Sleep represents the period when our body is relaxing and preparing for the forthcoming periods of intense physical and mental activity. The sleep drive builds up progressively, with a peak in the evening. Nevertheless, a good sleep-wake cycle depends on many variables, including an appropriate exposure to light.
As in the UK more than two million people live with various degrees of visual impairment, it is important to have an idea as to whether they also suffer from various sleep disturbances that may have an effect on their treatment compliance, disease progression, management and quality of life.
Biological Rhythms
Biological rhythms are subdivided into three types based on their duration:
- Circadian rhythms – approximately 24 hours duration
- Ultradian rhythms – lasting some hours, minutes or seconds
- Infradian rhythms – may last over days or even months
Introduced by Halberg in 1959, the term circadian (from the Latin ‘circa’ meaning approximately and ‘diem’ meaning day) defines a biological cycle with a period of approximately 24 hours.¹ These rhythms are the most studied and represent an important biological regulator in every organism.²
In humans, the biologic clock is genetically inherited and the dominant pacemaker resides in the suprachiasmatic nuclei (SCN) found within the anterior hypothalamus.³ Environmental changes also influence the central clock, both via the light-dark cycle but also via various behavioural and social rhythms, enabling individuals to anticipate certain changes during their own routine.4 In addition to the SCN, most peripheral tissues also contain peripheral, molecular clocks that regulate, amongst other things, sympathetic function and vascular tone.5
Mammalian Anatomy
In the mammalian retina, light stimulates the photoreceptor cells (rods and cones) and a signal is transmitted via bipolar cells to ganglion cells, the majority of which send their axons via the optic nerves to the lateral geniculate nuclei (LGN).
In addition to this vertical pathway (photoreceptor cell, bipolar cell, ganglion cell), horizontal and amacrine cells run tangentially across the retina, and interplexiform cells connect the two plexiform layers. However, not all retinal ganglion cells (RGCs) direct their axons to the LGN. A quite separate class of RGCs, the ‘intrinsically photosensitive retinal ganglion cells’ (or ipRGCs) representing some 1 to 2% of the total RGC population, send their axons to the SCN.
ipRGCs do not depend on stimulation via the photoreceptor cells. They contain light-sensitive melanopsin which renders them intrinsically sensitive to photic stimulation. When light falls upon them (and remember, the mammalian retina is ‘inverted’), their membranes are depolarised and a signal is sent to the SCN. The RGCs also regulate a range of responses such as acute suppression of melatonin, pupillary constriction, alertness and masking.6,7
They send an input to the SCN through the retino-hypothalamic tract (RHT), thus influencing various rhythms, such as body temperature, hormone levels, and activity. They also send signals to the intergeniculate leaflet (IGL), the olivary pretectal nucleus (OPN), the lateral hypothalamus and the preoptic regions.
In turn, SCN acts on the endocrine system by timing the ‘sleep-dependent’ hormonal secretion as well as through a ‘sleep-independent’ mechanism; the latter allows secretion of important hormones, such as melatonin and cortisone to continue even during sleep deprivation periods.4
As the day progresses, the SCN transmits signals that contribute to change levels of cortisol and initiates the secretion of melatonin from the pineal gland8 along with other sleep hormones. Advanced age is thought by some to contribute to an abnormal melatonin production which, in turn, will result in abnormal circadian rhythmicity and accelerated ageing-related processes with an impact throughout the human body, including upon the eye and cardiovascular system.
Ocular Impact
Melatonin, a truly pleiotropic molecule (producing more than one effect), contributes not only to the regulation of circadian rhythms and sleep patterns but also to the maintenance of retinal health by influencing the retinal pigment epithelium and controlling the amount of light reaching the photoreceptors.
In addition, it also acts as a potent antioxidant at both ocular and systemic levels, reducing the risk of ocular diseases (such as age-related macular degeneration) as well as systemic diseases (such as cardiovascular disease).9
Melatonin production can be affected by ageing, as pupil diameter decreases and light absorption by the crystalline lens increases with age.10 Indeed, age-related pupillary miosis and crystalline lens transmission changes are the two main contributory factors which affect the circadian cycle, both reducing the light available for melanopsin stimulation.
Sleep Disorders
Sleep disorders are many, and their complete description is beyond the scope of this article. A selection of the most common ones is presented in table 1.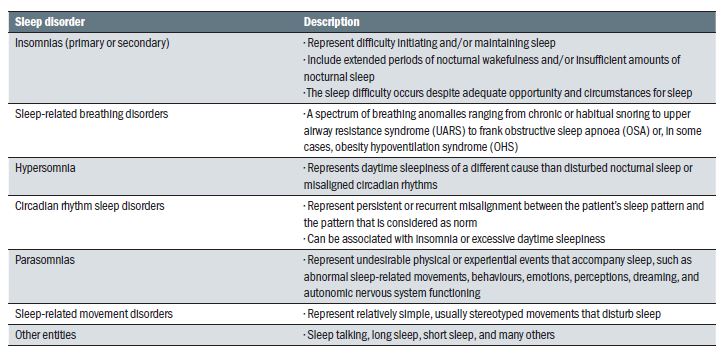
Table 1: Some common sleep disorders (adapted from Thorpy30)
A disturbed circadian rhythm due to abnormal light stimulation can lead to insomnia, short sleep duration, sleep fragmentation and exhaustion, which, in turn, will result in social isolation, depression, anxiety, increased morbidity and even mortality. Indeed, a recent study found that, after adjusting for all the variables such as depression, presence of sleep apnoea and socioeconomic status, the presence of visual impairments was a predictor for abnormal sleep durations.11
In addition, fatigue, as a result of these patients’ difficulty of processing visual stimuli, is also present in association with irreversible visual impairments.12
Sleep disturbances and sight loss
When the eye is exposed to light, the pineal melatonin secretion is inhibited. Abnormal melatonin rhythms in the sight impaired were first reported in 1981. Based on a study of ‘four blind men,’ it was found that melatonin levels were higher in the day compared to night time.13 Leger et al found that 26 subjects without light perception (with free running rhythms as based on melatonin secretion) had reduced total sleep time when compared to a normally sighted control group.14
Two other epidemiological studies by Leger et al found a higher prevalence of insomnia in non-sighted individuals than in normal controls (36 % versus 26%). Similarly, the prevalence of severe insomnia was also higher in the non-sighted group (28%) relative to the control group (15%).15,16
Tabanedeh et al also looked into this topic. Of the 388 non-sighted participants included in their study 48.7% suffered from sleep problems. Individuals with sleep problems also reported several other sleep related complications, including:
- interruptions in sleep (46.8%)
- increased sleep latency (40.3%)
- reduced duration of sleep (36.0%)
- daytime sleepiness (17.3%)
- irregular sleep (9.4%)
Subjects with no light perception had a higher incidence of severe disturbances of sleep (12.1%) when compared to subjects with some degree of light perception (6.4%). The overall conclusion of the study was that there was a highly significant relationship between the degree of sleep abnormalities and the level of visual loss.17
Sleep disturbances and ocular diseases
Cataract (figure 1)
Although it has been proposed that cataract is associated with a disturbed sleep cycle, more recent studies did not find such an association after adjusting for all the confounders.18 Nevertheless, other research showed that cataract surgery significantly improved subjective sleep quality irrespective of the IOL type implanted, therefore contributing to an increase in overall health.19
Figure 1
Glaucoma (figure 2)
It has been demonstrated that in patients suffering from glaucoma, oxidative stress, high levels of glutamate, abnormal nitrous oxide synthesis and mitochondrial damage all contribute to neuronal damage and ipRGC death.20 By affecting the ipRGS and their neurones, glaucoma could potentially interfere with the body’s circadian rhythm. Indeed, sleep disorders, anxiety and depression have all been reported in glaucoma patients as well.21
Moreover, glaucoma patients also seem to demonstrate changes in the plasma melatonin levels with subsequent alterations in various circadian rhythms, including those of the intraocular pressure and, possibly, ocular blood flow due to an influence upon systemic blood pressure.22
As a result, glaucoma is increasingly regarded as the ocular disease most likely to lead to photo-dependent circadian rhythm alterations and melatonin production impairment.23 In addition, it has been shown that glaucoma patients with severe visual field defects have higher risk of depression, anxiety and overall sleep disturbances compared to those with minor field loss.24
Figure 2
Age-related macular degeneration (figure 3)
It has been demonstrated that AMD is associated with abnormal sleep patterns. This could be the result of the fact that all three photoreceptor types (rods, cones, and ipRGCs) are affected in advanced AMD.25 In addition, it has also been proposed that, when present in AMD, sleep apnoea hinders the response of exudative AMD to intravitreal bevacizumab.26 Moreover, insomnia has been identified as a marker for an increased risk of developing AMD. It is also important to add that, as melatonin has been shown to have the capacity to control eye pigmentation, regulate the amount of light reaching the photoreceptors, and to protect the retinal pigment epithelium (RPE) from oxidative damage, treatment with melatonin in patients with AMD could not only regulate their sleep patterns but also protect the retina and to delay macular degeneration.27
Figure 3
Retinitis pigmentosa (figure 4)
A study of patients suffering from retinitis pigmentosa (RP) found that of a total of 177 participants suffering from this disease, 76% had sleep disturbances. Nevertheless, the authors were sceptical as to whether this was totally related to the ocular pathology itself.28 In addition, another study reported that patients with RP presented with increased daytime sleepiness, reduced alertness and night-time sleep of poorer quality than the normal controls. Melatonin was also suggested as possible treatment to help with the outcome of these patients.29
Figure 4
Conclusion
Sleep disturbances, as well as their complications such as depression and anxiety, are important to detect in visually impaired patients as they have a potentially negative influence on treatment compliance, disease progression and patients’ quality of life.
Visually impaired patients tend to compensate for their vision loss by adopting various other coping routines in order to maintain their daily functioning. These usually require a high level of concentration and mental preparation, therefore resulting in even more fatigue, mood alterations and sleep disruptions. Low-vision professionals should be aware of these issues and adapt their interventions to each patient’s capacity to cope. Patients should also be advised on other techniques and help, such as relaxation, socialising, external support and acceptance.23
Patients should be trained to practice good sleep hygiene when possible, such as going to bed at around the same time, creating a relaxing routine and avoiding food or drink before bed. In addition, totally blind patients will need to be referred for behavioural modification interventions and treatment with melatonin.
All this advice is simple but useful, and should be reinforced by all the health professionals that care for these patients because it can make a big difference.
It is debatable, whether specific questions regarding the patients sleeping patterns should be a part of a routine history and symptoms taking by the average optometrist. Nevertheless, by picking up on the existence of such problems, optometrists can play an important role in ensuring a better outcome for their patients. Further work is necessary to increase awareness and additional professional training may be needed for better management and treatment of our patients with such risks.
Dr Doina Gherghel is an academic ophthalmologist with special interest in inter-professional learning for optometrists. She also leads the new Geriatric Optometry module at Aston University.
For further information, go to pgadmissions@aston.ac.uk
References
- Halberg, F. Physiologic 24-hours periodicity: General and procedural considerations with reference to the adrenal cycle. Z Vitamin-Hormon-Ferment-Forsch, 1959; 10: 225.
- Smolensky M. H., Haus, E. Circadian rhythms and clinical medicine with applications to hypertension. American journal of hypertension, 2001; 14: 280S.
- Rensing, L., Meyer-Grahle, U., Ruoff, P. Biological timing and the clock metaphor: Oscillatory and hourglass mechanisms. Chronobiology international, 2001; 18: 329-69
- Hastings, M., O’Neill, J., Maywood, E. Circadian clocks: Regulators of endocrine and metabolic rhythms. The Journal of endocrinology, 2007; 195: 187-98.
- Agarwal, R. Regulation of circadian blood pressure: From mice to astronauts. Current opinion in nephrology and hypertension, 2010; 19: 51.
- Hattar, S., Lucas, R. J., Mrosovsky, N., et al. Melanopsin and rod-cone photoreceptive systems account for all major accessory visual functions in mice. Nature, 2003; 424: 75-81.
- Cajochen, C., Munch, M., Kobialka, et al. High sensitivity of human melatonin, alertness, thermoregulation, and heart rate to short wavelength light. The Journal of clinical endocrinology and metabolism, 2004; 90: 1311-6.
- Lubkin V, Beizai P, Sadun AA. The eye as metronome of the body. Surv Ophthalmol 2002; 47: 17-26
- Reiter, R. J., Tan, D. X., Paredes, S. D. and Fuentes-Broto, L. Beneficial effects of melatonin in cardiovascular disease. Ann Med, 2010; 42: 276-85.
- Turner, P. L. and Mainster, M. A. Circadian photoreception: Ageing and the eye’s important role in systemic health. Br J Ophthalmol, 2008; 92: 1439-44.
- Ramos AR, Wallace DM, Williams NJ et al. Association between visual impairment and sleep duration: analysis of the 2009 National Health Interview Survey (NHIS). BMC Ophthalmology, 2014; 14: 115
- Schakel W, Bode C, van der Aa HPA et al. Exploring the patient perspective of fatigue in adults with visual impairment: a qualitative study. BMJ Open 2017; 7:e015023
- Smith JA, O’Hara J., Schiff AA. Altered diurnal serum melatonin rhythm in blind men. Lancet. 1981: 933
- Léger D, Guillemïnault C, Santos C, et al. Sleep/wake cycles in the dark: sleep recorded by polysomnography in 26 totally blind subjects compared to controls. Clin Neurophysiol. 2002; 113: 1607-1614.
- Léger D, Guillemïnault C, Defrance R, et al. High frequency of sleep/wake disorders in blind individuals. Lancet. 1996; 348: 830-831
- Léger D, Guillemïnault C, Defrance R, et al. Prevalence of sleep/wake disorders in persons with blindness. Clin Sci. 1999; 97: 193– 199
- Tabandeh H, Lockley SW, Buttery R, et al. Disturbance of sleep in blindness. Am J Ophthamol. 1998; 126: 707–712
- Chen Y, Nondahl DM, Schubert CR, et al. The relation between sleep disruption and cataract in a large population study. Ophthalmic Epidemiol 2017; 24:111-115
- Zeng L, Wu XH, Lin HT. The effect of cataract surgery on sleep quality: a systematic review and Meta-analysis. Int J Ophthalmol 2017; 10: 1734-1741
- Agorastos A, Huber CG. The role of melatonin in glaucoma. Implications concerning pathophysiological relevance and therapeutic potential. J Pineal Res 2011; 50: 1-7.
- Mabuchi F, Yoshimura K, Kashiwagi K. 2008: High prevalence of anxiety and depression in patients with primary open-angle glaucoma. J Glaucoma 17: 552-557
- Chiquet, C., Gronfier, C., Rieux, C., et al. 2004. Reduced sensitivity to light suppression of nocturnal plasma melatonin in glaucoma patients. Invest Ophthalmol Vis Sci, 45, U425.
- Drouyer E, Dkhlssl-Benyahya O, Chiquet C, et al. Glaucoma alters the circadian timing system. PLoS ONE 2008; 3: e3931
- Agorastos A, Skevas C, Matthaei M, et al. Depression, anxiety and disturbed sleep in glaucoma. J Neuropsychiatry Clin Neurosci 2013; 25: 205-213
- Maynard ML, Zele AJ, Kwan AS et al. Intrinsically Photosensitive Retinal Ganglion Cell Function, Sleep Efficiency and Depression in Advanced Age-Related Macular Degeneration. IOVS 2017; 58:990-996
- Schaal S, Sherman MP, Nesmith B, et al. Untreated obstructive sleep apnea hinders response to bevacizumab in age-related macular degeneration. Retina 2016; 36:791-797
- Yi C, Pan X, Yan H, et al. Effects of Melatonin in Age-Related Macular Degeneration. Ann NY Acad Sci 2006 https://doi.org/10.1196/annals.1356.029
- Gordo MA, Recio J, Sanchez-Barcelo EJ. Decreased sleep quality in patients suffering from retinitis pigmentosa. J. Sleep Res. 2001; 10: 159-164
- Ionescu D, Driver HS, Heon E, et al. Sleep and daytime sleepiness in retinitis pigmentosa patients. Journal of Sleep Research 2001 https://doi.org/10.1046/j.1365-2869.2001.00271.x
- Thorpy MJ. Classification of Sleep Disorders. Neurotherapeutics. 2012 Oct; 9(4): 687–701.
