It has been well reported that cognitive functionality, visual functioning, awareness and memory are tightly bound within the brain’s processing centres, working in a harmonious and faultless manner in order to provide an effective and accurate image of the world around us.
Cognition and Ageing
Vision, and subsequent visual perception, is created via a network of 30 different brain areas across a number of associated cortices working simultaneously by combining various elements into what has been called the ‘blackboard of the mind’.1 Some neuroscientists view working memory (a form of short term memory, which mediates visual awareness) as the blackboard which holds visual precepts and other sensory inputs to create real time conscious awareness. Working memory allows us to make sense of what we see by combining visual stimuli with memories. Therefore, when vision declines, cognition is impaired as the brain simply cannot create a clear interpretation of the world around us.2
The normal cognitive changes associated with ageing have been well documented in the literature and they involve a gradual decline in the conceptual reasoning, memory, and processing speed (figure 1).

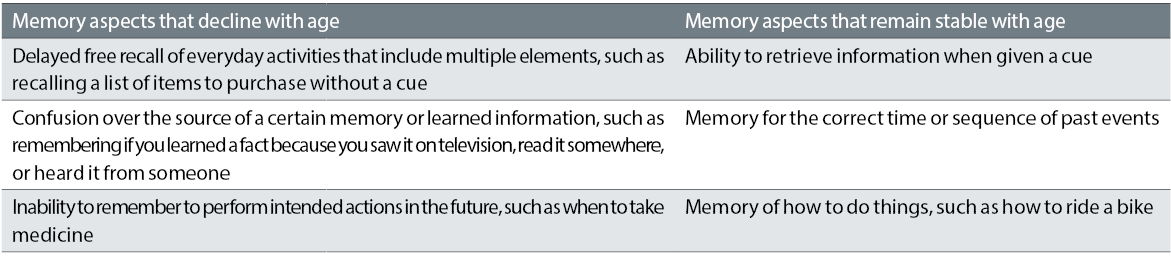
One of the most common cognitive complaints among older adults is change in memory (see table 1).
Both memory and vision are normally affected as we get older. Slowing in visual processing speed is a common characteristic of ageing. Many older adults require more time than younger adults to detect, discriminate, recognise, or identify visual targets. This contributes to higher-order processing problems characteristic of cognitive ageing and occur even in individuals that will not develop dementia. Studies have demonstrated that ageing related slowing in visual processing speed is exacerbated by increasing attentional task demands (such as distracting stimuli).4 In addition, many older adults have deficits in visually processing temporal information, which can hamper the visual performance of everyday tasks. For example, slower visual processing speed in older adults increases their risk for motor vehicle collision involvement even in the absence of an impaired visual acuity, contrast sensitivity or visual sensitivity. It also increases the time it takes them to complete visual tasks of everyday living such as finding an item on a shelf or reading a prescription bottle.
Vision and Cognitive Impairment
Various ocular conditions associated with ageing, such as AMD and cataract, result in various degrees of visual impairment. ‘Low vision’ is one of many terms used to describe the visual deficit experienced by somebody while wearing the best correction possible, which affects their performance in visual tasks to a point whereby it is inadequate for the person’s needs.5 Vision loss affects approximately two million individuals in the United Kingdom, with 18% of those registered as sight impaired or severe sight impaired.6
Among other adverse influences upon the general health of the affected individual, it has also been suggested that low vision is linked, to various degrees, with deficits in memory and cognition. Moreover, it has been suggested that subjects with good visual acuity (VA) are at a 63% decreased risk for developing dementia over an 8.5 year period than those with reduced vision.7 Even in children, word meanings are more limited in those with visual impairments than for normally sighted children.8
The Beijing Eye study performed in 2018 concluded that higher cognitive function correlates with the following:
- Younger age
- Higher level of education
- Rural region of habitation
- Lower scores of depression
- Greater physical activity
- Higher level of best corrected visual acuity
- Lower levels of under-corrected visual acuity
- Lower prevalence of primary angle-closure glaucoma
- Lower degree of fundus tessellation (or with thicker subfoveal choroidal thickness as a surrogate)
Nevertheless, the study also showed that prevalence of AMD, open-angle glaucoma, diabetic retinopathy, nuclear cataract, cortical cataract, posterior subcapsular cataract, retinal vein occlusions or pseudoexfoliation were not significantly associated with cognitive function.9 The authors concluded that the major ocular factors associated with cognitive dysfunction were a reduced best corrected visual acuity and a higher amount of under-corrected visual acuity (figure 2).

Figure 2: Cognitive dysfunction is associated with a reduced best corrected visual acuity
Poorer cognition was also correlated with the presence of primary angle-closure glaucoma. The authors found the only possible explanation for this was that these patients have generally a lower body height, and that this was associated with a lower socioeconomic background and so correlates with a lower cognitive function score.10 However, this study only considered Northern Chinese subjects and, therefore, the results cannot be generalised.
What can we do?
To be effective, any treatment should be introduced at an early stage in the condition and not when conditions such as Alzheimer’s disease (AD) or other dementias have already occurred. The National Institute of Health and Clinical Excellence (NICE) has identified a specific need (NG16) for early identification and easy access to interventions.14 In addition, the College of Optometrists highlighted the need to adapt assessments for people with both vision impairment and dementia.15 However, these are recommendations for patients with dementia and vision loss and do not refer to individuals with early cognitive decline.
Most of optometric practices now have an OCT that optometrists use to assess various posterior segment structures, including the RNFL (figure 4).
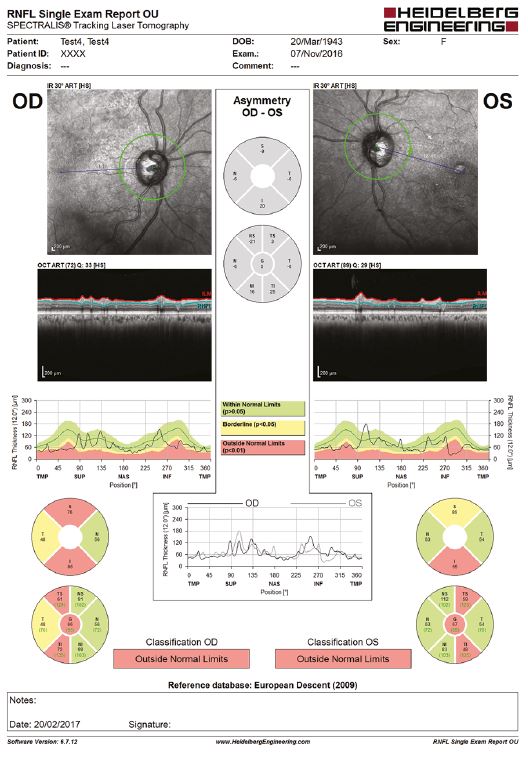
Figure 4: Thinning of the RNFL, as measured by an OCT, has been associated with worse baseline cognitive performance
It has been demonstrated that a thinner RNFL was associated with worse baseline cognitive performance. In addition, at three years follow-up, subjects in the two thinnest RNFL quintiles were nearly twice as likely to fail one or more cognitive tests. Retinal vascular analysis is also anticipated to serve as a biomarker for early detection of AD.20
Vascular attenuation, increasing standard deviation of the vessel widths, abnormal branching pattern, increase in venular width gradient and decrease in arterial fractal dimension were all reported in patients with AD.21 Patients with AD or mild cognitive impairment (MCI), also demonstrated lower retinal blood flow rate and velocity in precapillary arterioles and postcapillary venules in patients with AD and MCI compared with controls.22 Although blood flow is difficult to assess in the average optometric practice, retinal vascular architecture is easier to analyse.
Subjective cognitive decline (SCD) assessment, which is the recording of self-reported experience of worsening or more frequent confusion or any memory loss within the past 12 months, is a good method to quickly spot people suffering from such problems attending an optometric practice. The correlation between results obtained using SCD and those using objective methods are inconsistent. However, it has been highlighted that, in practice, detecting cognitive decline in individuals with vision or hearing loss using objective methods is limited due to the fact that many of these cognitive tests, such as Mini-Mental State Exam (MMSE), rely on visual skills.16 Therefore, SCD could be used as a surrogate tool in order to quickly determine the effect of sensory loss on patients’ cognition when they attend an optometric practice and not a memory clinic. To quote one researcher: ‘Assessing for memory and confusion complaints may also provide insight into a patient’s awareness and perception of cognitive health, psychological status, and overall well-being, allowing ophthalmologists and other providers to give more individualised advice regarding health promotion and medication adherence.’17
There are other psychometric tests that were developed with low vision in mind, such as the Blind Learning Aptitude Test (BLAT),18 the Cognitive Test for the Blind (CTB), and the Interim Hayes-Binet intelligence scale. Some of these tests can be used with both children and adults, but have various limitations and cannot be used by patients with dual sensory loss. This is an area than needs to be improved by technologies of the future.
Meanwhile, SCD can be used as an alternative. The SCD questionnaire usually starts with ‘During the past 12 months, have you experienced confusion or memory loss that is happening more often or is getting worse?’ Respondents with SCD are then asked two follow-up questions:
- ‘During the past 12 months, as a result of confusion or memory loss, how often have you given up day-to-day household activities or chores you used to do, such as cooking, cleaning, taking medications, driving, or paying bills?’
- ‘During the past 12 months, how often has confusion or memory loss interfered with your ability to work, volunteer, or engage in social activities outside the home?’
Responses of ‘always,’ ‘usually,’ and ‘sometimes’ are classified as positive responses, and responses of ‘rarely’ and ‘never’ are classified as negative responses.19
Hearing loss is also often associated with vision impairment in older adults and this dual sensory loss contributes even more to a high prevalence to cognitive decline and dementia in the affected individuals. Indeed, about 70% of patients attending memory clinics complain of one or another and report the disastrous effect these disabilities have on their quality of life.11 Therefore, even simple measures, such as wearing reading glasses or hearing aids when needed, could contribute to the prevention of cognitive decline due to old age (figure 3).12 Although considered somewhat controversial in the past, more recent research has suggested that cataract surgery may have a positive impact on cognition. Indeed, improvements in the visual and cognitive-related brain areas were reported three months after cataract surgery in elderly patients.13

Figure 3: Even simple measures such as the introduction of a hearing aid may help slow cognitive decline
Eye care providers could play an important role in detecting risk of cognitive decline in patients at risk, even more than the audiologists, because eye tests are performed more often than hearing tests. Nevertheless, optometrists lack training, awareness and also rarely work together with other specialities, such as audiologists, geriatricians or psychiatrists. In addition, there is a growing need for the memory clinics to adopt a patient-centred care and include vision and hearing testing into their practice.23
A survey of European and North American professionals representing all three domains of hearing, vision and dementia care identified that need of having a ‘sensory-cognitive’ team approach, with professionals from all three domains working seamlessly together and sharing a common knowledge base.’23 And although there is yet no consistent scientific evidence for the impact of hearing/vision interventions on cognitive function, rate of cognitive decline, quality of life, or caregiver burden, clinical evidences are often pointing to more positive conclusions. Therefore, every effort should be made to offer a personalised care to all our patients. In addition, further training and interdisciplinary work could also contribute to better outcomes.
Dr Doina Gherghel is an academic ophthalmologist with special interest in inter-professional learning for optometrists. She also leads the new Geriatric Optometry module at Aston University. For further information, email pgadmissions@aston.ac.uk
References
- Rosen PN. Vision Screening for Alzheimer’s Disease: Prevention from an Ophthalmologist’s Perspective (There is More to Vision than Meets the Eye). The Permanente Journal. Winter 2004. 8 (1) :15-21
- Asensio-Sánchez VM, Torreblanca-Agüera B, Martínez-Calvo S, Calvo M J, Rodríguez R. Visual symptoms as the first manifestation of Alzheimer’s disease. Arch soc Esp Oftalmol 2006; 81: 169-172
- Harada CN, Natelson Love MC, Triebel K. Normal cognitive aging. Clin Geriatr Med 2013; 29:737-752
- Owsley C, Sloane M, McGwin G, Ball K. Timed Instrumental Activities of Daily Living Tasks: Relationship to Cognitive Function and Everyday Performance Assessments in Older Adults. Gerontology 2002;48:254–265
- Jackson A, Wolffsohn, J. Low Vision Manual. Philidelphia: Elesevier; 2007. Pg1-13
- Leamon S, Davies M. Research briefing: Number of adults and children certified with sight impairment and severe sight impairment in England and Wales: April 2012 to March 2013. RNIGB and Moorfields Hospital NHS Foundation Trust; 2013. Report No.: 5.
- Rogers MA, Langa KM. Untreated poor vision: a contributing factor to late-life dementia. Am J Epidemiol 2010; 171:728–735
- Anderson, ED, Dunlea, A, Kekelis, LS. (1984) ‘Blind children’s language: resolving some differences.’ Journal of Child Language, 11, pp 45-64
- Jonas JB, Wei WB, Ping Zhu L, Xu L, Wang YX. Cognitive Function and Ophthalmological Diseases: The Beijing Eye Study. Scientific reports Nature 2018; 4816
- Xie, XW, Xu, L, Wang, YX. & Jonas, JB. Body height and ocular diseases. The Beijing Eye Study. Graefes Arch. Clin. Exp. Ophthalmol. 2009; 247, 1651–1657
- Leroi I, Pye P, Simkin Z, Hann M, Regan J, Dawes P. SENSE-Cog: Exploring the needs of individuals living with dementia and sensory impairment. Abstract. 5th annual BIT conference on Geriatrics and Gerontology, Fukuoka, 2017
- Spierer O, Fischer N, Barak A, Belkin M. Correlation Between Vision and Cognitive Function in the Elderly. A Cross-Sectional Study. Medicine (Baltimore). 2016 Jan; 95(3): e2423.
- Lin H, Zhang L, Lin D, Chen W, Zhu Y, Chen C, Chan KC, Liu Y, Chen W. Visual Restoration after Cataract Surgery Promotes Functional and Structural Brain Recovery. EBioMedicine. 2018 Apr;30:52-61
- NICE Guidelines. Dementia, disability and frailty in later life – mid-life approaches to delay or prevent onset. https://www.nice.org.uk/guidance/ng16/chapter/1-Recommendations. Accessed 25th August 2019
- Bowen M, Edgar DF, Hancock B, Haque S, Shah R, Buchanan S, Iliffe S, Maskell S, Pickett J, Taylor JP, O’Leary N. The Prevalence of Visual Impairment in People with Dementia (the PrOVIDe study): a cross-sectional study of people aged 60–89 years with dementia and qualitative exploration of individual, carer and professional perspectives. Southampton (UK): NIHR Journals Library; 2016 Jul.
- Mayniel S, Samri D, Stefano F et al. COGEVIS: A New Scale to Evaluate Cognition in Patients with Visual Deficiency. Behavioural Neurology Volume 2018, Article ID 4295184
- Lee MJ, Varadaraj V, Ramulu PY et al. Memory and Confusion Complaints in Visually Impaired Older Adults: An Understudied Aspect of Well-Being. Gerontol Geriatr Med. 2019 Jan-Dec; 5: 2333721418818944
- Mason, HL, and Skukla, SR. The Use of the Blind Learning Aptitude Test in England and Wales, India and the USA. British Journal of Visual Impairment, 1992; 10: 95-99
- Taylor CA, Bouldin ED, McGuire LC. Subjective cognitive decline among adults aged ≥45 years—United States, 2015–2016. MMWR Morb Mortal Wkly Rep 2018;67:753–7
- Frost S, Kanagasingam Y, Sohrabi H, et al. Retinal vascular biomarkers for early detection and monitoring of Alzheimer’s disease. Transl Psychiatry. 2013;3:e233
- Csincsik L, MacGillivray TJ, Flynn E, et al. Peripheral retinal imaging biomarkers for Alzheimer’s disease: a pilot study. Ophthalmic Res. 2018;59:182–192
- Jiang H, Liu Y, Wei Y, et al. Impaired retinal microcirculation in patients with Alzheimer’s disease. PLoS ONE. 2018;13:e0192154
- Leroi A, Himmelsbach I, Wolski L et al. Assessing and managing concurrent hearing, vision and cognitive impairments in older people: an international perspective from healthcare professionals. Age and Ageing 2019; 48: 580–587
