TFOS and DEWS
In recent decades the Tear Film and Ocular Surface Society (TFOS) sought to bring together experts from around the world in an international research effort, with the aim of better understanding the composition and regulation of the pre-ocular tear film.1 The launch of the original DEWS (Dry Eye Workshop) report in 2007 began what was to become the latest revolution in our understanding of dry eye disease and its management. This was followed by the MGD (meibomian gland dysfunction) report in 20112 and later the CLD (contact lens discomfort) report in 2013.3
The launch of the DEWS II report in July 2017 brings together the very latest in global consensus regarding many aspects of dry eye disease. It has taken those involved two-and-a-half years to develop and has resulted in the latest ‘DED (dry eye disease) Manual’. Like many manuals, it is a sizable volume containing a lot of evidence based information. This series of articles will attempt to concisely summarise the key elements relevant to a eye care practitioner.
The entire report is available as a free download. In this feature, we offer an overview of three main areas covered in the report;
- Definition and classification of dry eye disease
- Assessment techniques and diagnosis
- Management options
DEFINITION
The original DEWS report definition was the first-time dry eye was identified as a disease, with many underlying causes, that was deemed to result in symptoms and signs, in association with tear film hyperosmolarity and ocular surface inflammation.
The latest definition from DEWS II is as follows:
Dry eye is a multifactorial disease of the ocular surface characterized by a loss of homeostasis of the tear film, and accompanied by ocular symptoms, in which tear film instability and hyperosmolarity, ocular surface inflammation and damage, and neurosensory abnormalities play etiological roles.
To pick the definition apart, the main element in the outcome of dry eye disease is the loss of homeostasis, the normal status quo, of the tear film. What it really highlights is that the disease is actually caused by the key elements of inflammation, tear film instability and hyperosmolarity (figure 1).
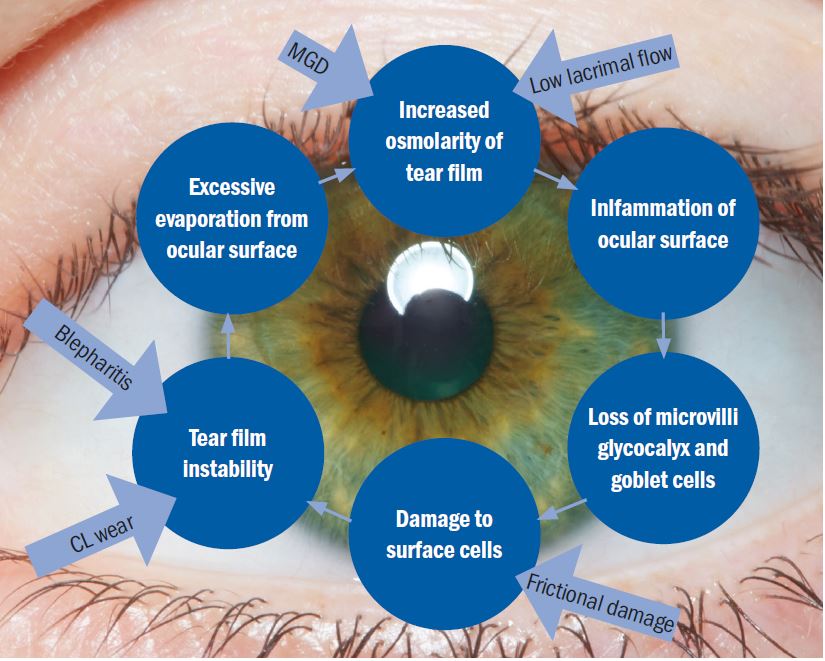
Figure 1: The basic concept of the Vicious Circle (adapted by the author). The core mechanism is hyperosmolarity. The form of dry eye disease determines where on the cycle a patient will enter but, once on the circle, the resultant effect is the same and self-perpetuating
The core mechanism of dry eye disease is tear hyperosmolarity which damages the ocular surface both directly and by initiating inflammation. Tear hyperosmolarity is universally present and ultimately results from excessive evaporation of the water component from the ocular surface. It is this hyperosmolarity that leads to the ‘Vicious Circle’ of dry eye (figure 1). The exact nature of an individual’s dry eye disease determines where they enter the cycle of the disease. The vicious circular nature of the disease means that, once an individual enters the circle, the resulting instability, inflammation and hyperosmolarity further accelerate adverse changes. TFOS DEWS II is the first time the definition has included a signalling role for the disease from issues arising in ocular receptors and nerves.
CLASSIFICATION
The historical classification of dry eye disease has typically considered two distinct disease entities:
- Aqueous deficient
- Evaporative
The latest thinking from the report suggests moving away from this separation and more towards a blurring of the lines between the two classically considered sub-types. In other words, we should consider the disease as more of a continuum of these two sub-types rather than separate entities.
The latest classification scheme encourages a simplified diagnostic strategy to help triage patients (figure 2). The scheme also importantly considers the cases where patients exhibit dry eye symptoms without evidence of obvious signs or, at the opposite end of the spectrum, present with marked signs but have no dry eye symptoms. It also includes a clinical decision guide for practitioners. The classification also recognises the necessity of symptomatic involvement and the need for presence of associated ocular surface signs in making an actual diagnosis of DED.
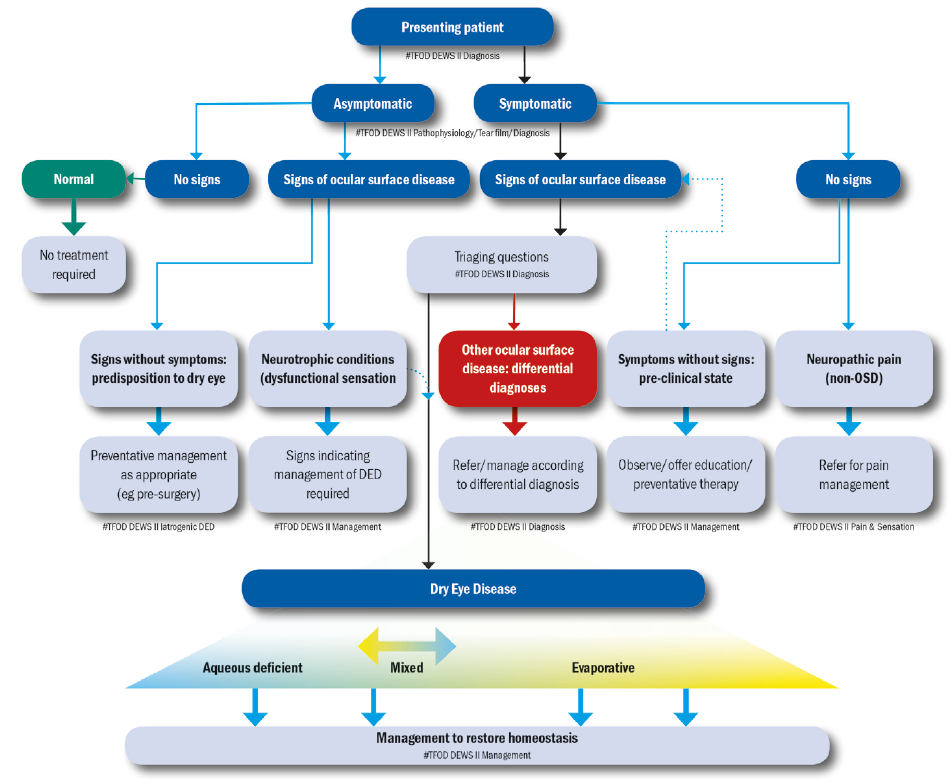
Figure 2: The DEWS II classification of dry eye disease. The upper part of the diagram represents a clinical triage guide, including assessing symptoms, followed by reviewing signs of ocular surface disease. DED exhibits both symptoms and signs and can be treated with appropriate strategies. Symptomatic patients without signs do not fall into the DED group. Asymptomatic patients with signs of DED are then separated into patients with poor corneal sensitivity or those with prodromal signs at risk of developing DED with time or a trigger such as surgery. The lower portion shows the categories of dry eye, now considered as more of a blend or hybrid of the two traditional categories of aqueous deficient (affecting lacrimal gland function) or evaporative (lid related and ocular surface related). More of this sliding scale is devoted to evaporative to reflect the greater proportion of DED attributable to this category. It is acknowledged that, as the disease progresses, it is more likely that both components will become apparent clinically
Symptoms without signs
It is now more widely recognised that often there is a significant mismatch between signs and symptoms in dry eye disease patients. The fact that the latest definition recognises the role of neurosensory abnormalities helps to explain why many patients who present with significant symptoms have very little signs and vice-versa.
The emerging role of neurosensory abnormalities is now considered so important that it is a key part of the new definition. Nociceptors are the receptors designed to detect stimuli that may cause harm to the body. They signal tissue irritation, impending injury or actual injury. On activation, they transmit pain signals via the peripheral nerves to the brain. Nociceptors in the cornea have the potential to be sensitised by repeated physiological stimulation or by noxious stimuli such as hyperosmolarity or inflammation.
Neuropathic pain due to a lesion or disease in the somatosensory system (the sensory system concerned with conscious perception of sensations including pain) results in the scenario where ocular pain symptoms disproportionately outweigh the clinical signs. In these cases, the patient requires pain management and this falls outside the scope of DED therapy. If symptoms of DED are present without signs, it can alternatively indicate a pre-clinical dry eye or episodic dry eye.
Signs without symptoms
A long-debated consideration in managing dry eye disease is for those patients who present in practice with signs of DED and no symptoms. As a practitioner, it can be difficult to decide whether to try to convince the patient to begin a rigorous regime for a problem that they are not aware of. The likely causes are either reduced corneal sensitivity or a predisposition towards dry eye.
Corneal nerve damage secondary to longstanding DED is a recognised issue resulting in reduced sensitivity and will mask patient discomfort. If a patient has early disease, then this might place them at risk of developing symptomatic DED if they undergo ocular surgery such as cataract or refractive surgery. Asymptomatic MGD has recently been reported with a prevalence double that of symptomatic MGD. It is known that symptoms of MGD become more common and more severe with age and, as such, in these cases preventative management should at least be discussed with the patient
Risk factors
The current understanding of the risk factors likely to be associated with dry eye disease can be split into groups according to how likely the association is (see table 1).
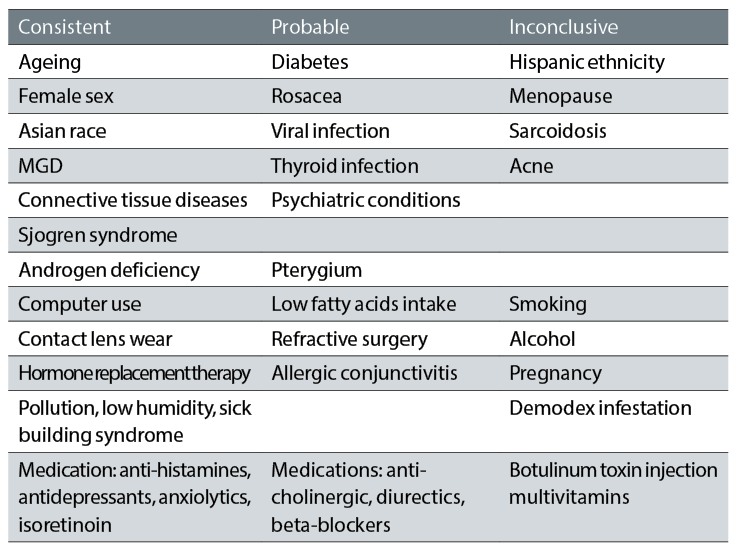
Table 1: Evidence based risk factors associated with dry eye disease (courtesy of Professor C Purslow)
DIAGNOSTIC TECHNIQUES
Key Diagnostic 1: History
The best diagnostic work up begins with a good history, and the assessment of possible dry eye disease is no exception. Although the relationship between signs and symptoms is variable between individuals, assessing symptoms accurately allows decisions to be made regarding whether further evaluation is important, as well as monitoring response to treatments. Dry eye questionnaires exist to aid the specific diagnosis of dry eye disease and grade the severity to set a baseline for future reference. The OSDI (Ocular Surface Disease Index) is the most widely used but the DEQ-5 (Dry Eye Questionnaire 5) is shorter but still accurate, and can be useful in a practice setting. They both ask a number of questions about visual disturbance and function, as well as discomfort. The final score can be graded to set a baseline and for monitoring treatment.
In addition to this, a list of specific questions to ask during a work up has also been developed, with the intention of allowing the practitioner to differentially diagnose dry eye disease compared to other conditions that can mimic the symptoms (table 2).

Table 2: Questions to ask to aid differential diagnosis of dry eye disease
Clinical tests
The report has highlighted a number of key tests that are essential in the work up of a dry eye patient. The dry eye diagnostic test battery is summarised in figure 3.
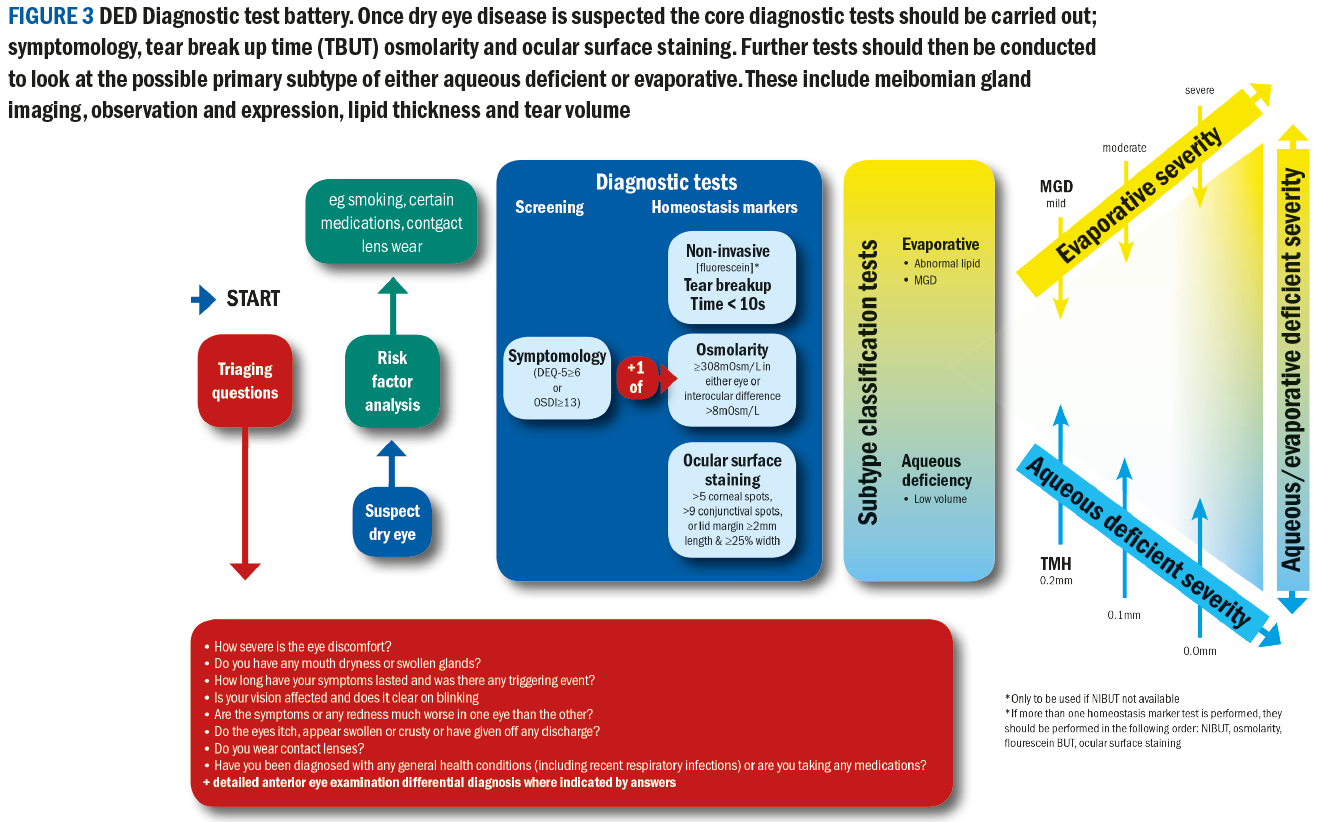
The critical diagnostic tests and their order are as follows:
- Symptoms
- Non-invasive tear break-up time (NITBUT)
- Osmolarity
- Fluorescein break up time (FBUT) if not NITBUT
- Ocular surface staining
Further tests to inform about the sub-type of a presentation include:
- Meibomian gland imaging (meibography), observation and expression
- Lipid layer thickness measurement
- Tear volume measurement
These are highlighted in table 3, which has been adapted by the author from the report. The table highlights common diagnostic tests and the normal versus dry eye values expected in a practice clinical setting. It also indicates in the right column the amount of change in a test reading required after treatment before a patient will experience a beneficial improvement in symptoms.
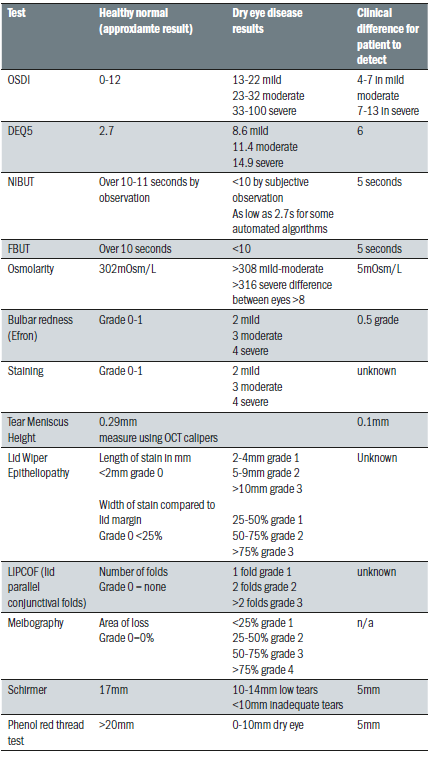
Table 3: Common diagnostic tests and normal and dry eye values
Key Diagnostic 2: Tear film stability
Impaired tear film stability is a key diagnostic feature. Tear break up time (TBUT) is a direct indicator and can be measured with no drop instillation using a tear-scope or automated topography based system (non-invasive NIBUT) or using fluorescein (FBUT). The fluorescein itself reduces the stability of the tears and means the technique is less sensitive and less specific than NIBUT, but its ease of use makes it a very commonly used technique in practice.
Key Diagnostic 3: Osmolarity
Tear osmolarity has been shown to have the greatest correlation to disease severity of the clinical DED tests, and has been reported as the single best metric to diagnose and classify DED. An osmolarity of 308mOsm/L or more and/or an interocular difference of eight or more is considered positive for dry eye disease. The two values that are important to note are the higher value of the two eyes, which is considered more indicative of the DED process, and the difference in value between the two eyes, which provides insight about the instability of the tear film. There is greater inter-eye variability in osmolarity in dry eye, which increase with severity and decrease with successful management. The tears of DED individuals show increased variability generally due to possible poor mixing of the tears between blinks. Osmolarity measurement is the least variable of all the common signs of dry eye disease over time.
Key Diagnostic 4: Ocular surface staining
Punctate staining of the ocular surface is a feature of many diseases. In DED, the use of fluorescein and lissamine green is currently advised in the report to highlight corneal and conjunctival/eyelid margin tissue damage respectively. In the UK, lissamine green is a medical device and is an unlicensed product and is currently not recommended for use in practice. Fluorescein stains viable cells with a compromised integrity.
Lissamine green stains epithelial cells only if the cell membrane is damaged. When using strips to instil dye, it is recommended to instil a drop from the strip inside the lower
temporal lid with the patient in up gaze, with the lid pulled slightly temporally to avoid damage to the conjunctival or lid wiper tissue. Both fluorescein and lissamine green should be observed roughly one to three minutes after instillation. Lid wiper epitheliopathy can be observed with either lissamine green or fluorescein. Corneal staining is considered to probably be a later stage sign of DED, and staining in mild to moderate cases shows poor correlation with disease severity. It is also worth bearing in mind that severity of DED can change with the time of day, so this should be considered when interpreting results and monitoring the condition.
Tests to aid subclassification
Tear Volume
Most of the tear fluid sits in the tear menisci, which serve as reservoirs supplying tears to the pre-corneal tear film. In a practice setting this can be measured using the slit lamp beam height, but is poorly repeatable. Schirmer test without anaesthetic remains the recommended diagnostic test for confirming severe aqueous deficiency, but its variability and invasiveness preclude it as a routine diagnostic test of tear volume. Anterior optical coherence tomography (OCT) assessment involves taking an image in the centre of the lower eyelid without lid manipulation shortly after a blink is advised. The image can then be measured with callipers allowing a non-invasive simple measure, but this can easily be disrupted by issues such as LIPCOF (lid parallel conjunctival folds) or conjunctivochalasis.
LIPCOF
Lid parallel conjunctival folds are folds in the lateral lower quadrant of the bulbar conjunctiva. They are thought to be associated with decreased mucin production, and correlate with the presence of lid wiper epitheliopathy (lissamine green staining along the lid margin associated with friction). They are observed with white light and can be graded by counting the permanent folds seen.
Inflammation
Inflammation is a recognised component of the mechanism of DED and can indicate its severity. However, inflammation is not specific to dry eye disease and can occur in other ocular or systemic disease. The tests most commonly employed to record it are conjunctival redness grading, either directly by the clinician or using digital image analysis, or directly measuring an inflammatory marker, such as matrix metalloproteinases or cytokines, with a commercial device, although it must be considered that these markers tend to increase with age and age specific normal values have not yet been published.
Lid Wiper Epitheliopathy (LWE)
The lid wiper is the area of the upper and lower lid that wipes the tear film over the ocular surface. It appears to be the most sensitive conjunctival ocular tissue. LWE is thought to be related to increased friction throughout blinks, although it may also involve tear film viscosity induced hydrodynamic forces at the start of a blink. It is detected using either lissamine green or fluorescein or both by everting the lid and grading the staining length in mm, and width relative to the lid margin.
Meibography
This allows observation of the meibomian gland structure directly. It is best carried out using infrared light, either in a mobile device, dedicated device or on a slit lamp. It alone is not sufficient for the diagnosis of MGD, but is a useful adjunct to build up the clinical picture. Unsurprisingly, changes in the glands are less pronounced in aqueous deficiency compared to evaporative issues. The lid is everted and observed. The grading is scored according to the area of loss of defined gland structure.
MG expression
Meibum quality, quantity and expressibility are thought to reflect gland function. It is carried out in practice by digital expression of the glands along the length of the eyelid, usually with meibomian gland forceps. Normal meibum is clear and readily expressed, but in MGD it loses its clarity and the viscosity increases and becomes increasingly difficult to express in severe MGD. The diagnostic value has not yet been established in DED.
Blink analysis
Blinking action clears debris, provides mechanical protection and reforms the tear film, as well as being vital for meibum distribution. Blink rate and the completeness of the blinks can be evaluated when the patient is performing a task such as questionnaire completion, unaware that they are being observed. The normal blink rate is reported to occur from 10-15 blinks per minute. Incomplete blinking can result in DED symptoms and staining.
Comorbidities
When diagnosing DED an important part of the work up is to consider co-morbidities that may make the issue more than just the primary DED discussed so far. Typical slit lamp examinations to consider include:
- Eyelashes for anterior blepharitis and Demodex spp
- Eyelid position (ectropion or entropion) and closure
- Eyelid palpebral conjunctiva for MGD, follicles or swelling
- Bulbar conjunctiva for conjunctivitis, redness pattern and swelling
- Cornea for ulceration and staining to detect possible trauma or dystrophies
- Anterior chamber for presence of cells or flare
Blepharitis
Recurrent or persistent blepharitis can cause DED so it is important to examine the eyelid to aid diagnosis. Staphylococcal or seborrheic anterior blepharitis are linked to aqueous deficient dry eye, possibly due to the decreased tear volume supporting less lysozyme or immunoglobulins. Demodex infestation which occurs in 100% of people aged over 70 can spread from the face to the eyelids, leading to blepharitis and rosacea, although Demodex can also be found in asymptomatic individuals. Demodex
consume sebum, and may consume follicular and glandular epithelial cells, which could lead to direct damage to the lid margin. They are also thought to cause blepharitis by carrying bacteria on their surface, also the internal protein and their waste products may trigger inflammatory responses.
Iatrogenics
When seeking a diagnosis, it is important to bear in mind that dry eye can be caused inadvertently by many types of intervention. Topical medications can cause DED due to their allergic, toxic and immuno-inflammatory effects on the ocular surface. Preservatives such as benzalkonium chloride may further aggravate DED. Systemic drugs can also induce DED via numerous mechanisms (see table 4).
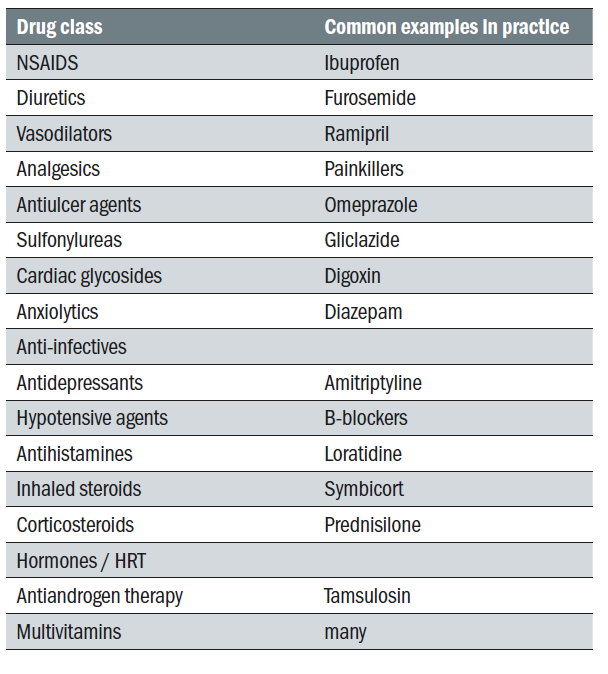
Table 4: Common systemic drugs that possibly cause or contribute to dry eye. A large number have anticholinergic activity and reduce aqueous and mucous and possibly meibomian gland mucin production
The use of contact lenses is also associated with DED. One of the other risk factors is from surgical procedures such as corneal refractive surgery due to corneal nerve cutting, cataract surgery, lid surgery, Botox application and cosmetic procedures. Efforts to aid early diagnosis prior to surgeries, and to better regulate and guide patients in self-medication could help alleviate this common issue.
MANAGEMENT
The TFOS DEWSII states, ‘Management is ultimately aimed at restoring the homeostasis of the ocular surface by breaking the vicious cycle of the disease and offering long-term options to prevent a return to the vicious cycle and a resurgence of symptoms.’
Evaporative or deficient?
It is now generally believed that most people with symptoms relating to ocular surface disease (OSD) suffer from variable combinations of both abnormal meibomian gland function (evaporative eye disease – EDE) and tear underproduction (aqueous deficient eye disease – ADDE) rather than them being entirely separate conditions. Patients with DED have been shown to be over three times more likely to have evaporative dry eye than aqueous deficient dry eye and 30% of patients had both types.
Staged management and treatment recommendations for dry eye disease
The basis of effective management begins with accurate diagnosis. It is important to categorise an individuals dry eye disease in terms of the degree to which EDE, ADDE and any other ocular surface conditions are contributing to the overall picture for that patient. The concept of determining the major causitive factor is key to appropriate and effective management. While there are treatments which are indicated for multiple aspects of DED, others are specific to one particular aspect of an individuals ocular surface condition.
The steps outlined below (and in figure 4) show the most evidence-based staged management of DED depending on severity. The exact nature of an individual’s DED will dictate the range and number of management options selected from one or more steps. Options within a category are not ranked according to importance and may be equally valid.
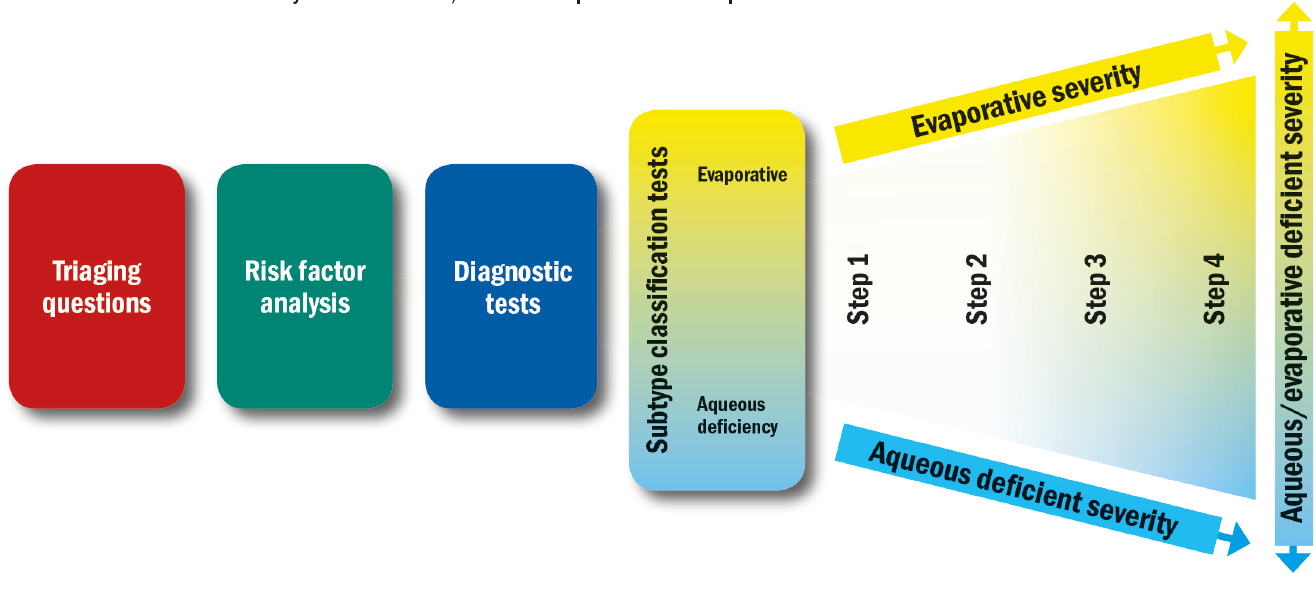
Figure 4: The DEWS II approach to managing DED. Once the working differential diagnosis and disease severity has been reached, the treatment plan can be developed
Step 1:
- Education regarding the condition, its management, treatment and prognosis
- Modification of local environment
- Education regarding potential dietary modifications (including oral essential fatty acid supplementation)
- Identification and potential modification/elimination of offending systemic and topical medications
- Ocular lubricants of various types (if MGD is present, then consider lipid containing supplements)
- Lid hygiene and warm compresses of various types
Step 2: If above options are inadequate consider:
- Non-preserved ocular lubricants to minimise preservative-induced toxicity
- Tea tree oil treatment for Demodex (if present)
- Tear conservation
- Punctal occlusion
- Moisture chamber spectacles/goggles
- Overnight treatments (such as ointment or moisture chamber devices)
- In-office, physical heating and expression of the meibomian glands (including device-assisted therapies)
- In-office intense pulsed light therapy for MGD
- Prescription drugs to manage DED
- Topical antibiotic or antibiotic/steroid combination applied to the lid margins for anterior blepharitis (if
- present)
- Topical corticosteroid (limited-duration)
- Topical secretagogues
- Topical non-glucocorticoid immunomodulatory drugs (such as cyclosporine)
- Topical LFA-1 antagonist drugs (such as lifitegrast)
- Oral macrolide or tetracycline antibiotics
Step 3: If above options are inadequate consider:
- Oral secretagogues
- Autologous/allogeneic serum eye drops
- Therapeutic contact lens options
- Soft bandage lenses
- Rigid scleral lenses
Step 4: If above options are inadequate consider:
- Topical corticosteroid for longer duration
- Amniotic membrane grafts
- Surgical punctal occlusion
- Other surgical approaches (eg tarsorrhaphy, salivary gland transplantation)
It is now more understood that symptoms and signs do not always correlate, and this needs to also be considered when determining the severity of the condition. It is important to bear in mind that in cases where there are chronic symptoms but no signs that neuropathic pain rather than DED should be considered. Conversely, in situations where the individual has few symptoms but clear signs, reduced corneal sensitivity may be a factor and treatment is important to avoid ongoing damage.
A Brief Summary of The Main In-Practice Options
Ocular lubricants
- Lubricants remain the mainstay of early treatment for DED. There are numerous options available, but they do not specifically target the underlying pathophysiology of DED. Formulations vary enormously in composition; osmolarity, viscosity and pH can have similar major components:
- Viscosity enhancing agents have a range of mechanisms including increasing tear film thickness, protecting the ocular surface, promoting tear retention and improving goblet cell density. Higher viscosity drops can increase retention time but also cause transient visual disturbance. Examples are carbomer and hyaluronic acid.
- Osmotic agents that are hypotonic may have an impact on improving goblet cell (mucous producing) density.
- Osmoprotectants are a group of solutes that protect cells under osmotic stress by balancing the osmotic pressure without disturbing the cell metabolism. An example is trehalose (found in Thealoz).
- Antioxidants are considered potentially beneficial due to the presence of oxygen free radicals in the tears of patients with DED. An example is vitamin A (in Hycosan Night).
- Preservatives are increasingly being removed from formulations, due to well founded evidence that chronic exposure to preservatives such as benzalkonium chloride result in toxicity and adverse changes to the ocular surface. Formulations are now more commonly being developed to be either preservative free or using newer variants of preservatives designed to have a lower impact on the ocular surface.
- Inactive agents (such as buffers) to maintain pH and keep the drop comfortable on instillation or electrolytes to reflect the tear profile and increase goblet cell density (for example TheraTears).
- Lipid supplements incorporate oils in the formulation as an emulsion to help restore the lipid layer. Different types of emulsion and lipids have been explored to try to mimic the natural meibum. Phospholipids are thought to help thicken and stabilize the lipid layer and are found in formulations (such as Systane Balance and liposomal sprays).
Biological tear substitutes
This category describes serums derived from the human body and is typically reserved for management of more severe cases such as recurrent corneal erosions, chemical burns or Stevens-Johnson syndrome.
Autologous serum is the fluid component of a patient’s own blood that remains after clotting and has the advantage of being biochemically similar to tears. Studies show that serum can enhance corneal epithelial wound healing. It has also been found to inhibit the release of inflammatory cytokines and to increase the number of goblet cells and mucin expression in the conjunctiva. Production of the serum is usually carried out at blood banks and is a costly process. It is also difficult to store, and is only stable at minus 20ºC for up to nine months. Allogenic serum and umbilical cord serum are derived from donors rather than the patient’s own blood.
Essential Fatty Acid (EFA) Supplementation
Two key EFAs are omega-3 and 6. Omega-3s exist as short chain (alpha-linolenic acid) ALA and long-chain eicosapentaenoic acid EPA and docosahexaenoic acid DHA. ALA is found in foods like flaxseed and walnuts and EPA and DHA is present in oily fish. Short chains can be converted into long chains by the body. Omega-6 is commonly derived from vegetable oils in the form
of linolenic acid LA. In the body, omega-3 and 6 compete with each other for the enzymes that regulate their metabolism and modulate inflammation. Most omega-6 derivatives are pro-inflammatory, but some are anti-inflammatory. Omega-3 EFA derivatives are anti-inflammatory, and essential for limiting and resolving inflammation. The relative ratio of 3 to 6 influences the overall inflammatory status of the body. Supplementing to improve the ratio can yield systemic anti-inflammatory benefits that can help inflammatory conditions like DED. A number of studies exist to support the use of EFA supplementation in DED, although exact dosage and protocol is still yet to be established.
Lid hygiene
The value of lid hygiene is widely accepted, but evidence is limited in terms of the correct exact strategy and optimal combinations of treatment. If used appropriately it can reduce lipid by-products and lipolytic bacteria associated with these conditions. A recent level 1 study demonstrated the efficacy of lid scrubs for removal of crusts in blepharitis with both commercial lid cleanser and baby shampoo. However, the dedicated cleanser showed reduced surface inflammatory markers, improved lipid layer quality and was better tolerated than the baby shampoo. Baby shampoo was also found to potentially have an adverse effect on goblet cell function by reducing ocular surface mucin levels. Compliance with lid hygiene instruction is notoriously poor with a study showing only 55% of patients compliant after six weeks of use.
Demodex infestation and Tea Tree Treatment
Demodex infestation is a causative factor in many cases of blepharitis and is associated with dry eye symptoms, but there is currently no evidence to link it directly with MGD. Appropriate management is with the use of topical products containing tea tree oil, which is toxic to Demodex, and has been shown to be more effective than baby shampoo at eradicating ocular Demodex. It can be toxic to the eye and causes stinging and irritation so needs to be used with caution. There is emerging evidence for the role of oral ivermectin, an anti-parasitic drug which is low cost and a single dose has been shown to reduce the number of Demodex on patients with blepharitis.
Punctal occlusion
Punctal occlusion mostly takes the form of plugs, which can be either absorbable (to test and determine the efficacy of occlusion) or non-absorbable. They are either at the level of the punctual opening or deeper within the canaliculus. Surgical occlusion can also be achieved, usually with cautery. Occlusion is a widely used tear conservation approach, and the rationale for ADDE can be understood, however its use in EDE remains controversial and the results are equivocal as to the effectiveness in improving meibomian gland status or lipid layer. Also, given the acceptance of the importance of inflammation to the continued cycle of DED, there is limited research on the potential for such intervention to impact on ocular surface inflammation by retaining inflammatory mediators on the ocular surface that needs to be considered. Although punctual plugs provide symptomatic improvement, few studies demonstrate a benefit of plugs over a comparison intervention, and no current level 1 large scale studies exist to support the contention that punctual occlusion of any form is effective in the management of DED.
MGD management
Warm compresses are proven to be effective, but compliance is often poor due to the time required and difficulty maintaining the temperature of the compress. The current suggestion is that heating the individual gland to at least 40ºC with optimal contact between the compress and the eyelid. Some studies conclude that a wet surface is best to transmit heat through to the lid, but others find that this tends to allow for more rapid cooling after heating due to evaporation from the wet lid surface. Commercial devices like Blephasteam goggles and MGDRx Eyebag have been shown to be more effective than warm towel treatment.
Expression can be used both diagnostically to assess the glands and therapeutically to help remove obstructed material. Forceful expression to help remove material is limited by the pain the patient can tolerate. A study investigating over a six month period involving four in office expressions with at home warm compressing showed the number of expressible glands, quality of secretion and lipid layer thickness significantly improved and all patients reported improved comfort and decreased symptoms. Intraductal probing can also be used to open the obstructed orifice.
Debridement scaling
One of the primary drivers of MGD is hyperkeratinization of the lid margin and duct orifices. As keratinized material builds up around and within the orifice the gland is obstructed and meibum cannot be delivered from the gland. Debridement of the lid edge along the mucocutaneous junction is believed to mechanically removing accumulated debris and keratinized cells from the lid margin, thus allowing increased flow of meibum.
In-practice devices: Lipiflow and IPL
Lipiflow is a heating and massaging device applied to the eyelid in office. An intense pulsed light (IPL) device uses light to treat the area around MG to promote activity in the gland. These have both been shown to offer potential relief when used in conjunction with at home warm compressing management.
Prescription drugs
The role of systemic antibiotics is still poorly understood and optimal dosing remains uncertain. Short doses of topical antibiotics such as ofloxacin based ointment has been shown to help in patients with obstructive MGD. Azithromycin is believed to have anti-inflammatory action rather than simply reducing the bacterial lid flora.
Tear stimulation approaches: topical and oral secretagogues
These agents trigger tear stimulation, and can be categorised as agents that stimulate aqueous, mucin and or lipids. They are not currently available in the UK.
Therapeutic contact lenses
Despite their association with dryness, contact lenses have a potential role in the management of DED. Their therapeutic role is thought to stem from the mechanical protection and reduction in corneal desiccation. CL for DED are often used on an extended wear basis and the risks of microbial keratitis must be considered. Both soft ‘bandage’ CL and scleral RGP lenses with a tear reservoir.
Anti-inflammatory therapy
Inflammation has a definite role in the vicious circle of dry eye, so it makes sense that anti-inflammatory treatment would have a role in its management. Studies have shown the clinical value of short-term use of topical corticosteroid preparations, but their long-term use is not without risk of complications. These include ocular hypertension, cataract and opportunistic infection.
Cyclosporine is an immunomodulating medication with anti-inflammatory properties, and is used typically as anti-rejection treatment in organ transplant patients, and in auto-immune diseases. Its use for dry eye has been shown to reduce inflammation and improve elevated osmolarity, and lead to recovery of goblet cell density.
Tetracycline analogues
These are broad-spectrum antibiotics that inhibit protein synthesis. It is thought that they may act to decrease bacteria-producing lipolytic exoenzymes and inhibit lipase production, which result in meibomian lipid breakdown, thus improving clinical parameters in MGD and anterior blepharitis. These agents also have anti-inflammatory properties by decreasing the production or activity of various inflammatory mediators. Studies show that they seem to be most effective when blepharitis and rosacea are present. There are currently no robust studies that demonstrate the efficacy of these antibiotics over lid hygiene or other treatments.
Azithromycin
Reports exist to highlight the positive impact of systemic azithromycin macrolide to treat MGD. A short five-day course appears to actually stimulate function of meibomian glands. Its anti-inflammatory properties may help to also control bacterial flora and lid inflammation. Topical azithromycin has also been shown to be potentially effective in MGD management.
Environmental factors
Thought should be given to modifying environmental elements that could have a role in the DED. These include chronic topical preserved medications (such as glaucoma management), systemic medications, blink rates, air conditioning and pollutants, contact lens wear.
INTERACTIVE EXERCISE
This exercise is designed to encourage discussion between all categories of registrant as each group might be expected to deal with the challenges either within or outside the consulting room. Obviously, each would be expected to answer according to their particular professional responsibility.
Before you attempt the exercise, there are six multiple choice questions which assess an overall understanding of this subject.
