation, and is based purely upon the fusional reserves being balanced, ie the patient must be operating within the ‘middle third’ of their total vergence range. If any of these criteria are not met, the relevant formulae will calculate how much relieving prism is required. There will not be total agreement between the criteria, but the values are a good guide to be used in conjunction with the practitioner’s judgement.
The weight of evidence for decompensation
The dynamic nature of the vergence system, along with the variability of stress put upon it by varying work demands and life styles, means that no single measure or approach can give a definitive answer to the degree of compensation, or to the amount of remedial action required. Instead, the practitioner must use their judgement in weighing up all relevant sources of evidence.
Asthenopic symptoms are a strong clue, but can be non-specific in that other conditions can lead to similar symptoms. Also, suppression can lead to the elimination of most symptoms, perhaps only leaving hints of clumsiness when accurate depth perception is required, due to the reduction or total loss of stereopsis that suppression inevitably brings. Intermittent diplopia in the primary or reading position is a very strong indication of decompensation, being almost pathognomonic. In the long run, however, elimination of symptoms can be seen as good evidence that compensation has been adequately restored.
As decompensation increases, evidence of the fusional reserves being inadequate for the size of the phoria would be expected, and increasing levels of associated phoria may also occur, both leading to a higher value of prism being proposed. Application of the fusional reserves criteria and the associated phoria are the two generally recognised approaches to establishing the value of prism to be prescribed. An improvement in a poor recovery movement on the cover test can be used as supporting evidence, or in isolation as the only objective evidence in a patient with poor communication or compliance. Restoration of compromised stereopsis can also be supportive. In both cases, look for the minimum prism to give results suggesting that compensation has been restored. However, cover test recovery and stereopsis are not always compromised in the first place, and absence of compromise should not be considered strong evidence of compensation. As decompensation tends to occur towards the end of the day, the timing of any investigations needs to be considered. If clinical findings suggest a better state of compensation that the patient’s symptoms, it may be appropriate to retest the patient as late in the day as possible in order to allow fatigue to have its full impact. If any of the test results worsen, then some level of decompensation can be assumed.
Management of decompensation
Prescribing the minimum amount of relieving prism, primarily based on fusional reserves and/or the associated phoria, but supported by secondary sources of evidence, should restore compensation by effectively reducing the size of the phoria. However, this does not cure the problem. It merely provides a crutch which the patient may become more and more dependent on. For this reason, prescribing prism should be approached with caution.
But prescribing prism is not the only way to reduce the size of a deviation: this can also be achieved by spherical manipulation, where the spherical component of the patient’s prescription is deliberately changed bilaterally. The amount of spherical change required is calculated by dividing the required prism by the gradient AC/A ratio, which in turn is easily calculated as the change in vergence position recorded on the Maddox wing divided by the change in accommodation that drives this change in vergence. If bilateral +1.50 and -1.50 spheres are used, the AC/A ratio = (change in vergence)/3, typically giving values in the range of 2 to 4.
Overminusing will drive accommodation, thereby increasing accommodative convergence, thereby reducing the size of an exo-deviation: but consideration must be given as to whether the patient has enough accommodation to cope with this increased demand, and is typically limited to dealing with distance exos.
Overplussing will have the opposite effect of relaxing accommodation and therefore reducing accommodative vergence and an eso-deviation will become smaller. This cannot be done for distance esos, as it will blur the distance vision, but can be used successfully for near esos, when it is typically prescribed as a reading addition in a bifocal or varifocal. Whether the deviation is reduced by prism or sphere, once robust compensation has been re-established, the practitioner may aim to reduce the correction over time. This slowly reintroduces the full deviation under controlled conditions, allowing the vergence mechanism to slowly exercise itself back to normal and full compensation. This may only be successful in cases of relatively mild and recent decompensation, and a constant heterotropia is unlikely to respond adequately.
The alternative to reducing the deviation is to improve the vergence mechanism’s ability to cope with the deviation by exercises, and this should be the first treatment option to be considered. There are a multitude of different exercise types to choose from, but they should be varied and targeted at the distance and direction of the phoria. They work best for exo-deviations, at near more so than distance, and are least effective for distance esos and vertical deviations. They can be prescribed instead of prism or spherical manipulation in mild decompensation, especially for near exo-deviations. They can also be used in conjunction prism or spheres in more advanced decompensation, where successful exercising may result in accelerated elimination of the physical prescription.
Note that motor and sensory fusion must be present for exercises to work, hence they will not work for heterotropia unless it has been reduced and robust bifoveality re-established. Also, good cooperation and compliance are essential for success, and this can be a major issue if extensive exercises are required for significant decompensation.
Whatever approach is taken to management, regular review is important in the short term, with the aim of tailing off exercises or reducing physical prescriptions. This monitoring can then be reduced when the problem has been resolved or stabilised, and either no further exercises are required or no further reduction in prescription is feasible.
Conclusion
Decompensating phorias, especially exos at near in adults, are a relatively common occurrence that optometrists should be able to manage. The inherent complexity and variability means that no single test or parameter is wholly reliable and can only act as a guide, and therefore the fullest range of relevant clinical information should be sought.
Management options should consider a combination of the following, all with the aim of long term relieving of symptoms;
- Exercise (the first choice)
- Spherical manipulation of the refractive correction where appropriate (applying the gradient AC/A ratio)
- And finally prism prescription (with the associated phoria being the most accessible method for most practitioners to establish the required value)
Whatever the management options chosen, short term review is necessary as changes are likely to be required in tailing-off exercises and/or altering prescriptions. Although this requires some judgement from the practitioner, this is no more onerous than many clinical decisions, and the risks are low with the potential of good patient satisfaction.
Dr Fergal Ennis is a Senior Lecturer (Teaching and Scholarship) at Cardiff University.
Bibliography
- Anomalies of Binocular Vision: Diagnosis and Management. Rutstein RP, Daum KM. (1998) Mosby.
- Clinical Management of Binocular Vision: Heterophoric, Accommodative, and Eye Movement Disorders. 4th edition. Scheiman M, Wick B. (2013) Lippincott Williams and Wilkins.
- Clinical Orthoptics. 3rd edition. Rowe FJ. (2012) Wiley-Blackwell.
- Ocular Accommodation, Convergence, and Fixation Disparity. 2nd edition. Goss DA. (1995) Butterworth-Heinemann.
- Pickwell’s Binocular Vision Anomalies, 5th edition. Evans BJW. (2007) Butterworth-Heinemann.
- Vergence Eye Movements: Basic and Clinical Aspects. Schor CM, Ciuffreda KJ. (1983) Butterworth.
