In this article, I will discuss the latest developments in laser treatment of glaucoma. I will also argue that we might consider selective laser trabeculoplasty (or SLT) as a technique of laser trabecular modulation rather than surgery, and therefore one that falls well within the scope of treatments to be undertaken by specialist optometrists (figure 1). Perhaps LTM would be a better description?
Figure 1: The author undertaking selective laser trabeculoplasty.
Overview
Classically, it has been standard practice initially to treat glaucoma and ocular hypertension with eye drops, such as prostaglandin analogues used alone or as a combination with a second or third drop, such as a beta-blocker, carbonic anhydrase inhibitor or alpha agonist. The exact treatment is patient dependent, and each type has a different mechanism of lowering IOP, so chosen as appropriate if further pressure lowering effect is needed beyond that offered by the first choice drop.
However, pharmaceutical options have potential side effects, such as ocular surface toxicity, and are also dependent upon patient compliance. Maintaining regular dosing and correct instillation of drops often prove challenging. Many patients being treated for glaucoma still will still progress towards blindness in one eye. There could be several possible reasons for this, but one of the most likely is poor or non-compliance with medical treatment. Reasons for this include the fact that older patients are known to forget to instil their drops, or that patients dislike the side effects, such as discomfort, caused by drop use.
Clinicians can turn firstly to laser treatment, and then surgery such as trabeculectomy, if adequate control of IOP cannot be obtained with drops alone, or where there is evidence of ocular allergy, toxicity, or perhaps serious systemic side effects indicating the drops should be discontinued. A full discussion of surgical treatment of glaucoma is outside the scope of this feature – see Optician 10.07.15 for a useful review.
It was in the 1970s that a range of relatively high power lasers were first used in an attempt to lower IOP by puncturing through the trabecular meshwork to Schlemm’s canal. This treatment went by the name of goniopuncture. Unfortunately, these holes closed within weeks due to fibrous scarring, so benefit was short-lived.
In 1979, Wise and Witter developed the argon laser trabeculoplasty (ALT) technique, in which much lower power laser burns were made to the trabecular meshwork to cause coagulation and tension in the trabecular meshwork. This change to the structure of the trabecular meshwork was described as trabeculoplasty and ALT produced a meaningful and relatively sustained reduction in IOP. Since the early 2000s, selective laser trabeculoplasty (SLT), which uses far less energy, has largely taken over from ALT.
In addition to avoiding the problems associated with drops as described, patients are less affected by nocturnal IOP spikes after laser trabeculoplasty as the effect on drainage is constant for the full 24-hour period.1 Generally, ALT or SLT can replace one glaucoma medication, often the prostaglandin analogue.2
ALT utilises an argon green gas laser, whereas the Nd:YAG laser used in SLT is a solid state Q-switched, frequency-doubled laser. The Q-switched Nd:YAG laser can deliver the very short pulses of light required for SLT. The same laser Nd:YAG can also be used in its non-frequency doubled, 1064nm mode for photo-disruptive treatments, such as YAG capsulotomy and peripheral iridotomy.
We will now look at ALT and SLT in greater detail.
Laser Treatment Overview
Laser trabeculoplasty is the most common laser procedure in treating open angle glaucoma. Several studies show the effectiveness of laser trabeculoplasty in IOP control in glaucoma.3 Initially, we had ALT in the 1990s through to the early 2000s. Since then, selective laser trabeculoplasty (SLT) has largely taken over. Two other variants are micropulse diode laser trabeculoplasty (MDLT) and titanium/sapphire laser trabeculoplasty (TSLT) which are not discussed further in this article.
Another novel variation of SLT is its use via the trans-scleral route, though I have not seen this performed personally. In this technique, the laser is applied to the sclera overlying the trabecular meshwork. This is reported to produce an equivalent IOP reduction to conventional SLT.
Expected IOP reduction with ALT or SLT is normally 20 to 30% when used as a primary procedure. At five years, it is effective in maintaining target IOP in 50% of cases, and 30% at 10 years. It tends to be very effective for the first one to three years, with the effect waning after this period. This means that a repeat procedure is required in these cases.4 It should be noted, however, that with ALT, any repeat procedure can only be performed on areas not already treated. ALT treatment initially involves applying a series of small high energy laser burns in the anterior portion of the pigmented trabecular meshwork. These cause thermal damage and shrinkage of the trabecular meshwork, opening up intra-trabecular spaces, improving aqueous drainage and lowering the intra-ocular pressure.5
Electron microscopy of the trabecular meshwork after ALT (see the left hand image of figure 2) clearly shows the laser burn, whereas following SLT there is no coagulation or destruction, merely an open trabecular meshwork (see the right hand image of figure 2).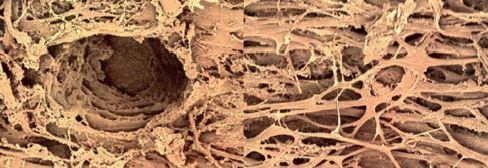
Figure 2: Scanning electron microscope images of the trabecular meshwork after trabeculoplasty showing the damage after ALT (left) and after SLT (right).
The destruction caused by ALT is key to its pressure lowering effect, as the contraction of scar tissue pulls the remaining untreated trabecular structure open. An SLT treated eye, by comparison, has a normal trabecular meshwork appearance. The IOP lowering in this instance is due to the mobilisation of macrophages which ‘clean up’ the pigment and other cellular debris within the meshwork, so increasing its permeability.
Because the treated area has been permanently modified in ALT, once burnt the area cannot be retreated. In fact, retreating could eventually lead to synechial angle closure and a decrease in outflow facility.6-10
Additional problems, specific to ALT, include anterior synechiae and pain which can result if the high intensity burn is placed too close to the iris or ciliary body. Rarely, ALT can cause bleeding within the angle. This is normally transient and is controlled by exerting pressure (with the goniolens) until clotting occurs. Corneal clouding may occur if high energy ALT burns are placed too far anteriorly, especially where there is a narrow angle.
Contraindications to trabeculoplasty
Both ALT and SLT require less energy if used in heavily pigmented trabecular meshworks. In cases of pigment dispersion glaucoma and pseudo-exfoliation, extra care is required to ensure that over-treatment does not occur. Many clinicians advocate initially treating only a few degrees to judge the reaction to the laser before proceeding to treat a further section. These patients often will end up with between 45 to 180 degrees being treated.11
Other contraindications to laser trabecular treatments include the following:12
- Advanced POAG, where a post-treatment pressure spike might ‘snuff out’ part of the remaining visual field
- Angles too narrow to view the trabecular meshwork (even with miotics)
- Neovascular or inflammatory glaucoma
- Angle recession
- Excessively hazy cornea
SLT can act as a stimulus for increased vascular proliferation in neovascular glaucoma. Corneal opacification, due to scarring or dystrophy for example, can make visualisation of the angle difficult during treatment, and might absorb laser energy and cause further corneal damage. In cases of excessive corneal oedema, such as in bullous keratopathy, hypertonic saline can be used for some days beforehand to improve corneal clarity.
Adverse reactions
The most common adverse reactions after either ALT or SLT are an IOP spike or anterior uveitis. Normally, apraclonidine or brimonidine drops are instilled before and after treatment to reduce immediate post-surgical pressure spikes. Some practitioners instil ketorolac (a non-steroidal anti-inflammatory drug) or dexamethasone (steroid) drops afterwards, to reduce anterior chamber inflammatory activity. Conversely, others recommend not doing so unless absolutely necessary, as the SLT treatment is attempting to up-regulate inflammatory activity in the trabecular meshwork and anti-inflammatories, steroids in particular, may interfere with this process.
Selective Laser Trabeculoplasty
The recent NICE Light Study,3 led by Moorfields Eye Hospital glaucoma consultant Professor Gus Gazzard (figure 3), recommends that SLT be considered as an early intervention and, in many cases, instead of eye drops to treat POAG and ocular hypertension. The American Academy of Ophthalmology preferred practice guidelines also state ‘laser trabeculoplasty can be considered as initial therapy in selected patients’. Figure 3: Professor Gus Gazzard, Moorfields Eye Hospital glaucoma consultant.
Figure 3: Professor Gus Gazzard, Moorfields Eye Hospital glaucoma consultant.
Not only is SLT found to be as effective or better than many existing first line drug treatments, it represents a major saving compared to the cost of eye drops and a significant improvement in patient lifestyle with freedom from eye drops.3,13,14 It has been estimated that, if all newly diagnosed cases were treated with SLT rather than drops, the NHS would save £1.5m annually.3 The savings would be many times more if SLT was introduced for existing glaucoma patients already under treatment. It has also been found that SLT is more effective if eye drops have not been used beforehand.
Figure 4, adapted from a study by Nagar et al,15 shows how the effect upon the IOP of 360° SLT is comparable to treatment with topical latanaprost over the course of one year. It can also be seen that the full IOP lowering effect is often not seen until four to six weeks after treatment. In an established glaucoma patient under treatment, any medication currently in use is often continued for the first month or so and then phased out if no longer required. Some clinicians advocate stopping prostaglandin analogue use prior to SLT, as the drugs have a similar physiological effect upon the trabecular meshwork as SLT. They suggest that, instead, the patient should change to a drop with a different mode of action until the full effect of the SLT has been realised. The use of pilocarpine is also common practice during SLT, as it may improve the clinician’s access to the angle structures as well as mitigate postoperative elevations in IOP.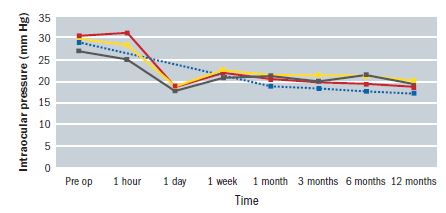 Figure 4: The impact upon IOP of treatment with topical latanoprost (blue line), 90°SLT (grey line), 180°SLT (yellow line) and 360°SLT (red line)15
Figure 4: The impact upon IOP of treatment with topical latanoprost (blue line), 90°SLT (grey line), 180°SLT (yellow line) and 360°SLT (red line)15
Because of their similar physiological effect, the impact of prostaglandin analogues can be used to predict those patients who are likely to be successful with SLT treatment.12 Newly diagnosed glaucoma and ocular hypertensive patients, whose pressure drops well in response to evaluative prostaglandin drops, are likely to do well with SLT.2 In the same vein, patients already on prostaglandin analogues are unlikely to get as much additional pressure lowering benefit from SLT treatment when compared with those on another drop, such as a beta-blocker. Also, patients who need further IOP reduction after SLT are probably better treated with a different family of glaucoma drops to the prostaglandins.
SLT works better in patients who have a higher presenting IOP. Several SLT studies have shown an average reduction in IOP of only 15% in normal tension glaucoma patients, which is probably to be expected. However, a reduction in nocturnal IOP spikes was still thought to be highly beneficial in these patients.16
Energy Levels in SLT – is it Surgery?
Figure 5 offers a summary of differences between ALT and SLT. Many clinicians currently consider SLT a form of ‘surgery’. This may be a valid opinion regarding ALT, as it requires a high power spot of laser energy (normally of the order of 600W or 600 mJ s-1 over a 50 micron target area) to be focused onto the trabecular meshwork where it causes burns, then scarring, then shrinkage of the adjacent trabecular meshwork, so opening it up to improve drainage. This tightening effect is not dissimilar to the effect upon the trabecular meshwork of cataract extraction.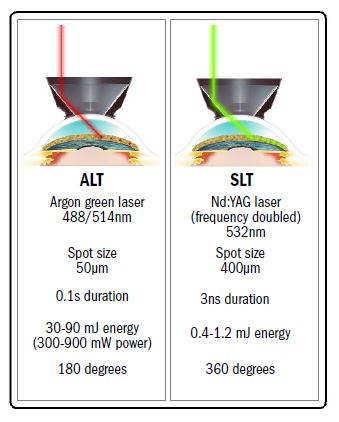
Figure 5: Some key differences between ALT and SLT.
The laser stimulation in SLT is, however, of significantly lower energy levels, often less than 0.5 mJ for only a three nanosecond treatment time, and over a much larger target area of some 400 microns. This, by my calculation, means that the spot intensity is an amazing 1012 (a trillion) times ‘weaker’ than with ALT.
The short treatment pulse means there is not enough time for the trabecular melanin pigment to convert the electromagnetic laser energy into heat energy. This is because the thermal relaxation time of melanin is one microsecond, many times longer than the three nanoseconds duration of the SLT pulse. The laser exerts a ‘cold biological’ effect whereby the laser energy activates chemical mediators that attract macrophages and other phagocytes. These then clean up the debris in the trabecular meshwork, thereby improving its throughput of aqueous, and so lowering the IOP.
The Equipment Used
Two of the main laser gonio lenses used for SLT are:
Figure 6: The original single mirror Latina lens.
- Single mirror Latina lens (figure 6) – developed by Mark Latina, one of the original pioneers of SLT.17
- Volk Rapid SLT 4 mirror lens (figure 7) – this amazing new lens does exactly what it says on the box, enabling you to rapidly treat the whole 360 degrees of the trabecular meshwork, only having to rotate the lens 22.5 degrees once. The multiple angle view makes the whole procedure very quick and improves the patient experience. As there is no blanching with SLT treatment, the new lens makes it easier not to lose the location you are treating because the lens is only rotated once during treatment.
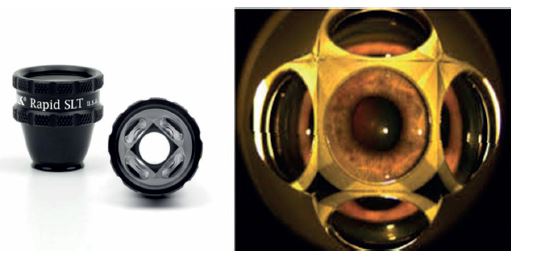
Figure 6: The new Volk Rapid SLT lens.
The Technique – Step-by-Step
- Anterior eye exam and gonioscopy, to rule out contraindications, including:
- Neovascular glaucoma
- Uveitis
- Plateau iris
- Angle recession
- Anterior synechiae
- Educate and counsel patient
- Patient signs an informed consent document
- Instil apraclonidine or brimonidine drops, 15 minutes before the treatment
- Instil pilocarpine if the angle is narrow or the iris has a convex profile
- Instil topical anaesthetic, typically benoxinate
- Set the microscope eyepieces to between 16x to 25x magnification
- Position the SLT lens on cornea and then initiate laser application
- Apply laser to the whole 360 degrees of trabecular meshwork, with approximately 25 treatment spots per quadrant
- Use the minimum laser energy setting required to obtain small bubble formation, and then reduce the energy level by 0.1mJ so bubble formation ceases
- Regularly repeat titration of power setting, as requirements will vary depending on the pigmentation in different quadrants of the trabecular meshwork
- Avoid pigment blanching
- In more heavily pigmented eyes, fewer clock hours are treated initially as the treatment is likely to have greater impact
- Avoid areas of anterior synechiae and blood vessels
- After the SLT lens is removed, instil a further drop of apraclonidine or brimonidine
- Check IOP 15 to 30 minutes later
- Optional ketorolac or dexamethasone drops for one week, or as required
- Review the patient again in one to two weeks, and confirm a reduced IOP and lack of anterior chamber activity
- Review after six weeks, at which point the IOP measurement will dictate whether to reduce the use of existing glaucoma drops
- The second eye may now be treated, once the full effect on first eye has been evaluated.
Optometrists and SLT
SLT is considered by many to be relatively straightforward to perform, as the precise location of the patch of laser stimulation is not so critical owing to its use of a larger spot size and a lower energy laser. The whole width of the trabecular meshwork is irradiated with the low intensity laser light during SLT. In ALT, a small, high intensity spot must be focused on the anterior aspect of the pigmented trabecular meshwork. In ALT usually 180 degrees is initially treated with 50 to 60 burns, approximately two spot diameters apart. SLT, by comparison, usually treats 360 degrees with 100 treatment spots.
There have been studies that show how SLT can work effectively at even lower energy levels than the usual 0.8 mJ. In one such study,18 very low energy SLT (0.2 to 0.4 mJ) was performed annually and gave effective IOP control in 85% of patients after five to seven years, compared to 47% with conventional SLT on an ‘as needed’ basis and 38% with ALT treatment.
The two main stumbling blocks to the universal adoption of SLT as a first line treatment are the initial cost of the laser and the professional time demands to implement it. As with intra-vitreal injections, I feel this is where therapeutic optometrists can rise to the occasion, requiring only a moderate amount of additional training to build on their already impressive slit-lamp skills. Glaucoma specialist therapeutically qualified optometrists are an obvious first cohort to include in a pilot study to prove to ophthalmology that this group can provide an SLT service that is both safe and cost effectively.
Over 300 optometrists are therapeutically qualified (as DipTpIP) and 30 to 40 have a specialist higher glaucoma management qualification (DipGlauc). If they have both qualifications, they are entitled independently to treat ocular hypertension (raised IOP likely to progress to glaucoma if left untreated) and to manage established glaucoma, once an ophthalmologist has made the initial diagnosis and referred the patient to them.
Hopefully, if enough therapeutic optometrists get accreditation and experience in these techniques and enough of our broader minded ophthalmology colleagues get behind us, optometrist-led glaucoma/ocular hypertension treatment with laser can become a reality.
Owing to the low power used, and the fact that the accuracy of placing the treatment spot is less critical, as well as the knowledge that gonioscopy is a skill most specialist optometrists already possess, SLT by optometrists should be considered a safe procedure. A study by Nisha and Teng19 evaluated how long the learning curve was to perform SLT. They found that there was no difference in outcomes between a trainee doctor’s performance during their first SLT procedure when compared to later treatments. This would, again, suggest that SLT would be well within the remit of a suitably trained specialist optometrist.
I would argue that SLT should be redefined as being non-surgical, as there is no photocoagulation, burning, or photodisruption during treatment and no major change in structure is induced. Perhaps, it should have a new name – LTM or Laser Trabecular Modulation. This reflects more accurately what is actually happening. If this redefinition is accepted by the various authorities concerned, it is likely that LTM (or whatever it ends up being called), could be reclassified as a non-surgical procedure, just as is the case with the high energy light pulse treatment of the lids (IPL), able to be performed by suitably accredited optometrists.
Glaucoma specialist therapeutic optometrists currently treat and manage established glaucoma patients referred to them by ophthalmologists, currently with drugs, and independently diagnose and preventively treat patients with elevated eye pressure that has not progressed to glaucoma as yet (ie ocular hypertensives), thereby avoiding them suffering unnecessary loss of sight. Current treatment is mainly with eye drops, however, using SLT means that a patient may have adequate pressure control to prevent glaucoma medication for two to five years, after which time it can be repeated as no laser damage has been done to the trabecular meshwork during treatment.
In many ways, SLT is similar to the light and laser therapies offered by many beauticians in high streets. Indeed, SLT uses much lower energy levels than optometrists currently use in IPL to treat dry eye and meibomian gland dysfunction.
Often therapeutic optometrists are the first port of call for ocular emergencies, using steroid and NSAIDs to treat inflammatory conditions such as cystoid macular oedema and anterior uvietis, antibiotics for infective disease and ocular hypertensives (both oral and topical) to control both chronic and acute raised IOP. In the course of anti-VEGF treatment, I use drugs such as iopidine and brimonidine to prevent pressure spikes. I am proficient at lowering IOP post injection with ocular massage. In the hospital environment, I have injected intra-vitreal pellets and performed paracentesis. I work closely with ophthalmology both in the NHS and private sectors.
All these things are clinically more demanding than performing and managing SLT. Drugs that are commonly used during SLT include miotics, steroids and ocular hypertensives; all well within the remit of a suitably trained and experienced therapeutic optometrist.
SLT (possibly renamed LTM?) gives us a tool to improve patients’ lives, often by completely freeing them from the need to instil eye drops, for possibly up to five years between treatments. Its adoption will reduce the number of adverse reactions to medication, enable better nocturnal control of IOP fluctuations, eliminate non-compliance problems and save the patient or the NHS massive expenditure.
In order to achieve full benefit for the patient and the NHS, it is vital that there are enough people able to provide the service. Suitably trained and qualified optometrists are able to provide this treatment conveniently, safely and cost effectively. This will reduce the need for so many hospital review appointments and free our ophthalmology colleagues up for more demanding tasks.
We need to push for this to happen, both in the HES and in private practice.
Andrew Matheson is a Glaucoma Specialist Therapeutic Optometrist involved in glaucoma management and providing hospital laser surgery services.
References
- Lee AC, Mosaed S, Weinreb RN, Kripke DF, Liu JH. Effect of laser trabeculoplasty on nocturnal intraocular pressure in medically treated glaucoma patients. Ophthalmology. 2007;114(4):666-670.
- Francis BA, Ianchulev T, Schofield JK, Minckler DS. Selective laser trabeculoplasty as a replacement for medical therapy in open angle glaucoma. Am J Ophthalmol. 2005;140(3):524-525.
- Gazzard G et al. Laser in Glaucoma and Ocular Hypertension (LIGHT) Study. Lancet, March 2019.
- The Glaucoma Laser Trial Research Group. The Glaucoma Laser Trial (GLT). 2. Results of argon laser trabeculoplasty versus topical medicines. Ophthalmology. 1990;97(11):1403-1413.
- Kramer TR, Noecker RJ. Comparison of the morphologic changes after selective laser trabeculoplasty and argon laser trabeculoplasty in human eye bank eyes. Ophthalmology. 2001;108(4):773-779.
- Bradley JM, Anderssohn AM, Colvis CM, et al. Mediation of laser trabeculoplasty-induced matrix metalloproteinase expression by IL-1beta and TNF-alpha. Invest Ophthalmol Vis Sci. 2000;41(2):422-430.
- Acott TS, Samples JR, Bradley JM, Bacon DR, Bylsma SS, Van Buskirk EM. Trabecular repopulation by anterior trabecular meshwork cells after laser trabeculoplasty. Am J Ophthalmol. 1989;107(1):1-6.
- Dueker DK, Norberg M, Johnson DH, Tschumper RC, Feeney-Burns L. Stimulation of cell division by argon and Nd:YAG laser trabeculoplasty in cynomolgus monkeys. Invest Ophthalmol Vis Sci. 1990;31(1):115-124.
- Alvarado JA, Alvarado RG, Yeh RF, Franse-Carman L, Marcellino GR, Brownstein MJ. A new insight into the cellular regulation of aqueous outflow: how trabecular meshwork endothelial cells drive a mechanism that regulates the permeability of Schlemm’s canal endothelial cells. Br J Ophthalmol. 2005;89(11):1500-1505.
- Kramer TR, Noecker RJ. Comparison of the morphologic changes after selective laser trabeculoplasty and argon laser trabeculoplasty in human eye bank eyes. Ophthalmology. 2001;108(4):773-779.
- Ayala M, Chen E. Comparison of selective laser trabeculoplasty (SLT) in primary open angle glaucoma and pseudoexfoliation glaucoma. Clin Ophthalmol. 2011;5:1469-1473.
- Ayala M, Chen E. Predictive factors of success in selective laser trabeculoplasty (SLT) treatment. Clin Ophthalmol. 2011;5:573-576.
- Katz LJ, Steinmann WC, Marcellino G, SLT/MED Study Group. Presented at the American Academy of Ophthalmology annual meeting. November 2006.
- The SLT Med Study, Katz LJ et al, Journal of Glaucoma, sept 2012, 460-468
- Nagar M, Ogunyomade A, O’Brart DP, et al. A randomized, prospective study comparing selective laser trabeculoplasty with latanoprost for the control of intraocular pressure in ocular hypertension and open angle glaucoma. Br J Ophthalmol. 2005;89(11):1413-1417.
- Tojo N, Oka M, Miyakoshi A, Ozaki H, Hayashi A. Comparison of fluctuations of intraocular pressure before and after selective laser trabeculoplasty in normal-tension glaucoma patients. J Glaucoma 2014; 23(8): e138–e143.
- G Clews. Has the SLT light study shed new light on treating eye conditions? Acuity, summer 2019
- Gandolfi & Fechtner. Low-power Annual SLT Effective for Ocular Hypertension. Medscape, May 19th, 2014.
- Nisha C & Teng C, resident performed SLT in patients with OAG, Glaucoma Today, Jan/Feb 2015.
