Neurological lesions that produce visual field defects might be defined as those that affect the intracranial visual pathway (the pathway from the intracranial part of the optic nerve back to the visual cortex). However, it may be more practical to include the whole of the optic nerve within the definition. Disorders that affect the visual pathway produce characteristic visual loss, which together with the associated neurological deficits, can help to identify the location of the problem.
The analysis of visual field defects has an anatomical basis and a topographical classification. A basic working knowledge of the anatomy of the visual pathway is vital to the task of visual field analysis. The major elements that make up the pathway, and particularly the distribution of nerve fibres within them, are the link between the defects you have plotted and your assessment of the position of the lesion producing them.
Anatomy
The visual pathway starts at the photoreceptors in the retina and finishes at the neural cells in the visual cortex. It forms an integral part of the central nervous system (CNS) and, as such, has features that are common to all CNS structures.
The extraocular part of the pathway is made up of a number of distinct and well- recognised anatomical components (figure 1). They are the:
- optic nerve
- optic chiasma
- optic tract
- lateral geniculate nucleus (or body)
- optic radiations
- visual (or striate) cortex
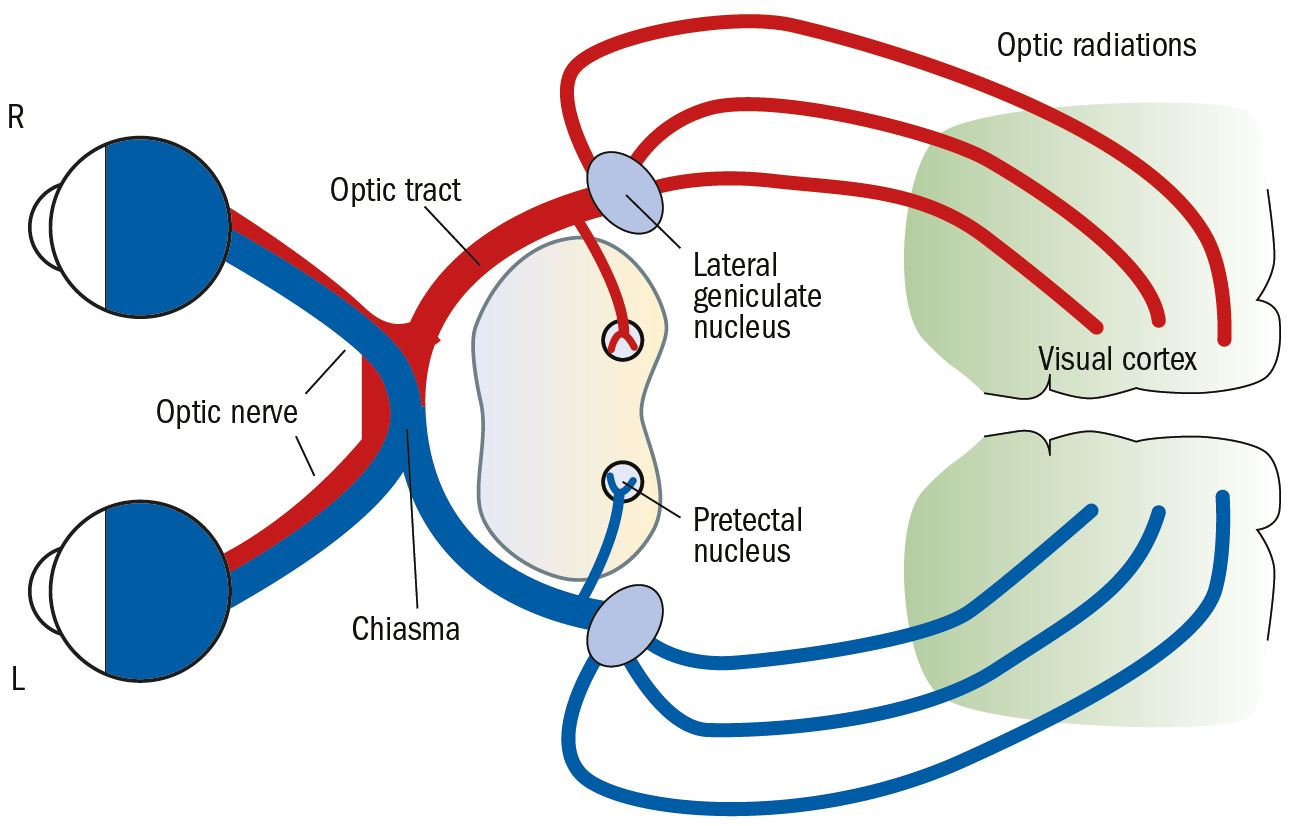
Figure 1: Main components of the visual pathway
We will now take a look at each of these in more detail.
Optic nerve
The optic nerve is approximately 50mm long and 3mm in diameter. The intraorbital portion is about 30mm long and the intracranial portion of the nerve is about 10mm long. The nerve is made up of about one million fibres, which are divided into approximately 1000 bundles. The intraorbital portion of the nerve is more or less oval in section and, as the distance from the globe to the back of the orbit is only about 18 to 20mm, it curves in an S shape to pass through the optic canal in the sphenoid bone. The extra length and slight zigzag in the nerve allows for flexing during ocular movements.
In the main, the fibres of the optic nerve are afferent visual fibres that comprise the myelinated axons of retinal ganglion cells. There are, however, some afferent pupillary fibres and some efferent fibres, the function of which is not well understood. The organisation of fibres within the optic nerve initially follows the arrangement of nerve fibres at the optic nerve head with the macular fibres from the papillomacular bundle on the lateral or temporal aspect of the nerve (figure 2).
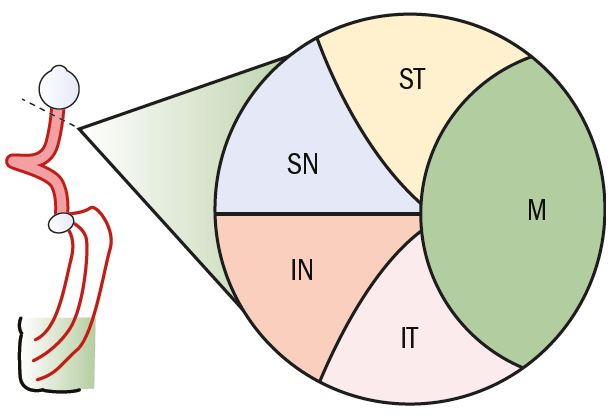
Figure 2: Cross-section of the anterior optic nerve showing the distribution of nerve fibres (SN = Superior nasal; ST = Superior temporal; M = Macular; IT = Inferior temporal; IN = Inferior nasel)
About one-third of the way along the nerve, at the point where the central retinal artery and vein move into the nerve, the fibres are reorganised so that the macular fibres move into a central position, with the peripheral fibres arranged in the same orientation to the portion of retina where they originated (figure 3).
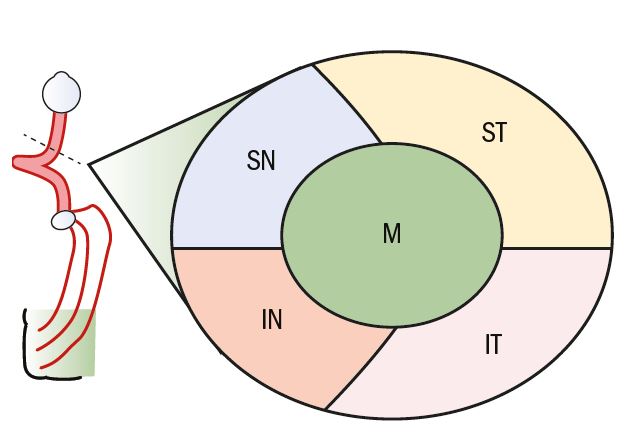
Figure 3: Cross-section of the posterior optic nerve showing the distribution of nerve fibres
Chiasma
At the chiasma, which is approximately 12mm wide by 8mm deep, the two optic nerves meet and some fibres cross over to the other side of the pathway. The crossing of just over half of the nerve fibres enables the stimulation of corresponding points in the visual field of the two eyes to send signals to the same half of the visual cortex. This arrangement is common in animals with frontward facing eyes, such as cats and primates, and allows binocular vision with the perception of stereopsis. In animals with little overlap of the monocular visual fields, such as rodents, nearly all the fibres (perhaps 90 to 95 per cent) cross.
At the chiasma, fibres that originate in the temporal retina, relating to the nasal visual field, remain at the outside of the pathway, while those that originate in the nasal retina, relating to the temporal visual field, cross over or decussate. The number of decussating fibres is greater than the number of non-decussating fibres, which reflects the larger peripheral temporal field. Fibres from the nasal half of the macula also decussate in the same way as the peripheral fibres. The nerve fibres in the chiasma are arranged in a recognisable format and retain groupings based on the area of the retina where they originated (figure 4).
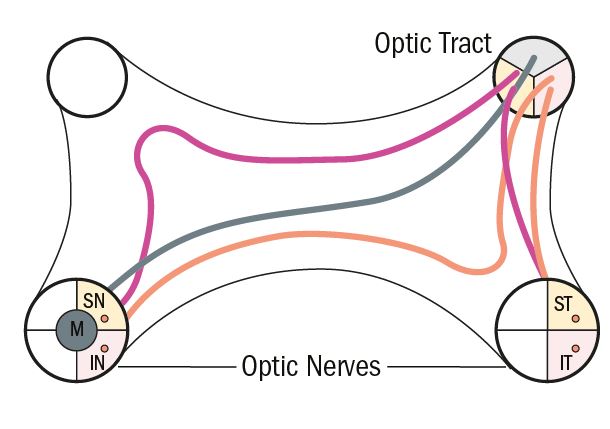
Figure 4: General arrangement of fibres at the chiasma
Fibres that originate from the inferior nasal part of the retina and decussate in the more anterior portion of the chiasma tend to loop slightly forward into the posterior part of the contralateral optic nerve. This forward looping is called the anterior knee or loop of Willebrand. Fibres that pass more posteriorly through the chiasma tend to loop posteriorly into the ipsilateral optic tract.
A number of important structures that do not form part of the visual pathway are situated near to the chiasma. Among these are a number of important blood vessels (see later) and the pituitary gland which resides in its bony fossa. The length of the intracranial optic nerves dictates the position of the optic chiasma in relation to the pituitary fossa. In most cases it is underneath and slightly anterior to the chiasma. In about 10 per cent of cases it is further forwards and, in about 10 per cent, it is further back.
Optic tract
The optic tract consists of nerve fibres from one-half of the visual field only, but still retain a recognisable organisation, with the macular fibres uppermost, the upper peripheral fibres on the inferior medial aspect and the lower peripheral fibres occupying the inferior lateral aspect (figure 5). The optic tract bends the pathway around the brainstem. About 10 per cent of the fibres leave each half of the visual pathway at the posterior end of the optic tract on the medial side (see figure 1). These are predominantly fibres that pass to the pretectal nucleus in the brain stem and are associated with the pupillary light reflex. The remainder of the optic tract ends at the lateral geniculate nucleus.
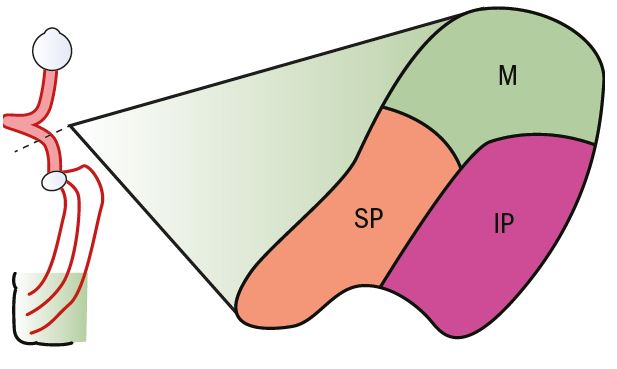
Figure 5: Cross-section of the optic tract showing the distribution of nerve fibres (M = Macular; IP = Inferior periphery; SP = Superior periphery)
Lateral geniculate nucleus
The lateral geniculate nucleus (LGN), sometimes called the lateral geniculate body, lies on the posterolateral aspect of the brain stem and next to the thalamus. It is generally thought of as a relay station at which nerve fibres that originate from the retinal ganglion cells synapse with fibres that originate from cells in the visual cortex, although there is evidence that some degree of low-levelvisual processing does go on within it.
It has a complex six- layered structure that contains nerve fibres and cell nuclei (figure 6). Fibres from the two eyes are clearly segregated in the LGN. Those that passed through the chiasma uncrossed, that is from the temporal retina on the same side, go to layers 2, 3 and 5, and the fibres from the nasal retina, which have crossed at the chiasma, go to layers 1, 4 and 6.
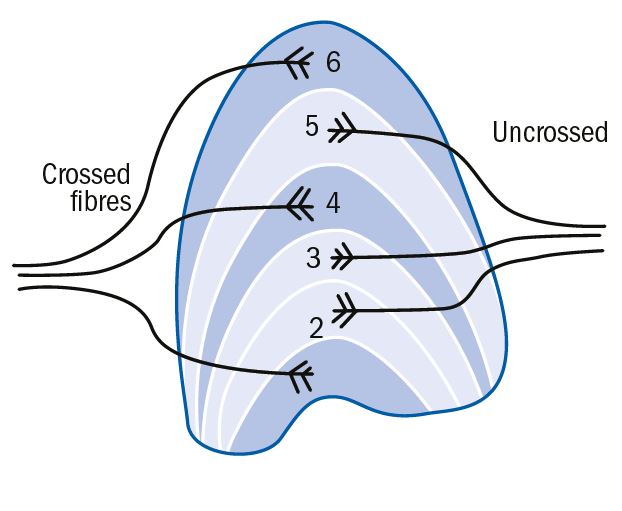
Figure 6: Six-layered structure of lateral geniculate nucleus showing the destination of crossed and uncrossed fibres
Each LGN neurone makes contact with a similar number of retinal nerve fibres, always from the same eye, and receives inputs from retinal cells adjacent to each other in a circular pattern. The LGN’s prime function is to sort out fibres that have become mixed up in the optic tract so that there is almost perfect ‘point-to-point’ representation of the retina in the LGN, albeit in adjacent layers.
Optic radiations
The optic radiations continue the visual pathway from the LGN to its end at the neurones of the visual cortex. The fibres leave from the lateral aspect of the LGN and spread out in a fan formation, still retaining a retinotopic projection (figure 7). The upper fibres of the optic radiations from the superior peripheral retina move around the lateral ventricle and steadily back to the visual cortex, ending in the area above the calcarine fissure. The fibres from the lower peripheral retina pass underneath and around the bottom of the lateral ventricle, looping backwards and ending in the area of the visual cortex below the calcarine fissure. The fibres from the macula pass to the cortex in between the upper and lower peripheral fibres.
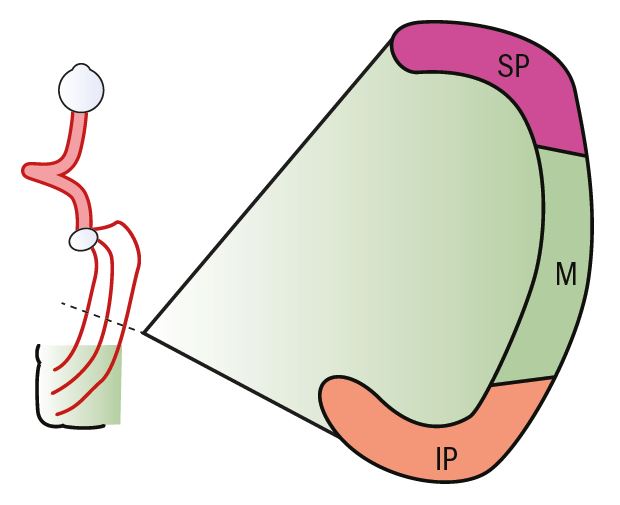
Figure 7: Cross-section of the optic radiations showing the distribution of nerve fibres
Visual cortex
Visual information is processed in the cortex in three main areas. The primary visual cortex is Brodmann’s area 17 with two secondary areas, Brodmann’s areas 18 and 19. The visual cortex, area 17, is situated on the medial aspect of the occipital lobe. The visual cortex retains the retinotopic organisation seen in the more anterior parts of the pathway. The peripheral parts of the retina are represented in the more anterior parts of area 17, with the upper and lower retina represented either side of the calcarine fissure (figure 8). The macular area is represented by a larger portion of the visual cortex occupying the posterior part of area 17 and extending around the posterior pole slightly. Again, the upper and lower fibres are separated by the calcarine fissure.
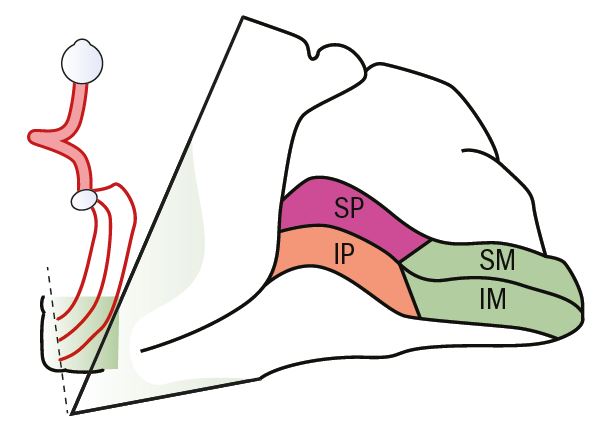
Figure 8: Visual cortex showing the general organisation of nerve fibres (SP = Superior periphery; IP Inferior periphery; SM Superior macular; IM inferior macular)
In the visual cortex corresponding retinal points finally meet and become perfectly matched by location. The point-to-point relationship between retinal receptors and cortical cells is also maintained. Most cortical cells (about 70 per cent) have directional sensitivity and are also sensitive to a variety of other characteristics, only responding to particular stimuli, such as light or dark edges. Cells in the visual cortex are organised into functional groups, each with a specialised purpose; some only respond to stimuli orientated in one particular direction, others to a specific colour response.
There is some evidence of cross linking of receptive fields in the right and left halves of the visual cortex, either directly or indirectly through the corpus callosum, which might help explain the clinical phenomenon of macular sparing.
Blood supply
An understanding of the blood supply to the visual pathway is important for the clinician. Many of the major blood vessels that supply the visual pathway are physically close to the individual components of the pathway. Thus, not only may clinically significant problems arise if the blood supply is interrupted or disturbed, but a physical abnormality of the blood vessel itself can also cause direct mechanical effects on the pathway.
The blood supply to the visual pathway is derived from three major intracranial vessels, the two internal carotid arteries and the basilar artery, which comes up the front of the brainstem from an anastomosis of the two vertebral arteries. These three vessels are joined by a system of smaller vessels into a complete circuit of interconnecting arteries called the Circle of Willis (figure 9). The more anterior parts of the Circle of Willis are very closely situated to the optic chiasma.
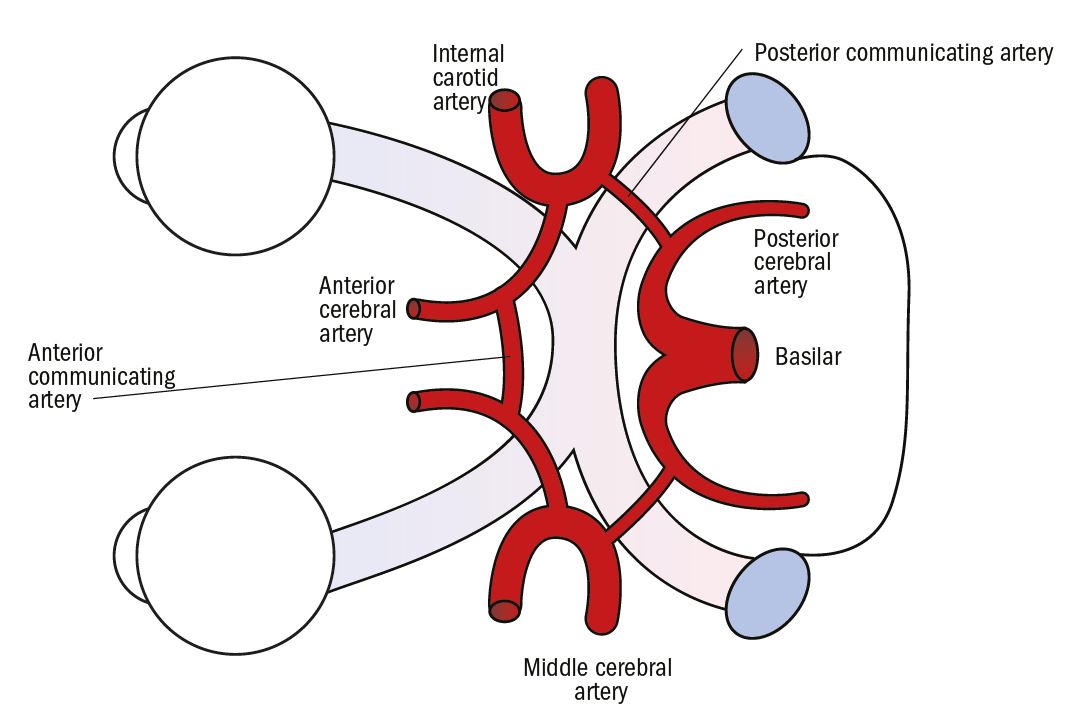
Figure 9: Blood vessels of the Circle of Willis seen from underneath
The LGN is supplied by branches of the anterior choroidal and posterior cerebral arteries. The anterior parts of the optic radiations are supplied by branches of the anterior choroidal artery and the more posterior parts are supplied by a long branch of the middle cerebral artery, called the deep optic artery.
The visual cortex is supplied mainly by the calcarine artery, which is a branch of the posterior cerebral artery. Some supply is also derived from the middle cerebral artery, which has an anastomosis with the posterior cerebral artery (figure 10). This ‘dual’ blood supply may help to maintain arterial circulation to the visual cortex in the event of a disruption to the supply from one of the vessels, and is also thought to be another possible explanation for macular sparing.
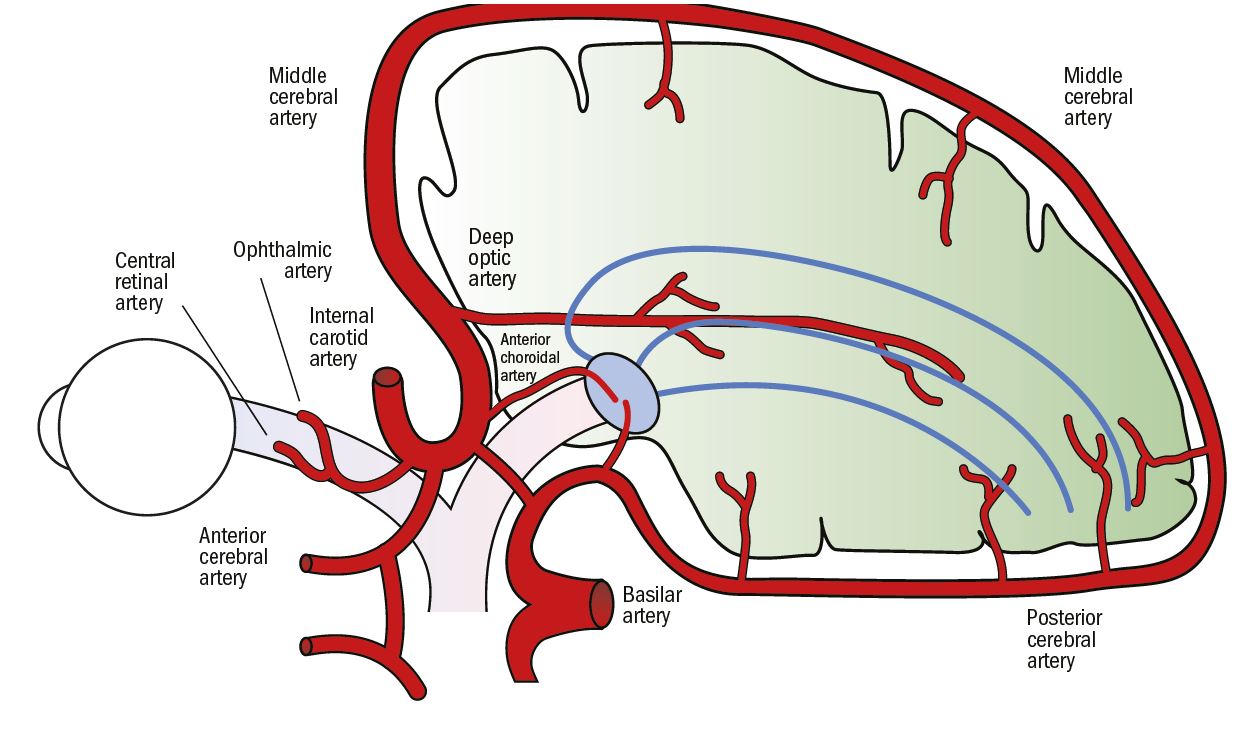
Figure 10: Vascular supply to the visual pathway
Visual field analysis
Before deciding that a visual field defect results from a neurological lesion, all of the available clinical information must be considered. The importance and relevance of signs, symptoms, history and other findings may be key to the correct interpretation of visual field defects. As in the case of glaucoma, for which the appearance of the optic discs, intraocular pressure and family history are taken into consideration when deciding if field defects are glaucomatous, the presence or absence of other factors may be the key to deciding if a defect is genuinely caused by a neurological abnormality or not. Many non-neurological abnormalities, such as tilted discs, can give rise to ‘pseudo’ neurological field defects, such as irregular temporal loss in each field.
History may indicate the time of onset and duration of a dramatic event like a vascular accident, although history may be so vague as to be of little diagnostic value. Personal history, such as stroke or cranial trauma, may also be directly relevant to findings.
Ultimately, the diagnosis must be made by a neuro-ophthalmologist or a neurologist with the aid of sophisticated, modern neuro-imaging techniques, but it is important that the correct degree of urgency is attached to the initial referral.
The results recorded may show genuine abnormalities or artefacts. A stimulus point seen at 1 or 2 decibels below the retinal threshold would not, in normal circumstances, be considered significant. However, a small group of stimuli that are missed close to threshold might signify a defect. In comparison, one isolated point seen at a markedly lower setting than all others is probably not a defect despite its low sensitivity, but an anatomical feature such as a blood vessel. Consideration of a specific defect in the field plot should look at four separate components:
- Shape. The shape of the field defect plotted is very important, as it may be directly related to anatomy and physiology. In the case of neurological lesions, a comparison of the shape of both defects is an element in judging congruity.
- Size. The similarity of size and shape is helpful in neurological defects. Field defects of different size, even if they are the same shape, do not indicate congruity. Size is also important when considering the extent of the lesion and its age.
- Margins. The steepness of the margins of a defect gives specific information primarily about the type of causative lesion. Shallow margins suggest a slowly developing lesion, such as a space- occupying lesion, while steep margins are more likely to be found with a vascular accident.
- Depth. Deep, almost absolute, scotomata are usually associated with vascular accidents.
A number of other clinical findings may need to be taken into account in the analysis of neurological defects;
- symptoms and history
- visual acuity (VA)
- ophthalmoscopy
- pupil responses
- colour vision
Symptoms of diagnostic value, if present, are either related to the actual field loss, such as mobility problems, clumsiness and positive scotomata, or unrelated, such as headaches. Vascular accidents are of rapid onset and, therefore, the field defects are more likely to be noticeable to the patient, while those caused by slowly developing mass lesions are not.
The topographical classification simply subdivides the extraocular visual pathway into three general sections:
- pre-chiasmal
- chiasmal
- post-chiasmal
The predictable grouping and organisation of the visual pathway nerve fibres, which steadily increases as the pathway moves towards the visual cortex, is helpful for analysis.
Pre-chiasmal
Defects in this category are essentially caused by optic nerve lesions, and are likely to show all or some of the following characteristics:
- One eye only. This is true of almost all pre-chiasmal defects. Classically, a central or centrocoecal defect is found (figure 11). A lesion near the posterior end of the optic nerve may produce a defect in both eyes, when anterior looping nasal fibres from the contralateral nerve become affected. In this case a significant defect is present on the affected side, combined with an upper temporal crescent-shaped defect in the periphery on the other side; the so- called ‘junction’ defect.
- Reduced VA. This is likely, as macular fibres are much more susceptible to damage because of their fineness and thinner sheathing.
- Afferent pupillary defect. A relative afferent pupillary defect (RAPD) is highly likely because any lesion serious enough to produce a field defect will affect the pupillary fibres.
- Colour vision defect. This is likely to be present if there is reduced VA.
- Normal ophthalmoscopy. Retrobulbar optic nerve lesions won’t produce any fundus changes. Optic atrophy may give rise to an abnormal disc appearance on ophthalmoscopy. However, the onset of optic atrophy will not occur for many weeks after the initial symptoms. Defects may be caused by meningiomas (of the optic nerve sheath or the sphenoid bone), arise as a result of previous radiation therapy in the region, or (less commonly) as a result of intrinsic disease such as demyelination.
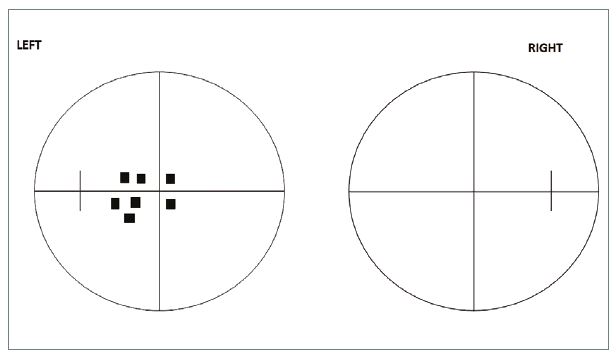
Figure 11: Centrocoecal defect
Chiasmal
The chiasmal part of the pathway is much more likely to be affected by abnormalities than any other pathway, and the vast majority are compressive lesions. Defects caused by lesions that affect the chiasma show the following general characteristics:
- In both eyes. As fibres from both eyes are now present in the pathway, there must be a defect of some sort present in both eyes. The ‘junction’ defect described above is sometimes classified as a chiasmal defect
- Heteronymous. Present in both eyes, but essentially on opposite sides of the field (both nasal halves, or both temporal halves of the field)
- Midline not respected. As a result of the juxtaposition of nasal and temporal fibres, eventually a lesion will have some influence on all the fibres from both of the eyes. Field defects therefore progress from one side to the other across the midline. Chiasmal defects cannot be true hemianopias
The most common chiasmal lesion is due to a pituitary tumour. As the chiasma is affected by pressure from underneath, the nasal fibres from each eye are primarily affected and a temporal defect is found in both eyes, the classic bitemporal hemianopia (figure 12). The hemianopia is often asymmetrical and may be associated with significant visual loss in one eye from involvement of an optic nerve. In most cases the field defect starts in the upper temporal regions and spreads to the lower temporal quadrants, lower nasal quadrants and eventually the whole field is lost. Patients rarely notice any direct symptoms, but may have general complaints about navigation or problems driving. Pituitary tumours are large by the time they affect the chiasma, and around two-thirds of them are associated with a variety of hormonal abnormalities including diabetes.
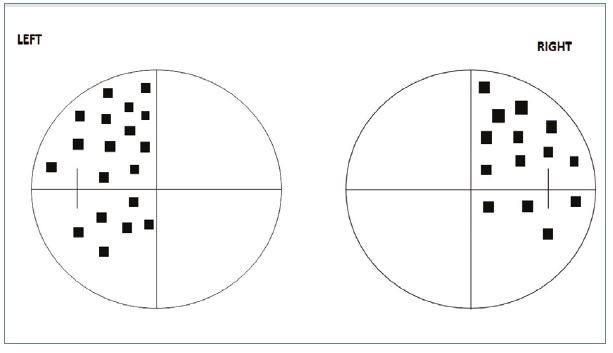
Figure 12: Bitemporal hemianopia
Post-chiasmal
Post-chiasmal neurological lesions affecting the visual pathway may produce defects that are hemianopic, quadrantanopic or scotomata. They show the following characteristics:
- In both eyes. The defect is present in both eyes
- Homonymous. The defect is always on the same side in both eyes (ie, the tem- poral field of the right eye and the nasal field of the left eye)
- Midline respected. Defects, if they are big enough, always show perfect demarcation down the vertical midline and not cross over
Post-chiasmal lesions may produce effects (such as reduced VA) if macular fibres are affected, and a pupil defect if the lesion is in the optic tract, but not necessarily. It is useful to be able to further subdivide post-chiasmal lesions. Although this is more difficult to do, some characteristics of the visual field defect and other clinical findings may help.
Pre-lateral geniculate nucleus
Here, field defect characteristics include:
- Incongruous. The defects are not similar in size and/or shape (figure 13).
- Possible macular splitting. The VA may be reduced because the macular fibres are affected.
- Pupil anomalies. If the lesion is in the tract, then the pupillary fibres may be affected and there will be a RAPD in the contralateral eye because of the greater number of crossed fibres.
- Possible optic atrophy. This is likely to take the form of localised rather than general atrophy of the optic disc. Its presence indicates a slow growing compressive lesion. Isolated optic tract lesions are rare and usually caused by a space-occupying lesion such as a glioma. Occasionally, defects result from demyelination or vascular abnormalities.
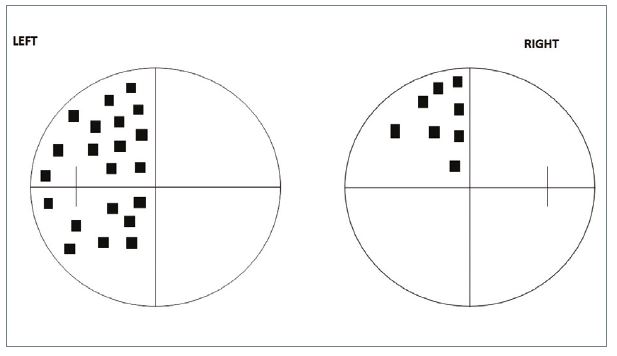
Figure 13: Incongruous defect
Post lateral geniculate nucleus
Defects here may show;
- Increasing congruity.
- Increasing macula sparing.
- Pupil reactions normal. Even in the presence of cortical blindness.
- No optic atrophy.
The optic radiations pass through the parietal and temporal lobes of the cortex, and patients with lesions in these regions tend to present with other neurological deficits that overshadow their visual loss. These include:
- Parietal lobe. Agnosia (the loss of the ability to recognise visually), agraphia (the inability to interpret writing), acalculia (the inability to do arithmetic), right and left confusion.
- Temporal lobe. Aphasic motor abnormalities (the muscular inability to form words), epilepsy (seizures characterised by unusual tastes and smells), déjâ-vu; hallucinations
As the superior peripheral fibres are found in the parietal lobe, the visual field defects tend to be found in the lower half, predominantly quadrantanopic.
The inferior peripheral fibres pass through the temporal lobe (described as Meyer’s loop) and the visual field defects are therefore found in the superior half of the field (figure 14). This type of defect is described by some authors as a ‘pie in the sky’ defect.

Figure 14: Field defect due to temporal lobe lesion
Parietal lobe lesions may also be characterised by visual neglect, a condition in which if two simultaneous stimuli are presented in the contralateral visual field, only one is seen at a time.
Visual cortex
Lesions in the visual cortex are characterised by:
- Complete congruity. Field defects, be they scotomata or hemianopias, show complete congruity because of the exact matching of corresponding retinal points in the visual cortex (figure 15).
- Macular sparing. The preservation of a small (2 to 3°) field around the macula. Patients will have normal acuity. There are a number of possible explanations as to why the clinical phenomenon of macular sparing is found. The dual blood supply to the occipital cortex already mentioned is likely to be a preserving factor.
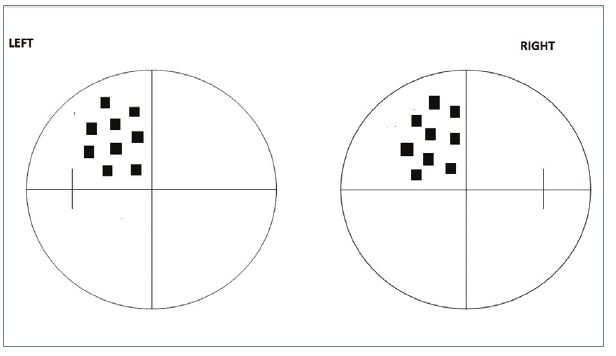
Figure 15: Full congruity
Also, the cross-linking of the right and left halves of the visual cortex and the sheer size of the macular representation in the visual cortex mean that a huge amount of cortex needs to be destroyed before macular function ceases. Some authors have also speculated that psychological factors associated with maintaining fixation are involved. It is likely that a combination of these mechanisms is responsible for macular sparing found in an individual patient.
Most lesions that affect the visual cortex are vascular and present as characteristic visual field loss without other neurological signs.
Conclusion
Neurological visual field defects can be analysed by the application of a simple knowledge of the anatomy of the visual pathway. A range of characteristic visual field defects, together with other clinical findings and specific signs and symptoms, can be used to target the location of the underlying disease process. Although final diagnosis must rest on the use of neuroimaging technology, a confident preliminary identification of neurological lesions that cause visual field defects, leading to an appropriate referral process, can be initiated by optometrists using visual field screeners in their own practice.
Geoff Roberson is an optometrist formerly in private practice, and former adviser to DOCET, visiting lecturer at City University and director of the Institute of Optometry.
